Abstract
Neonates are highly susceptible to infectious diseases and, in general, respond poorly to conventional vaccines due to immaturity of the immune system. In the present study, we hypothesized that the anti-tetanus toxoid (TT) vaccine response of neonatal mice could be enhanced by retinoic acid (RA), a bioactive retinoid, and polyriboinosinic:polyribocytidylic acid (PIC), an inducer of IFN. Early-life treatments with RA and/or PIC were well tolerated and stimulated both primary anti-TT IgG production in infancy and the memory response in adulthood. TT-specific lymphocyte proliferation and type 1/type 2 cytokine production were also significantly augmented. In addition, RA and PIC modulated the maturation and/or differentiation of neonatal B cells, natural killer (NK)/NKT cells, and antigen-presenting cells. Although RA alone increased the neonatal anti-TT antibody response, it selectively increased anti-TT IgG1 and IL-5, resulting in a skewed type 2 response. PIC, a potent adjuvant in adult mice, elevated neonatal anti-TT IgG as well as all IgG isotypes (IgG1, IgG2a, and IgG2b) and induced TT-specific IFN-γ, an important type 1 cytokine; however, PIC alone failed to benefit the memory response. The combination of RA plus PIC was more potent than either agent alone in elevating primary and secondary anti-TT IgG responses as well as IgG isotypes. Moreover, RA plus PIC increased TT-specific IFN-γ and IL-5, suggesting the combination effectively promoted both type 1 and type 2 responses in neonatal mice. Thus, RA combined with PIC, a nutritional-immunological intervention, seems promising as an adjuvant for early-life vaccination.
Keywords: CD80, IgG isotypes, macrophage, type 1/type 2 immunity, vaccination
Infants and neonates are well known to be at high risk of infectious diseases. According to estimates by the World Heath Organization (WHO), ≈2.5 million infants between 1 and 12 months of age die each year of infectious diseases (1). The high susceptibility of infants and neonates to infections is mostly attributed to relative immaturity of the immune system, which may involve several aspects. First, compared with adults, the cellularity of the peripheral lymphoid organs is lower in neonates, and the microarchitecture of secondary lymphoid organs is not well developed (2, 3). Second, neonatal immune cells are functionally immature as compared with similar cells from adults. The antibody response of B cells to T cell-dependent antigens and T cell-independent (TI) type-2 antigens is especially low in infants and children <2 y of age, and in the young of experimental animal models. The neonatal antibody response to T cell-dependent antigens is characterized by weak Ig isotype switching (lack of IgG2a), poor Ig affinity maturation, and restricted heterogeneity due to limited use of V region Ig genes (4). Moreover, T helper (Th)-1 cell and cytotoxic T lymphocyte responses are low in neonates, which are considered to be responsible for the increased susceptibility of neonates to intracellular pathogens (3). In addition, the limited immune response in neonates may also be due to an immaturity of antigen-presenting cells (APCs), as shown by a low expression of costimulatory molecules, impaired antigen-presenting ability, and reduced IL-12 production after infection or vaccination (3, 5, 6).
Currently, early-life vaccination is an important strategy for protecting neonates and infants from infectious diseases. In children >2 y old and in adults, conventional vaccines can generally induce sufficient production of specific protective antibodies to neutralize pathogens (or their toxins) and thereby protect the host from infections. However, the immaturity of the neonatal immune system significantly hampers the generation of a protective vaccine response (1). Therefore, strategies to enhance vaccination efficiency in early life are highly sought. Several recent studies have reported promising results with strategies using certain adjuvants, such as IL-12 and CpG-containing oligonucleotides, which can induce type-1 cytokine production as well as cytotoxic T cell responses and successfully increase antibody production in neonatal mice (7-9). However, in some of these strategies, weight loss was observed (10), and the potential adverse effects of these treatments would seem to limit their application in human neonates.
Vitamin A (VA) is known to play an important role in both innate and adaptive immunity. VA-deficient children are at increased risk of mortality and infection diseases; particularly, measles and diarrheal diseases have been reported to be more severe (11, 12). By providing VA supplements to VA-deficient children aged 6-72 months, all-cause mortality was reduced by 23%, measles-related mortality by 50%, and diarrheal disease mortality by 33% (13). Therefore, periodic high-dose supplementation with VA is considered as a highly cost-effective approach to prevent VA deficiency and save children's lives (14). To facilitate the delivery of VA supplements to young children, the WHO has recommended integrating VA supplementation as a part of the Expanded Program of Immunization (EPI) in countries where VA deficiency is prevalent, and supplementation has been implemented in children <12 months of age (15). The integration of VA administration into early-life vaccination programs has been shown to be as a safe and effective way to improve the VA status of infants (16). Moreover, coadministration of VA with measles or diphtheria-pertussistetanus vaccination significantly elevated vaccine-induced antibody responses in infants, suggesting the potential benefit of VA on the vaccine response in early life (15, 17, 18).
Polyriboinosinic:polyribocytidylic acid (PIC) is a synthetic dsRNA that triggers toll-like receptor-3 and its downstream signaling molecules (19), activating both innate and adaptive immunity. PIC is well known for its ability to induce type I/type II IFNs and enhance antiviral and antitumor reactions in several models (20-22). Furthermore, PIC is a potent immune adjuvant that can promote dendritic cell (DC) maturation, increase natural killer (NK)-mediated cytotoxicity, and enhance CD8 T cell response as well as T helper (Th) 1-cytokine production (23, 24).
Previously, we reported that coadministration of all-trans-retinoic acid (RA) and PIC with tetanus toxoid (TT) immunization cooperatively enhanced the anti-TT antibody response in VAadequate adult mice (25, 26). Moreover, the combination of RA plus PIC stimulated a robust, durable, and balanced increase in all of the anti-TT IgG isotypes (IgG1, IgG2a, and IgG2b). These results suggested to us that this combination might act as a promising strategy for enhancing the response to TT and similar vaccines in healthy populations. However, whether RA and PIC, alone or in combination, can effectively enhance antibody production in a neonatal vaccination model is still unknown. Therefore, in the present study we sought to evaluate the adjuvant effects of RA, PIC, and their combination on anti-TT vaccination response in neonatal mice.
Materials and Methods
Experimental Design. Animal protocols were approved by the Institutional Animal Use and Care Committee of Pennsylvania State University. Adult C57BL/6 mice purchased from Charles River Laboratories were bred under specific pathogen-free conditions, and the date of birth of offspring was recorded. Pups remained with their mothers until weaning at 4 weeks of age. During the experimental period, all of the mice were fed with a nutritionally complete diet (LabDiet 5001, containing VA 22 units/g, Purina).
Vaccine, Adjuvants, and Immunization Procedures. RA (Sigma) was prepared in canola oil at 4 mg/ml. PIC, stabilized with poly-l-lysine and carboxymethylcellulose, was used as described (25). The doses of TT (Connaught Laboratories), RA, and PIC for neonatal mice were calculated by adjusting the doses used for adult mice (25) according to metabolic body weight (BW0.75), resulting in doses of ≈3.5 μg, 12 μg, and 0.7 μg per neonate, respectively. One-week-old mice were randomly divided into four groups: control, RA, PIC, and RA plus PIC. One day before primary immunization (day-1), neonates were fed orally, by using a micropipet, with RA or canola oil only. The next day (day 0), each neonate was immunized i.p. with TT and cotreated with RA orally and/or PIC by i.p. injection. From days 1-5 after priming, the neonates were fed the same dose of RA or oil daily. To evaluate the effects of RA and/or PIC treatments on neonatal lymphocyte populations, some neonates were euthanized 3 days after priming and spleens were analyzed by flow cytometry. The rest of the neonates remained with their mothers until weaning and were reimmunized with TT (10 μg per mouse) at 6 weeks of age without further treatment with RA/PIC. Blood was collected 14 days after priming and 7 days after reimmunization for determination of primary and secondary antibody responses, respectively. Two weeks after reimmunization, spleens were collected for in vitro proliferation and cytokine response assays.
Serum Anti-TT Antibody Analysis. Serum anti-TT IgG and anti-TT IgG isotypes were quantified by ELISA by using serially diluted serum samples as described (27). Measurements in a linear doseresponse range were compared with a standard of serially diluted pooled immune serum, included on every ELISA plate, to calculate the titers of anti-TT IgG; 1 unit was defined as the dilution fold that produced 50% of the maximal optical density for the standard sample.
Lymphocyte Proliferation. Spleen mononuclear cells were isolated as reported (28) and suspended at 5 × 106 cells/ml in RPMI medium 1640 with 10% FBS. To assess TT-induced cell proliferation, 96-well plates were coated with TT (2.5 μg/ml) at 4°C overnight, followed by washing. Then, 5 × 105 cells per well were added in triplicate and incubated in the presence of soluble TT (2.5 μg/ml) at 37°C for 96 h. For comparison, cells were also incubated with plate-bound anti-mouse CD3 (145-2C11, BD Pharmingen) in 96-well plates at 37°C for 72 h. Cell proliferation was determined by the incorporation of [methyl-3H]thymidine (Amersham Pharmacia Biosciences) as described (28). The stimulation index (SI) was defined as the ratio of experimental cpm/control cpm (without stimulation).
Quantification of Cytokine Production. To assess TT-specific cytokine production, spleen mononuclear cells (5 × 106 cells/ml) were cultured in TT-coated 24-well plates, and incubated with soluble TT (2.5 μg/ml) at 37°C for 96 h. For anti-CD3-induced cytokine production, cells were also incubated in anti-CD3-bound 24-well plates at 37°C for 72 h. Cytokines (IL-4, IL-5, and IFN-γ) in culture supernatants were detected by a sandwich ELISA according to the protocol from BD Biosciences. Purified anti-mouse IL-4 (11b11), IL-5 (TRFK4), and IFN-γ (R4-6Α2) mAbs, as well as biotinylated anti-mouse IL-4 (BVD6-24G2), IL-5 (TRFK4), and IFN-γ (XMG1.2) mAbs were obtained from BD Pharmingen. Cytokine values were expressed by reference to a standard curve established by assaying serial dilutions of the respective mouse cytokine standards (10).
Flow Cytometry. Splenic mononuclear cells in RPMI medium 1640 containing 1% FBS were incubated with combinations of appropriately diluted fluorochrome-conjugated monoclonal antibodies (BD Pharmingen) at room temperature for 40 min. For T cell population analysis, the cells were double-labeled with phycoerythrin (PE)-anti-CD4 (H129.19) and FITC-anti-CD8 (53-6.7). B cells were stained with PE-anti-B220 (RA3.6B2) or FITC-anti- CD19 (1D3). For NK/NKT cell populations, the cells were double-labeled with FITC-anti-CD3 (17A2) and PE-anti-NK1.1 (PK136). For APCs, the cells were double-labeled with PE-anti-CD11b (M1/70) and FITC-anti-CD80 (16-10A1) or FITC-anti-CD86 (GL1). The cells were then washed and fixed, and live-gated cells were detected by using a Coulter XL-MLC flow cytometer. The results were analyzed with flow-jo software (Tree Star, Ashland, OR).
Statistical Analysis. Data are reported as mean ± SE. The main effects of RA, PIC, and their interaction were evaluated by two-way ANOVA. When group variances were unequal, data were subjected to log10 or square-root transformation before analysis. Differences among groups, P value <0.05, were determined by using Fisher's protected least significant difference (LSD) test (superanova, Abacus Software, Berkeley CA).
Results and Discussion
Having previously observed that the combination of RA, an active metabolite of VA, and PIC was well tolerated by adult mice and led to a robust and durably enhanced anti-TT antibody response (25), we considered it possible that this combination of nutritional-immunological intervention would also function as an adjuvant for neonatal vaccination.
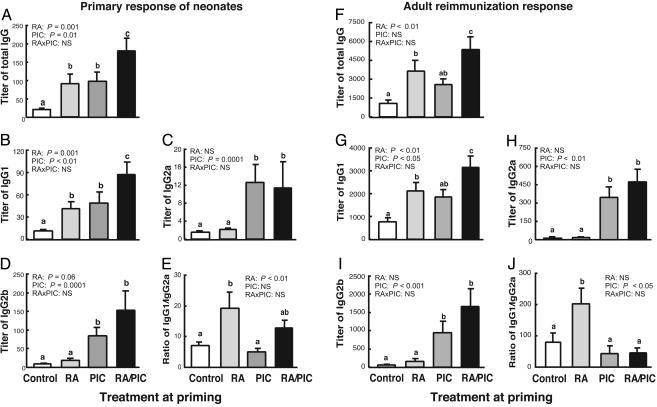
Early-Life Treatments with RA and/or PIC Augment both Primary and Secondary Anti-TT IgG Responses. Early-life treatments with RA and/or PIC were well tolerated and did not affect the growth of neonates (Fig. 6, which is published as supporting information on the PNAS web site). Although the primary anti-TT antibody response in neonatal mice was very weak, both RA and PIC alone significantly increased anti-TT IgG titers, and the combination of RA plus PIC further increased the response (Fig. 1A). Moreover, RA and/or PIC enhanced IgG isotype switching in neonatal mice as detected by the IgG isotype concentrations (Fig. 1 B-D) and ratios (Fig. 1E). RA alone selectively increased the titers of IgG1 and elevated the ratio of IgG1/Ig2a, an index reflecting the balance of type 2 to type 1 immune response. PIC significantly enhanced all of the IgG isotypes (IgG1, IgG2a, and IgG2b) without affecting the IgG1/IgG2a ratio. Compared with PIC alone, the combination of RA plus PIC further increased IgG1 and IgG2b, while still maintaining the IgG1/IgG2a ratio similar to the control level. Analysis by two-way ANOVA confirmed that RA was a positive regulator for IgG1, and PIC was a positive regulator for all of the IgG isotypes. Therefore, RA and PIC treatments significantly promoted the primary anti-TT antibody response and differentially regulated IgG isotypes in neonatal mice.
Fig. 1.
RA and/or PIC treatments enhance both primary anti-TT antibody response in neonatal mice and memory response in adulthood. One-week-old C57BL/6 mice were pretreated with RA or oil for one day, followed by TT immunization with or without RA and/or PIC as described in Materials and Methods. Five weeks after priming, mice were reimmunized with TT only. Blood samples were collected 1 week after priming and 2 weeks after reimmunization. Anti-TT IgG and IgG isotypes were measured by using ELISA. Shown are total IgG (A and F), IgG1 (B and G), IgG2a (C and H); IgG2b (D and I), and ratio of titers of IgG1 to IgG2a as indicator of type 2 to type 1 balance (E and J). Shown are means ± SE, n = 10-12 per group. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c). Results of two-way ANOVA for each factor (RA, PIC, and interaction) are also shown in each panel.
Of particular interest with respect to vaccination is the recall response to antigen. The coadministration of RA and/or PIC with TT immunization during the neonatal stage also significantly enhanced the secondary (recall) antibody response when the neonates grew to be 6-7 weeks old. The secondary anti-TT IgG level was slightly enhanced by PIC alone but significantly up-regulated by RA and by RA plus PIC (Fig. 1F). Similar to the primary response, RA alone selectively induced secondary anti-TT IgG1 (Fig, 1G) and elevated the ratio of IgG1/Ig2a. PIC alone selectively induced IgG2a and IgG2b without affecting the IgG1/IgG2a ratio (Fig. 1 H-J). Notably, RA plus PIC significantly increased all of the IgG isotypes, whereas the ratio of IgG1/IgG2a was equivalent to that in the control group. Overall, early treatment with RA and/or PIC successfully enhanced both the primary antibody response in neonates and their memory responses as young adults.
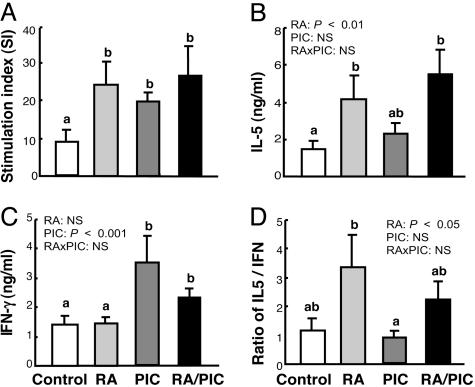
Early-Life Treatments with RA and/or PIC Increase TT-Specific Lymphocyte Proliferation and Cytokine Production. Because TT-induced antibody production requires the involvement of active T helper cells and type 1/type 2 cytokines, we evaluated the effect of RA and PIC on lymphocyte proliferation and cytokine production. RA, PIC, and RA plus PIC significantly doubled or tripled TT-induced lymphocyte proliferation (Fig. 2A). Moreover, RA and PIC significantly regulated TT-specific type 1/type 2 cytokines, which are key regulators of Ig isotype switching. In the present study, we measured IFN-γ, a major “signature” of type 1 cytokine responses, and IL-4 and IL-5, two type 2 cytokines. Because TT-induced IL-4 was not significantly detectable, we focused on the regulation of IFN-γ and IL-5. TT-induced IL-5 was increased by RA alone and by RA plus PIC (Fig. 2B). TT-induced IFN-γ, on the other hand, was markedly elevated by PIC alone. Although the combination of RA plus PIC slightly attenuated PIC-induced IFN-γ production, it still induced a higher level of IFN-γ compared with the control group (Fig. 2C). Consequently, the ratio of IL-5 to IFN-γ, an index of the balance of type 2 relative to type 1 response, was elevated ≈2-fold by RA, whereas RA plus PIC together rebalanced this ratio close to the control level (Fig. 2D). Therefore, RA and PIC promoted TT-specific type 1 and type 2 cytokine responses, respectively, whereas the combination of RA plus PIC promoted both type 1 and type 2 responses, resulting in a high-level, balanced response.
Fig. 2.
RA and/or PIC treatments enhance TT-specific lymphocyte proliferation and production of IL-5 and IFN-γ. One-week-old C57BL/6 mice were pretreated with RA or oil for 1 day, followed by TT with or without RA and/or PIC as described in Materials and Methods. Five weeks after priming, the mice were reimmunized with TT only. Two weeks later, spleen cells were isolated and stimulated with TT for 96 h in vitro.(A) Lymphocyte proliferation determined by [methyl-3H]thymidine incorporation. (B and C) Cytokines in supernatant detected by ELISA. (D) Ratio of IL-5:IFN-γ as an indicator of type 2 to type 1 cytokine balance. Shown are means ± SE, n = 10 per group. Different letters above bars within panels indicate significant differences (P < 0.05, a < b < c). Results of two-way ANOVA are also shown in each panel.
It is interesting that anti-CD3 induced T cell proliferation and cytokine production was similar regardless of prior RA/PIC treatments during the neonatal stage (Fig. 7, which is published as supporting information on the PNAS web site). To confirm this result, spleen cells were also collected 14 days after neonatal priming, and stimulated with anti-CD3. Similarly, RA and/or PIC did not significantly affect anti-CD3-induced T cell proliferation and cytokine production (data not shown). Together, these data implied that the immunoregulatory effects of RA/PIC were apparently antigen-specific, without affecting the general T cell response to TCR/CD3 stimulation. Because we used a restimulation model, the TT-responding cells showing regulation by RA and/or PIC were likely to have represented a memory pool, which might be increased in number or antigen responsiveness by prior treatment with RA and/or PIC in the neonatal period. These cells may have then survived and remained capable of responding to TT upon later restimulation. Anti-CD3, on the other hand, would be expected to stimulate both naive and memory CD3+ T cells, and apparently these cells or their progenitors were unaffected by the RA and PIC treatments that the neonates received. Currently, several agents, such as IL-12, CpG-containing oligonucleotides, and Freund's complete adjuvant, have been shown to effectively boost vaccine responses in neonates (3, 29, 30). However, a major concern is that these agents might lead to a state of heightened inflammation later on, increasing the risk of inflammation and autoimmune diseases (3). Although further studies are necessary, our current results suggest that the effects of RA and PIC could be focused on antigen-specific T cells, without having a long-lasting effect on the general population of T cells.
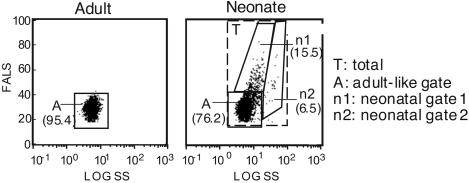
RA and/or PIC Treatments Promote Neonatal Lymphocyte Maturation and Differentiation. A lack of mature lymphocytes in peripheral lymphoid organs is believed to be an important factor in the weak immune response of neonates (3). Therefore, we determined whether RA and/or PIC treatments could directly affect the maturation and development of neonatal immune cells. Flow cytometry analysis by using forward angle light scatter (FALS) and side scatter (SS) showed that >20% of neonatal spleen cells were larger (increased FALS) and/or seemed to be more granular (increased SS) than the great majority of adult spleen cells (Fig. 3A). Thus, in addition to analyzing the effects of RA and PIC on the total spleen cell population, we separately quantified cells using the following three gates: an “A” (adult) gate set according to the distribution of live cells in adult mouse spleen; an “n1” (neonatal 1) gate, and an “n2” (neonatal 2) gate. In the A gate of neonatal mice, the percentages of CD3+ T cells, NK1.1+ cells, CD11b+ cells, and CD11c+ cells were all much lower than the adult levels (Table 1). Although the percentage of B220+ cells in the A gate was comparable with the adult level, the expression of CD19, a B cell coreceptor, was about half (34.5%) in neonates compared with adults (62.9%). In addition, the percentage of IgM+IgD- cells (indicating immature B cells) was >25%, suggesting the cell population in the A gate of neonates was relatively immature. The distribution of cells in the n1 gate was similar to that in the A gate, except that the CD11b+ cells were notably enriched in the n1 gate as compared with the A gate population. Also, the n1 gate contained 6% of IgM+IgD- cells (Table 1), and most of B220- positive cells expressed CD19 (data not shown). By these criteria, the n1 gate contained more mature cells than the A gate. Although the n2 gate covered only 6.5% of splenic mononuclear cells, nearly all of these cells were CD11b-positive. Moreover, the median fluorescence intensity (MdFI) of CD80 and CD86 of cells in the n2 gate were ≈1- to 2-fold higher than those of cells in the A and n1 gates (data not shown). Therefore, the n2 gate comprised mostly relatively mature macrophages. Retinoic acid and RA plus PIC significantly reduced the cell proportion in the A gate but increased the cell proportion in the n1 gate, suggesting that these treatments promoted lymphocyte maturation in neonatal mice (see Fig. 8, which is published as supporting information on the PNAS web site). The total number of spleen cells, however, was not significantly affected (Fig. 4A).
Fig. 3.
Neonatal splenocyte population is more heterogeneous than the adult cell population. One-week-old (neonate) and 6-week-old (adult) C57BL/6 mice were immunized with TT. Three days later, spleen cells were isolated and detected with fluorochrome-conjugated mAbs. Based on the light-scatter plots, the neonatal splenocyte population was gated into three gates: the A (adult-like) gate, the n1 (neonatal 1) gate, and the n2 (neonatal 2) gate. Values shown are the average cell percentages in each gate.
Table 1. The percentages of cells with different markers in each gate, mean ± SE (n = 4).
| Neonates
|
|||||
|---|---|---|---|---|---|
| Cell type | Adults | Total | A | n1 | n2 |
| CD3+ | 17.3 ± 2.2 | 7.2 ± 0.6 | 7.6 ± 0.7 | 7.9 ± 0.6 | 2.4 ± 0.3 |
| B220+ | 74.2 ± 2.3 | 63.0 ± 1.3 | 70.4 ± 1.4 | 56.6 ± 3.1 | 6.1 ± 0.7 |
| IgM+IgD− | 4.79 ± 0.2 | 20.2 ± 0.9 | 25.8 ± 0.8 | 6.3 ± 0.3 | 1.9 ± 0.2 |
| NK 1.1+ | 5.3 ± 0.6 | 2.3 ± 0.2 | 2.3 ± 0.2 | 1.3 ± 0.1 | 1.7 ± 0.2 |
| CD11b+ | 9.5 ± 0.7 | 16.1 ± 0.7 | 6.8 ± 0.3 | 32.9 ± 2.3 | 90.7 ± 1.3 |
| CD11c+ | 6.5 ± 0.4 | 2.8 ± 0.1 | 2.2 ± 0.1 | 5.1 ± 0.2 | 2.2 ± 0.4 |
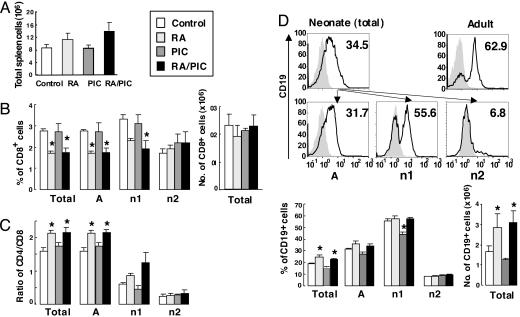
Fig. 4.
RA and/or PIC treatments regulate neonatal lymphocyte populations. One-week-old C57BL/6 mice were pretreated with RA or oil for 1 day, followed by TT with or without RA and/or PIC. Three days after priming, spleen cells were isolated and stained with fluorochrome-conjugated mAbs as described in Materials and Methods.(A) Number of total spleen cells. (B) The percentage of CD8+ T cells. (C) The ratio of CD4 to CD8 cells. (D) Histogram of the percentage of CD19+ B cells (control group is illustrated; the gray areas represent the isotype control) and the percentage of CD19+ B cells. Cells were quantified as the total cells and as cells residing in the A, n1, and n2 gates. The number of each cell type is calculated based on the number of total spleen cells. Shown are means ± SE, n = 6 per group. *, P < 0.05 versus control group.
Retinoic acid and/or PIC treatments at the neonatal stage significantly regulated neonatal lymphocyte populations. The percentage of CD3+, CD4+, and CD8+ T cells in neonatal mice was ≈60% less than those of adult mice (Table 1 and data not shown). RA and/or PIC treatments did not significantly affect neonatal CD4+ cells; however, the percentage of CD8+ cells was significantly reduced by RA and RA plus PIC in the total as well as in the A and the n1 gates (Fig. 4B). Consequently, the ratio of CD4+ cells to CD8+ cells in neonatal mice was significantly decreased by RA and RA plus PIC (Fig. 4C), even though the number of CD8+ cells was not significantly changed by the treatments, suggesting that the treatments in our study did not directly reduce CD8+ T cells. Instead, RA and RA plus PIC might promote the expansion of other cell types (such as B cells and NK/NKT cells, which will be described later), thereby indirectly reducing the percentage of CD8+ cells.
The B cell population (B220+ cells) in neonatal spleen, compared with adults, was only slightly reduced; however, neonatal B cells expressed much less CD19 (Fig. 4D). CD19 is a 95-kDa transmembrane protein expressed from the early stage of B cell development up to the stage of plasma cell differentiation. It is known as an essential downstream element of B cell receptor signaling required for B cell maturation and activation, T cell-dependent, antigen-specific antibody responses, and germinal center formation (31, 32). Notably, RA and RA plus PIC, but not PIC alone, up-regulated the percentage and number of CD19+ cells (Fig. 4D), which might in turn enhance antigen-triggered B cell receptor signaling transduction, thereby promoting anti-TT antibody response in neonatal mice.
Furthermore, RA and PIC also regulated NK/NKT cell populations in neonatal mice. The proportions of NK cells (NK1.1+CD3-) and NKT cells (NK1.1+CD3+) were much lower in neonates than adults (NK, 2.08% versus 4.33%; NKT, 0.7% versus 1.36%). However, RA plus PIC significantly increased the percentage of NK cells and number of NK and NKT cells (Fig. 9 A and B, which is published as supporting information on the PNAS web site). NK cells are known as an early source of IFN-γ, whereas NKT cells have been shown to secrete IL-4 and IL-10 (as well as IFN-γ) and, in general, to promote a type 2 response (33, 34). Therefore, the increase in NK and NKT cells could be responsible in part for the increased type-1/type-2 cytokine production by RA/PIC-treated neonatal mice.
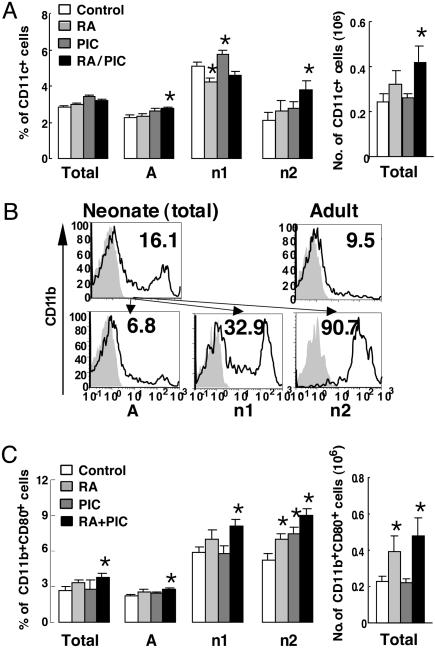
In addition to the lymphocyte population, RA and/or PIC regulated the generation and differentiation of CD11c+ cells (DCs) and CD11b+ cells (macrophages). The proportion of CD11c+ cells in neonatal mice was <50% of the adult level (Fig. 5A). In contrast, the proportion and median fluorescence intensity of CD11b+ cells in neonatal mice were about twice the adult level (Fig. 5B and data not shown). Because macrophages are major contributors to innate immunity, a relatively high proportion of macrophages in neonates implies their importance of innate immunity, which may help to control infections during the neonatal period when the ability to mount adaptive immune responses is not yet well developed. Although the percentage of CD11b+ cells was not significantly affected, the percentage of CD11c+ cells was significantly increased by PIC in total cells and in the n1 gate, and by RA plus PIC in the A and n2 gates (Fig. 5A and data not shown). Therefore, RA and/or PIC enhanced the generation of DCs in neonatal mice.
Fig. 5.
RA and/or PIC treatments regulate populations and differentiation of neonatal macrophages and DCs. One-week-old C57BL/6 mice were pretreated with RA or oil for 1 day, followed by TT with or without RA and/or PIC. Three days after priming, spleen cells were isolated and stained with fluorochrome-conjugated mAbs. (A) The percentage of CD11c+ cells in the total gate and each subgate is shown. (B) CD11b+ cells in total, A, n1, and n2 subgates; the gray areas represent the isotype control (control group illustrated). (C) CD11b+CD80+ cells. Bars show means ± SE, n = 6 per group. *, P < 0.05 versus control group.
Because macrophages and DC function as professional APCs, we therefore measured the CD80 and CD86 costimulatory molecules expressed on APCs. RA and/or PIC treatments slightly induced total expression of CD80, without affecting CD86 (data not shown). Notably, RA and PIC alone increased the percentage of CD11b+CD80+ cells in the n2 gate, and RA plus PIC combined significantly increased CD11b+CD80+ cells in all four gates. Moreover, the number of CD11b+CD80+ spleen cells was significantly elevated in RA- and RA plus PIC-treated neonates (Fig. 5C). Therefore, RA and/or PIC treatments induced CD80 expression on neonatal macrophages. Muthukkumar et al. (6) reported that the defective antigen-presenting ability of neonatal APCs was strongly associated with the absence of costimulatory molecules, such as CD80 and CD86. The up-regulation of CD80 on macrophages implies that RA and/or PIC may enhance the antigen-presenting capacity of neonatal macrophages, thereby contributing to augmentation of anti-TT lymphocyte responses and antibody production. Analysis by two-way ANOVA showed that RA was a positive regulator for neonatal CD19+ cells, NK cells and NKT cells, and CD11b+CD80+ cells (data not shown), indicating that RA treatment significantly increased a wide spectrum of immune cells in neonates. PIC, on the other hand, was a positive regulator for NK cells and for total CD11c+ cells (data not shown), suggesting that PIC enhanced the generation of NK cells and DCs. The regulation of immune cell populations by RA and/or PIC might directly affect functional outcomes in neonates.
Conclusion
In this study, early-life treatments with RA and/or PIC enhanced the TT-induced vaccine response in neonatal mice. RA alone given at the neonatal stage significantly enhanced both primary and secondary anti-TT IgG responses as well as TT-specific lymphocyte proliferation. However, it selectively increased type 2 responses as indicated by the induction of TT-specific IL-5 and IgG1 production, therefore further skewing the neonatal immune response toward a type 2 direction. Because the lack of immaturity type 1 responses is a major contributor to the increased susceptibility of neonates to intracellular pathogens (3), a selective induction of type 2 immunity by RA could be disadvantageous for the development of type 1 responses and cell-mediated immunity in neonates. On the other hand, PIC, shown to be a potent adjuvant in adult mice (25), strongly increased primary anti-TT IgG and all of the IgG isotypes (IgG1, IgG2a, and IgG2b) in neonatal mice. PIC alone also significantly enhanced TT-specific lymphocyte proliferation and IFN-γ production, but, unfortunately, PIC did not significantly benefit the secondary anti-TT IgG and IgG1 response, suggesting PIC alone did not provoke a good memory response. Therefore, PIC alone may not be sufficient as an adjuvant for neonatal vaccination. Compared with either RA or PIC alone, the combination of RA plus PIC was more potent in augmenting both primary and secondary anti-TT IgG responses as well as all IgG isotypes. Moreover, the balance of anti-TT IgG1/IgG2a was maintained close to that of vehicle-treated neonates. Furthermore, RA plus PIC significantly increased the production of TT-specific IFN-γ and IL-5, thereby effectively promoting both type 1 and type 2 cytokine responses. Somewhat to our surprise, RA given at the neonatal stage did not significantly attenuate TT-specific type 1 responses (e.g., anti-TT IgG2a and IFN-γ production), although we have reported that RA attenuated PIC-induced levels of type 1 cytokine mRNAs and IgG2a production in healthy adult mice (25). However, it seems that neonatal mice as compared with adults do not respond identically to RA in terms of the balance of IgG isotypes and cytokine production. This difference could be partly due to the significant effect of RA on neonatal immune-cell maturation and differentiation, such as by promoting the differentiation of NK/NKT cells, which are sources of IFN-γ and IL-4 (35, 36), and macrophages as well. Nevertheless, RA plus PIC at the neonatal stage was more effective and more durable than either of these agents alone in promoting anti-TT antibody production in infancy, stimulating both type 1 and type 2 cytokines, and providing long-term immunity (heightened recall response) in the young adult stage. Overall, the combination of an RA plus PIC nutritional-immunological intervention can act as a powerful adjuvant for neonatal vaccination.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 DK-41479, the Huck Institute for Life Sciences, and the Dorothy Foehr Huck Chair in Nutrition.
Abbreviations: APC, antigen-presenting cell; DC, dendritic cell; PIC, polyriboinosinic:polyribocytidylic acid; RA, all-trans-retinoic acid; TT, tetanus toxoid; VA, vitamin A; NK, natural killer; PE, phycoerythrin; A gate, adult gate; n1 gate, neonatal 1 gate; n2 gate, neonatal 2 gate.
References
- 1.Siegrist, C. A. (2001) Vaccine 19, 3331-3346. [DOI] [PubMed] [Google Scholar]
- 2.Ridge, J. P., Fuchs, E. J. & Matzinger, P. (1996) Science 271, 1723-1726. [DOI] [PubMed] [Google Scholar]
- 3.Adkins, B., Leclerc, C. & Marshall-Clarke, S. (2004) Nat. Rev. Immunol. 4, 553-564. [DOI] [PubMed] [Google Scholar]
- 4.Marshall-Clarke, S., Reen, D., Tasker, L. & Hassan, J. (2000) Immunol. Today 21, 35-41. [DOI] [PubMed] [Google Scholar]
- 5.Levin, D. & Gershon, H. (1989) Cell. Immunol. 120, 132-144. [DOI] [PubMed] [Google Scholar]
- 6.Muthukkumar, S., Goldstein, J. & Stein, K. E. (2000) J. Immunol. 165, 4803-4813. [DOI] [PubMed] [Google Scholar]
- 7.Arulanandam, B. P., Mittler, J. N., Lee, W. T., O'Toole, M. & Metzger, D. W. (2000) J. Immunol. 164, 3698-3704. [DOI] [PubMed] [Google Scholar]
- 8.Kovarik, J., Bozzotti, P., Love-Homan, L., Pihlgren, M., Davis, H. L., Lambert, P. H., Krieg, A. M. & Siegrist, C. A. (1999) J. Immunol. 162, 1611-1617. [PubMed] [Google Scholar]
- 9.Brazolot Millan, C. L., Weeratna, R., Krieg, A. M., Siegrist, C. A. & Davis, H. L. (1998) Proc. Natl. Acad. Sci. USA 95, 15553-15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovarik, J., Martinez, X., Pihlgren, M., Bozzotti, P., Tao, M. H., Kipps, T. J., Wild, T. F., Lambert, P. H. & Siegrist, C. A. (2000) Virology 268, 122-131. [DOI] [PubMed] [Google Scholar]
- 11.Semba, R. D. (2000) in Vitamin A and Retinoids: An Update of Biological Aspects and Clinical Application, ed. Livrea, M. A., (Birkhauser Verlag Basel/Switzerland), pp. 97-108.
- 12.Villamor, E. & Fawzi, W. W. (2000) J. Infect. Dis. 182, Suppl. 1, S122-S133. [DOI] [PubMed] [Google Scholar]
- 13.Alnwick, D., Aylward, B. Barbiero, V., de Benoist, B., Burger, S. Carrasco, P., Cervinskas, J., Clements, J., Csete, J., Coleman, T., et al. (1998) Integration of Vitamin A Supplementation with Immunization: Policy and Programme Implications (World Health Organization, Geneva), document WHO/EPI/GEN/98.07.
- 14.Sommer, A., Tarwotjo, I., Djunaedi, E., West, K. P., Jr., Loeden, A. A., Tilden, R. & Mele, L. (1986) Lancet 1, 1169-1173. [DOI] [PubMed] [Google Scholar]
- 15.Benn, C. S., Aaby, P., Bale, C., Olsen, J., Michaelsen, K. F., George, E. & Whittle, H. (1997) Lancet 350, 101-105. [DOI] [PubMed] [Google Scholar]
- 16.Martines, J., Underwood, B., Arthur, P., Bahl, R., Bhan, M. K., Kirkwood, B. R., Moulton, L. H., Penny, M. E., Ram, M., Kholhede, C. L., et al. (1998) Lancet 352, 1257-1263.9788455 [Google Scholar]
- 17.Benn, C. S., Balde, A., George, E., Kidd, M., Whittle, H., Lisse, I. M. & Aaby, P. (2002) Lancet 359, 1313-1314. [DOI] [PubMed] [Google Scholar]
- 18.Rahman, M. M., Mahalanabis, D., Hossain, S., Wahed, M. A., Alvarez, J. O., Siber, G. R., Thompson, C., Santosham, M. & Fuchs, G. J. (1999) J. Nutr. 129, 2192-2195. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732-738. [DOI] [PubMed] [Google Scholar]
- 20.Lepe-Zuniga, J. L., Rotbein, J. & Gutterman, J. U. (1989) J. Interferon Res. 9, 445-456. [DOI] [PubMed] [Google Scholar]
- 21.Levine, A. S. & Levy, H. B. (1978) Cancer Treat. Rep. 62, 1907-1912. [PubMed] [Google Scholar]
- 22.Saravolac, E. G., Sabuda, D., Crist, C., Blasetti, K., Schnell, G., Yang, H., Kende, M., Levy, H. B. & Wong, J. P. (2001) Vaccine 19, 2227-2232. [DOI] [PubMed] [Google Scholar]
- 23.Manetti, R., Annunziato, F., Tomasevic, L., Gianno, V., Parronchi, P., Romagnani, S. & Maggi, E. (1995) Eur. J. Immunol. 25, 2656-2660. [DOI] [PubMed] [Google Scholar]
- 24.Verdijk, R. M., Mutis, T., Esendam, B., Kamp, J., Melief, C. J., Brand, A. & Goulmy, E. (1999) J. Immunol. 163, 57-61. [PubMed] [Google Scholar]
- 25.Ma, Y., Chen, Q. & Ross, A. C. (2005) J. Immunol. 174, 7961-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeCicco, K. L. & Ross, A. C. (2000) Proc. Nutr. Soc. 59, 519-529. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita, M. & Ross, A. C. (1993) FASEB J. 7, 1277-1282. [DOI] [PubMed] [Google Scholar]
- 28.DeCicco, K. L., Youngdahl, J. D. & Ross, A. C. (2001) Immunology 104, 341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arulanandam, B. P., Van Cleave, V. H. & Metzger, D. W. (1999) Eur. J. Immunol. 29, 256-264. [DOI] [PubMed] [Google Scholar]
- 30.Pihlgren, M., Tougne, C., Schallert, N., Bozzotti, P., Lambert, P. H. & Siegrist, C. A. (2003) Vaccine 21, 2492-2499. [DOI] [PubMed] [Google Scholar]
- 31.Otero, D. C., Anzelon, A. N. & Rickert, R. C. (2003) J. Immunol. 170, 73-83. [DOI] [PubMed] [Google Scholar]
- 32.Otero, D. C. & Rickert, R. C. (2003) J. Immunol. 171, 5921-5930. [DOI] [PubMed] [Google Scholar]
- 33.Stein-Streilein, J., Sonoda, K. H., Faunce, D. & Zhang-Hoover, J. (2000) J. Leukocyte Biol. 67, 488-494. [DOI] [PubMed] [Google Scholar]
- 34.Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. (2000) Immunol. Today 21, 573-583. [DOI] [PubMed] [Google Scholar]
- 35.Biron, C. A. & Brossay, L. (2001) Curr. Opin. Immunol. 13, 458-464. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura, H., Ohta, A., Sekimoto, M., Sato, M., Iwakabe, K., Nakui, M., Yahata, T., Meng, H., Koda, T., Nishimura, S., et al. (2000) Cell. Immunol. 199, 37-42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.