Abstract
Early stages of B cell development take place in the bone marrow, resulting in formation of immature B cells, which migrate to the spleen for their final differentiation into mature cells. This final maturation step is essential for B cells to become responsive to antigens and to participate in the immune response. Previously, we showed that the MHC class II chaperone, invariant chain (Ii), controls the differentiation of B cells from the immature to the mature stage. In this study, by generating transgenic mice expressing truncated Ii lacking its luminal domain, we could dissect the chaperonin activity of Ii from its role in B cell maturation. We demonstrate in vivo that Ii N-terminal domain is directly involved in the maturation of B cells and is sufficient to promote B cell differentiation.
Keywords: transgenic mice‖N-terminal domain‖immature B cell‖mature B cell‖antigen presentation
B cell development involves the ordered progression of a stem cell through a number of stages, ultimately resulting in a mature B cell. There are various selective criteria that the cell must fulfill to complete this program. In the bone marrow, B cell development can be divided into different stages, based on the rearrangement status of the IgH and IgL chain loci (1, 2) and the expression of intracellular and surface-bound markers. This developmental program is controlled largely by a set of transcription factors and signaling pathways. The first cells expressing IgM at their surface during this developmental process are the immature B cells that leave the bone marrow and migrate to the spleen (3, 4). These immature B cells then penetrate the marginal zone sinus and reside in the outer zone of the periarteriolar lymphocytic sheath (5), where they become part of the B cell-rich follicular areas (5, 6). At this site in the spleen, B cells are still immature and can be distinguished from their mature counterparts (3, 7–9). The transition from immature to mature B cells is characterized by a series of changes in surface marker expression and in the activities of these cells. Recently, this transition stage was characterized further and divided into two populations. Transitional B cells of type 1 (T1) are the recent immigrants from the bone marrow. These cells develop into transitional B cells of type 2 (T2), which cycle and are found exclusively in the primary follicles of the spleen (10). Only 5–10% of the newly generated immature B cells are selected into the pool of long-lived, antigen-responsive mature B cells (9, 11). Why and how only such a small proportion of immature B cells is selected and by what molecular mechanism this selection occurs are largely unknown.
Previously, we have shown that invariant chain (Ii), a MHC class II chaperone, plays a crucial role in B cell maturation (12). MHC class II molecules are heterodimeric complexes that present foreign antigenic peptides on the cell surface of antigen-presenting cells (APCs) to CD4+ T cells (13–15). MHC class II synthesis and assembly begin in the endoplasmic reticulum (ER) with the noncovalent association of the α- and β-chains with trimers of Ii, a glycosylated type II protein (16). Analysis of the role of Ii in class II-positive cells has become possible through the generation of Ii-deficient mice by gene targeting. Cells from such mutant animals show aberrant transport of MHC class II molecules, resulting in reduced levels of class II complexes at the cell surface. These mice have poor in vivo immune responsiveness to protein antigens, and the ability of their splenic APCs to present exogenous protein antigen in vitro in a class II-restricted fashion is reduced (17–19).
Until recently, the Ii chain was thought to function mainly as MHC class II chaperone. However, in our recent study of mice defective in Ii expression, we were able to show that Ii plays an essential role in B cell maturation as well (12). In these mice, the development from immature to mature B cells is impaired and B cells are arrested at an immature stage characterized by low expression levels of IgD and CD23 and poor response to T-independent antigens (12, 20, 21). In addition, we have shown recently that Ii-induced B cell maturation involves activation of transcription mediated by NF-κB p65/RelA homodimer and requires the B cell-enriched coactivator TAFII105 (22).
In this study, we investigated further the role of Ii in B cell differentiation. Using transgenic mice, we demonstrate in vivo that Ii N-terminal domain is directly involved in the maturation of B cells in a process that is independent of its chaperonic activity.
Materials and Methods
Mice.
C57BL/6 (control), Ii deficient on a C57BL/6 background (Ii−/−) (19), and Ii class II double-deficient mice (23) were used in this study.
Generation of 1–82 Transgenic Mice.
The type II transmembrane 1–82 cDNA (see Fig. 2A) (the kind gift of R. N. Germain, National Institutes of Health, Bethesda) was inserted into the Bluescript plasmid, under the control of the κ light chain promoter. This construct was injected into fertilized B6C3H eggs. The transgenic founders were backcrossed to Ii-deficient mice. The Ii heterozygous mice were backcrossed again to generate Ii-deficient animals, which exclusively express the 1–82 segment.
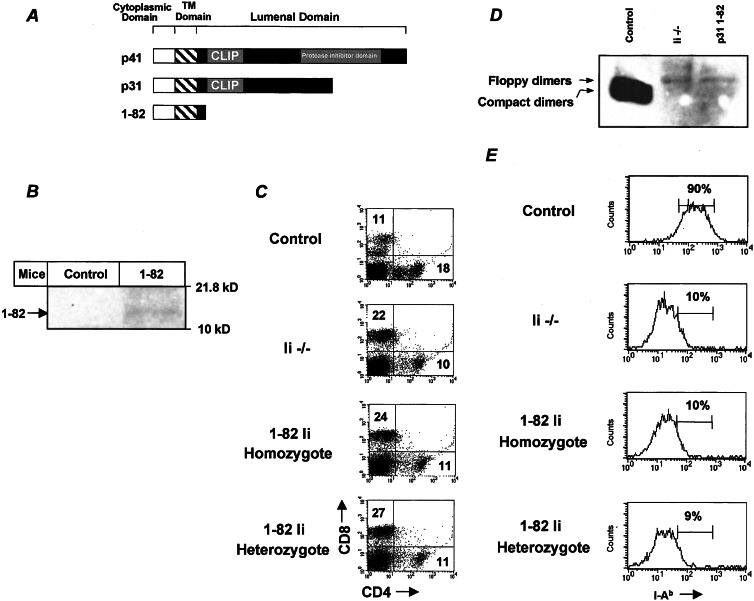
Figure 2.
Phenotype and lack of MHC class II expression of 1–82 transgenic mice. (A) A schematic illustration of Ii p31 and p41 isoforms and the 1–82 mutant used in this study. (B) Western blot analysis of 1–82 Ii expression in control and 1–82 mice. Splenocytes from control and 1–82 mice were lysed, and boiled lysates were separated on 15% (wt/vol) SDS/PAGE and analyzed with IN1 antibodies. (C) Splenocytes were double-stained with anti-CD8 and anti-CD4. Dot plots show the expression of these markers in each group of mice. The results presented are representative of five different experiments. (D) Analysis of steady-state levels of MHC class II expression. Splenocytes from control, Ii−/−, and 1–82 homozygotic mice were lysed, and unboiled lysates were separated on 12% (wt/vol) SDS/PAGE followed by Western blot analysis by using M5/114 antibodies. Floppy dimers and compact dimers are indicated by arrows. (E) Cells were double-stained with anti-B220 and anti-MHC class II. Histograms show the expression of MHC class II on B220-positive cells in each animal. The results presented are representative of seven different experiments.
Cells.
Spleen and lymph node (LN) cells were obtained from the various mice at 6–8 weeks of age as described (24). B cells were enriched by treating the splenocyte suspension with anti-Thy 1.2, CD4, and CD8 (Southern Biotechnology Associates) for 1 h followed by incubation with low Tox-M complement (Cedarlane Laboratories) for 1 h at 37°C.
Preparation of Cell Extracts and Western Blot Analysis.
Cells were pelleted and incubated with 50 μg/ml digitonin (Sigma). The pellet then was lysed as described (24). Lysates were resolved by SDS/PAGE and electroblotted onto nitrocellulose. The blots were blocked with 10% (vol/vol) skim milk for 1 h and then probed for 1 h with m5/114 (anti-MHC class II) or IN1 (anti-Ii chain cytoplasmic tail mAb) antibodies, followed by washing and 1-h incubation with horseradish peroxidase-conjugated goat anti-rat IgG (Jackson ImmunoResearch) and peroxidase visualization by enhanced chemiluminescence (Amersham Pharmacia).
Immunofluorescence and Flow Cytometry.
Staining was performed on freshly isolated splenocytes or peripheral blood cells as described (12). The following antibodies were purchased from Southern Biotechnology Associates: 53–6.7 anti-CD8, RA3–6B2 anti-CD45R/B220, and GK1.5 anti-CD4. The antibodies anti-I-Ab, AMS 9.1 anti-IgD, R6–60.2 anti-IgM, 7G6 anti-CD21/CD35, and B3B4 anti-CD23 were obtained from PharMingen.
T-Independent Response.
Control, Ii−/−, and the MHC class II and Ii−/− double-deficient animals 6–8 weeks of age were injected i.p. with 25 μg of (4-hydroxy-3-nitrophenyl)acetyl (NP)-Ficoll (NP-AECM-Ficoll; Biosearch) in 0.1 ml of 0.85% NaCl. After 6 days, blood was drawn and IgM titers to NP were quantitated by ELISA on plastic wells coated with NPBSA. Two-fold serum dilutions were made in PBS/1% FCS and applied to preblocked wells for 2 h. IgM detection was carried out with alkaline phosphatase-conjugated reagents and pNPP substrate (Southern Biotechnology Associates). For standard, we have used IgM antibodies against NP. All of the reagents were the generous gift of M. J. Shlomchik (Yale University School of Medicine, New Haven, CT).
BrdUrd Labeling of Cells.
Mice were fed with drinking water containing 1 mg/ml BrdUrd (Sigma) for 3 days. Spleen cells then were prepared and fixed with 70% ethanol. For determining the percentage of BrdUrd+ cells, cells were washed twice with PBS and resuspended in 0.5 ml of 2 M HCl/Triton X-100 and left for 20 min at room temperature. Cells were collected by centrifugation and washed. Cells were stained with FITC-labeled anti-BrdUrd (Becton Dickinson) and anti-B220 (Southern Biotechnology Associates) and were analyzed by FACS.
Adhesion Assay.
Adhesion assays were performed as described (25).
Proliferation of B Cells.
Purified B cells were cultured in 96-well plates at 2 × 105 cells per well in RPMI 1640 medium supplemented with 5% FCS/2 mM glutamate/100 units/ml penicillin/100 μg/ml streptomycin and several concentrations of anti-IgM (Jackson ImmunoResearch). DNA synthesis was assayed by pulsing the cells with 1 μCi of [3H]thymidine (International Chemical and Nuclear, Irvine, CA) in the last 18 h of a 2-day culture, after which the cells were harvested and counted. Assays were done in triplicate.
Results and Discussion
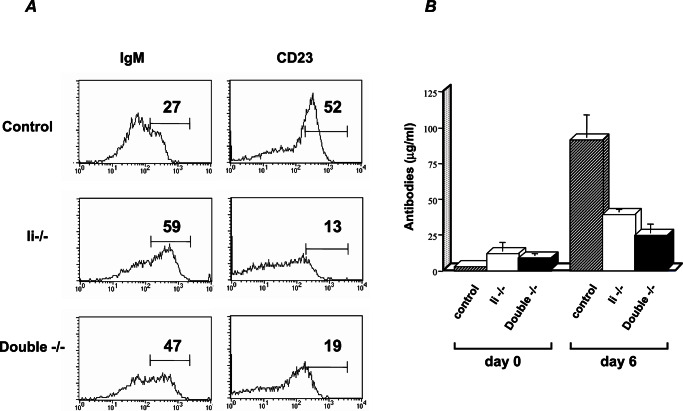
Recently, it has been proposed that mice lacking Ii are subjected to accumulation of MHC class II β-chain in the ER and that this β-chain build-up inhibits B cell maturation (26). To demonstrate that the inability of Ii-deficient cells to mature is not caused by accumulation of toxic MHC class II β-chain and independent of the chaperone activity of Ii, we analyzed the B cell population in mice that lacks both Ii and MHC class II β-chain [double knockout (KO)]. Splenocytes derived from control, Ii−/−, and double-KO mice were FACS-analyzed for expression of B cell maturation markers. As observed in the Ii-deficient B cell population, the double-negative cells exhibited higher levels of IgM and lower levels of CD23 (Fig. 1A). Thus, B cells from the double-KO mice exhibited an immature phenotype, suggesting that this phenotype does not depend on MHC class II β-chain accumulation. To further examine these Ii- and β-chain-deficient cells, we analyzed their ability to respond to thymic-independent antigen stimulation, using NP-Ficoll. Unlike the response in class II-deficient mice (27, 28), and similar to the response of Ii−/− mice, 6 days after immunization, concentrations of anti-NP IgM were low in the double-negative cells, suggesting that the primary response of B cells lacking both Ii and MHC class II β chain was impaired (Fig. 1B). These results indicate that B cells derived from the double-KO mice are arrested at an immature stage. Thus, absence of Ii and not accumulation of MHC class II β-chain in the ER causes the arrest in B cell maturation.
Figure 1.
Immature B cells accumulate in mice lacking both Ii and MHC class II β-chain. (A) Cytofluorimetric analysis of CD23 and IgM on B220+ cells. Control, Ii−/−, and double-deficient (double−/−) splenocytes were stained with both antibody to B220 (anti-B220) and anti-IgM or anti-CD23. Histograms show the expression of these molecules on B220 cells. (B) IgM response to TI antigen. Control, Ii−/−, and double-deficient (double−/−) mice were challenged with the type II-TI antigen NP90-Ficoll. Six days after injection, blood was drawn and IgM titers were quantitated by ELISA. The graph shows concentration of antibodies at days 0 and 6 from four control, Ii−/−, and double −/− mice. The results presented are the average of four injected mice.
It was shown previously that the contribution of Ii to the folding, assembly, and transport of class II molecules depends on its interaction with the MHC class II binding site (29). The luminal domain and especially the class II-associated Ii peptide is sufficient to induce class II folding and transport from the ER to the Golgi (30). The cytoplasmic tail of Ii was shown to contain targeting or retention signals that are responsible for endosomal/lysosomal localization (31–34). In an attempt to distinguish between the functions of Ii in antigen presentation and B cell maturation, we generated transgenic mice expressing a C-terminal truncated form of Ii lacking its luminal domain driven by the Ig κ promoter (Ii amino acids 1–82; Fig. 2A). This region is composed of Ii cytosolic and transmembrane domains and a part of the luminal domain lacking the class II-associated Ii peptide segment. The 1–82-positive transgenic mice were bred with the Ii-deficient mice to generate mice expressing exclusively the truncated Ii in their B cell population. To determine transgene expression, splenocytes were lysed and separated by SDS/PAGE followed by immunoblotting with anti-Ii (IN1) antibodies. The truncated form of Ii was detected only in the lysates of the transgenic cells (Fig. 2B). To increase levels of expression, mice positive for the transgene were bred to homozygosity. The expression of the transgene is targeted to the B cell population; therefore, CD4+ T cells are not expected to undergo positive selection in the thymus, because the dendritic cells are expected to be defective in Ii expression. To verify that the mice indeed have an Ii−/− background, we analyzed the T cells of these mice. As expected, the transgenic animals exhibited low CD4+ population because of the low positive selection of these cells in the thymus (Fig. 2C).
As reported previously, the absence of Ii alters the nature of peptide binding to class II molecules. Essentially, no MHC α and β “compact” dimers (dimers that bear tightly bound peptide) are formed; rather, floppy or unstable dimers are expressed (17–19). To elucidate the role of Ii 1–82 in assembly and peptide loading, nonboiled lysates of the control, Ii−/−, and 1–82 homozygous splenocytes were separated by SDS/PAGE followed by immunoblotting with anti-I-Ab (M5/114) antibodies. Compact dimers were not detected in the transgenic animals lacking the Ii luminal domain, and only the floppy form was observed (Fig. 2D). FACS analysis testing the ability of the Ii 1–82 B cells to express cell surface MHC class II revealed that, similar to Ii−/− B cells, the heterozygotic and homozygotic transgenic cells expressed only low levels of MHC class II (Fig. 2E). Thus, in agreement with previous studies, the lack of the class II-associated Ii peptide in the luminal domain prevents MHC class II folding and assembly and their presentation on the cell surface.
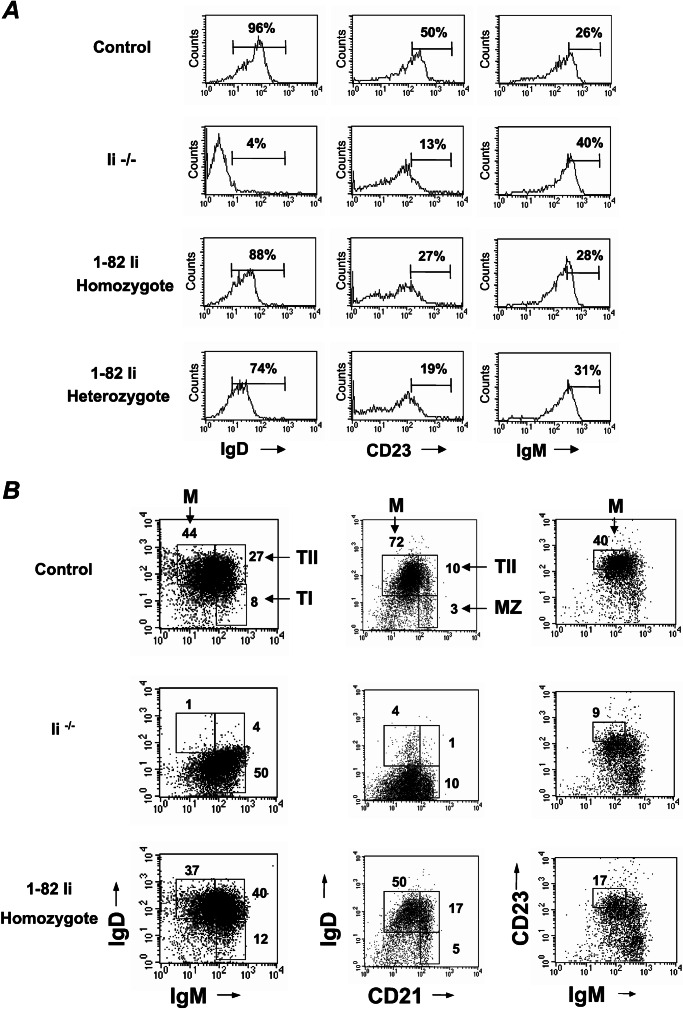
To determine whether the Ii 1–82 segment is involved in B cell maturation, we compared spleen cells from control, Ii−/−, and the heterozygotic and homozygotic 1–82 transgenic mice by using a panel of antibodies to B cell markers. Ii−/− B cells exhibit an immature phenotype. They express low levels of IgD and CD23 and higher levels of IgM and heat-stable antigen (12). These cells are arrested in the T1 stage, which has been characterized (10). However, unlike the immature phenotype observed in the Ii−/− B cells, B cells derived from the 1–82 transgenic mice exhibited high levels of IgD, lower levels of IgM, and only a slight elevation of CD23, indicating a more mature B cell phenotype in splenocytes (Fig. 3). Moreover, dot-plot analysis of IgM, CD23, and CD21 expression on 1–82 B cells revealed that these cells could differentiate to mature B cells. However, compared with the B population of a control mouse, a much larger proportion of T2 B cells was detected (Fig. 3B), implying that the maturation process was not complete, probably because of low levels of expression of the transgene. Thus, together, these results show that truncated Ii lacking its luminal domain enables B cell maturation but not MHC class II expression.
Figure 3.
B cells derived from the 1–82 transgenic mice differentiate to mature cells. (A) Splenocytes from control, Ii−/−, and 1–82 heterozygotic (hetero) or homozygotic (homo) mice were double-stained with anti-B220 and either anti-IgM, anti-CD23, or anti-IgD. Histograms show the expression of the various markers on B220-positive cells. (B) Splenocytes from control, Ii−/−, and 1–82 homozygotic mice were triple-stained with anti-B220 and anti-IgD, anti-IgM, anti-CD23, and anti-CD21. Dot plots show the expression of the markers on B220-positive cells. M, mature B cells; T1, transitional B cells 1; T2, transitional B cells 2; MZ, marginal zone B cells. The results presented are representative of five different experiments.
Immature B cells can be labeled easily with the thymidine analogue BrdUrd, because they are derived from a pool of actively cycling precursor B cells that replaces nearly half of the pool of immature B cells on a daily basis. Mature, small, resting B cells are labeled inefficiently with BrdUrd because they do not actively divide (4). To test whether the 1–82 B splenocytes have a mature phenotype, we analyzed their BrdUrd incorporation. Unlike the Ii-deficient B cells whose BrdUrd staining was elevated (3–4 times more), the transgenic B cells behaved similarly to control cells and inefficiently incorporated BrdUrd (Fig. 4A). Thus, the 1–82 B cells mainly are resting mature cells.
Figure 4.
B cells derived from the 1–82 mice behave as mature cells. (A) Adult control Ii−/− or 1–82 mice were fed BrdUrd in their drinking water for 3 days. Splenic B cells were analyzed for their BrdUrd incorporation. Histograms represent BrdUrd labeling of B220+-gated spleen cells. Numbers represent percentage of BrdUrd-positive cells. The results presented are representative of three different experiments. (B) LN cells from control, Ii−/−, and 1–82 mice were double-stained with anti-B220 and anti-CD8. Dot plots show the CD8 and B220 populations in the various mice. The results presented are representative of three different experiments. (C) Wild-type B cells (B con), immature B cells from Ii−/− mice (B −/−Ii), or 1–82-derived B cells were labeled with 51Cr, washed, resuspended in adhesion medium, and added to fibronectin-coated wells in the presence of phorbol 12-myristate 13-acetate or SDF-1 stimulation. The adherent cells were lysed, and radioactivity was determined. One experiment representative of four is depicted. (D) B cells purified from C57BL/6 (B6) Ii−/− and 1–82 transgenic mice were cultured in the presence of elevated concentrations of anti-IgM. Proliferation was determined by the addition of 1 μCi of [3H]thymidine for the last 18 h of a 2-day culture. One experiment representative of three is depicted.
To study further the functionality of the transgenic B cells, we analyzed the LNs of the 1–82 mice. As was described previously, in Ii−/− mice there is a striking decrease in the proportion of B cells populating the LN (20, 21, 25), because immature B cells negatively regulate their homing to these organs by secreting low levels of IFN-γ (25). To determine whether 1–82 B cells are able to migrate to the LNs, the B cell population in these compartments was characterized. As can be seen in Fig. 4B, the LN derived from the 1–82 mice are populated with significantly higher levels of B cells, further indicating a more mature population that efficiently homes to these organs. To migrate to the LN, leukocytes, including B cells, must interact with adhesive components of the extracellular matrix (ECM; ref. 35). We have shown previously that, unlike mature cells, immature B cells from control or Ii−/− mice are unable to respond to stimulation and increase their adhesion to ECM (25). We therefore compared the adhesion response of 1–82 B cells with that of control or Ii−/− B splenocytes by studying their ability to adhere, upon activation, to fibronectin, a major, ECM-based, cell-adhesive integrin ligand. 51Cr-labeled B cells were plated for 30 min on fibronectin-coated microtiter wells in the presence of phorbol 12-myristate 13-acetate, a potent agonist of integrin-mediated adhesion (36, 37), or SDF-1, a potent B cell chemoattractant (38). After stimulation, in contrast to the Ii−/− immature B cells, whose adhesion remained unchanged, the 1–82 B cells dramatically increased their adhesion to fibronectin-like, mature control cells (Fig. 4C). Moreover, the 1–82 B cells have a strong, T-independent response (data not shown), and these cells proliferate in response to anti-IgM stimulation, unlike Ii-deficient B cells (Fig. 4D). Thus, the 1–82-derived B cells function as mature cells.
The role of Ii as a chaperone for MHC class II molecules has been well characterized. We previously have described a novel role for Ii. In mice lacking this chain, B cell maturation is impaired and the periphery of these mice is enriched with immature B cells (12). In addition, recently, we have shown that Ii-induced B cell maturation involves activation of transcription mediated by NF-κB p65/RelA homodimer and requires the B cell-enriched coactivator TAFII105 (22).
In the present study, we demonstrated in vivo that Ii is directly involved in the maturation of B cells in a process that is independent of its chaperonic activity. In this study, we confirmed that Ii is crucial for B cell maturation by analyzing mice lacking both Ii and MHC class II β-chain. B cells obtained from these double-deficient mice remained arrested in the immature stage, indicating that the lack of Ii and not accumulation of β-chain is responsible for the defect in the maturation process. In our in vitro (22) and, currently, the in vivo studies, we identified the domain of Ii that mediates B cell maturation. Truncated Ii containing amino acids 1–82 and lacking the luminal domain, when expressed in transgenic mice, was unable to allow MHC class II cell surface expression and formation of compact dimers. However, this segment was sufficient to induce B cell maturation from T1 to T2 and less efficiently to mature cells. B cells derived from the 1–82 transgenic mouse expressed high levels of IgD and slightly higher levels of CD23 and lower levels of IgM, markers characteristic of the mature stage. Moreover, these cells exhibited several other properties associated with maturation: they were inefficient in their BrdUrd uptake and, like mature cells, were able to respond to chemokine stimulation and increase their adhesion to fibronectin and home to the LNs. Moreover, these cells proliferated in response to anti-IgM stimulation. These findings provide strong evidence that Ii induces B cell maturation by a mechanism that is independent of its activity in MHC class II folding and assembly. Thus, we could discern between the various functions of Ii as an MHC class II chaperone and its role in B cell differentiation.
Recently, a hypothesis was raised explaining the “low B cell” phenotype and the arrest in B cell maturation in Ii-deficient mice. This theory suggests that in these mice, Ii does not perform its normal chaperone function or facilitate proper formation of class II complexes. Thus, unpaired or mispaired class II β-chains that accumulate in the absence of Ii are toxic for the cells and cause a low B cell phenotype. According to this hypothesis, MHC class II and accessory molecules are not necessary per se for B cell differentiation or survival (39), but, rather, mispaired chains are toxic to mature B cells (26). Our results strongly argue against this hypothesis and directly demonstrate that truncated Ii, which has lost its MHC class II chaperone activity, can still induce the differentiation of B cells. Recent studies have suggested that the 1–82 fragment is involved in Ii chain trimerization and indeed can interact with MHC class II (40, 41). It therefore remains formally possible that Ii 1–82 is altering assembly or trafficking of MHC class II chains in such a way that relieves the “toxicity.” However, these compact dimers are not detectable by Western blot or FACS analysis in the 1–82 B cells, and most of the α- and β-chains are unassembled. The dramatic shift of the entire 1–82 B population to IgD-positive cells suggests that most of the cells and not a neglectable population have differentiated in the presence of the 1–82 segment. Yet, it should be still kept in mind that in the 1–82 transgenic mice there still may be some unpaired MHC class II α-chains that might contribute to the phenotype.
The trigger of Ii activity and the nature of the maturation pathway that is controlled by Ii are still being explored. However, our studies clearly show that Ii is a key molecule involved in B cell development.
Acknowledgments
We gratefully acknowledge members of the Shachar laboratory, Richard A. Flavell for his invaluable advice and generous gift of mice, and Ron Germain, Jim Miller, and Pippa Marrack for the generous gifts of reagents and mice. We thank the transgenic animal facility: Ahuva Knyszynski, Tatyana Burkova, and Judith Hermesh. This research was supported by the Minerva foundation (Germany), The Israel Science Foundation founded by the Academy of Sciences and Humanities, the German–Israeli Foundation for Scientific Research and Development, and the Israel Cancer Research Fund. I.S. is an incumbent of the Alvin and Gertrude Levine Career Development Chair of Cancer Research.
Abbreviations
- Ii
invariant chain
- ER
endoplasmic reticulum
- KO
knockout
- LN
lymph node
- ECM
extracellular matrix
References
- 1.Ehlich A, Martin V, Muller W, Rajewsky K. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 2.Boekel E, Melchers F, Rolink A. Int Immunol. 1995;7:1013–1019. doi: 10.1093/intimm/7.6.1013. [DOI] [PubMed] [Google Scholar]
- 3.Allman D M, Ferguson S E, Lentz V M, Cancro M P. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 4.Rolink A G, Andersson J, Melchers F. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Lortan J E, Roobottom C A, Oldfield S, MacLennan I C. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 6.MacLennan I C, Gray D. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 7.Allman D M, Ferguson S E, Cancro M P. J Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- 8.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas-Vaslin V, Freitas A A. Int Immunol. 1989;1:237–246. doi: 10.1093/intimm/1.3.237. [DOI] [PubMed] [Google Scholar]
- 10.Loder F, Mutschler B, Ray R J, Paige C J, Sideras P, Torres, Lamers M C, Carsetti R. J Exp Med. 1999;190:75–90. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajewsky K. Immunol Today. 1993;14:40–41. doi: 10.1016/0167-5699(93)90324-E. [DOI] [PubMed] [Google Scholar]
- 12.Shachar I, Flavell R A. Science. 1996;274:106–108. doi: 10.1126/science.274.5284.106. [DOI] [PubMed] [Google Scholar]
- 13.Unanue E R. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 14.Long E O. Immunol Today. 1989;10:232–234. doi: 10.1016/0167-5699(89)90259-4. [DOI] [PubMed] [Google Scholar]
- 15.Harding C V, Unanue E R. Cell Regul. 1990;1:499–509. doi: 10.1091/mbc.1.7.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cresswell P. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 17.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Cell. 1993;26:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 18.Bikoff E K, Huang L Y, Episkopou V, van Meerwijk J, Germain R N, Robertson E J. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott E A, Drake J R, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell R A. J Exp Med. 1994;179:681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenty G, Martin W D, Van Kaer L, Bikoff E K. J Immunol. 1998;160:606–614. [PubMed] [Google Scholar]
- 21.Kenty G, Bikoff E K. J Immunol. 1999;163:232–242. [PubMed] [Google Scholar]
- 22.Matza D, Wolstein O, Dikstein R, Shachar I. J Biol Chem. 2001;276:27203–27206. doi: 10.1074/jbc.M104684200. [DOI] [PubMed] [Google Scholar]
- 23.Ignatowicz L, Kappler J, Marrack P. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 24.Shachar I, Elliot E A, Chasnoff B, Grewal I S, Flavell R A. Immunity. 1995;3:373–383. doi: 10.1016/1074-7613(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 25.Flaishon L, Hershkovitz R, Lantner F, Lider O, Alon R, Levo Y, Flavell R A, Shachar I. J Exp Med. 2000;192:1381–1387. doi: 10.1084/jem.192.9.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrecque N, Madsen L, Fugger L, Benoist C, Mathis D. Immunity. 1999;11:515–516. doi: 10.1016/s1074-7613(00)80126-0. [DOI] [PubMed] [Google Scholar]
- 27.Gosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz J S, Rogers P R, Grusby M J, Parker D C, Glimcher L H. J Immunol. 1993;150:1223–1233. [PubMed] [Google Scholar]
- 29.Romagnoli P, Germain R N. J Exp Med. 1994;180:1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong G, Castellino F, Romagnoli P, Germain R N. J Exp Med. 1996;184:2061–2066. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakke O, Dobberstein B. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 32.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid S L, Quaranta V, Peterson P A. Nature (London) 1990;348:600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 33.Lamb C A, Yewdell J W, Bennink J R, Cresswell P. Proc Natl Acad Sci USA. 1991;88:5998–6002. doi: 10.1073/pnas.88.14.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pieters J, Bakke O, Dobberstein B. J Cell Sci. 1993;106:831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 35.Butcher E C, Picker l J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu Y, van Seventer G A, Ennis E, Newman W, Horgan K J, Shaw S. J Exp Med. 1992;175:577–582. doi: 10.1084/jem.175.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faull R J, Kovach N L, Harlan J M, Ginsberg M H. J Exp Med. 1994;179:1307–1316. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bleul C C, Schultze J L, Springer T A. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellino F, Han R, Germain R N. Eur J Immunol. 2001;31:841–850. doi: 10.1002/1521-4141(200103)31:3<841::aid-immu841>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 41.Ashman J B, Miller J. J Immunol. 1999;163:2704–2712. [PubMed] [Google Scholar]