Abstract
The objective of this study was to evaluate the influence of ingested l-arginine, l-citrulline, and antioxidants (vitamins C and E) on the progression of atherosclerosis in rabbits fed a high-cholesterol diet. The fatty diet caused a marked impairment of endothelium-dependent vasorelaxation in isolated thoracic aorta and blood flow in rabbit ear artery in vivo, the development of atheromatous lesions and increased superoxide anion production in thoracic aorta, and increased oxidation-sensitive gene expression [Elk-1 and phosphorylated cAMP response element-binding protein]. Rabbits were treated orally for 12 weeks with l-arginine, l-citrulline, and/or antioxidants. l-arginine plus l-citrulline, either alone or in combination with antioxidants, caused a marked improvement in endothelium-dependent vasorelaxation and blood flow, dramatic regression in atheromatous lesions, and decrease in superoxide production and oxidation-sensitive gene expression. These therapeutic effects were associated with concomitant increases in aortic endothelial NO synthase expression and plasma 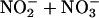 and cGMP levels. These observations indicate that ingestion of certain NO-boosting substances, including l-arginine, l-citrulline, and antioxidants, can abrogate the state of oxidative stress and reverse the progression of atherosclerosis. This approach may have clinical utility in the treatment of atherosclerosis in humans.
and cGMP levels. These observations indicate that ingestion of certain NO-boosting substances, including l-arginine, l-citrulline, and antioxidants, can abrogate the state of oxidative stress and reverse the progression of atherosclerosis. This approach may have clinical utility in the treatment of atherosclerosis in humans.
Keywords: antioxidant, nitric oxide, amino acids, endothelial nitric oxide synthase
Atherosclerosis is an inflammatory disease (1) characterized by vascular endothelial cell dysfunction and diminished production of NO (2-5). Endothelial NO synthase (eNOS) gene transfer can reduce atherogenesis in hypercholesterolemic animals (6). NO is a widespread signaling molecule in the cardiovascular system, which functions in multiple ways to protect against the initiation and progression of atherosclerosis (7-9). For example, NO aids in preventing the adhesion and aggregation of blood cells and platelets along the endothelial cell lining in blood vessels (7, 8) and is a potent inhibitor of vascular smooth muscle cell proliferation (10). NO is a potent antioxidant that can elicit antiinflammatory effects by scavenging certain reactive oxygen species (11-13), and it can prevent the oxidation of low-density lipoprotein cholesterol and thereby retard the progression of atherosclerosis (5, 14). Moreover, NO deficiency is generally associated with up-regulation of oxidation-sensitive genes, whereas increased NO production leads to decreased expression of oxidation-sensitive genes (7, 15). NO is synthesized by NOS, which utilizes l-arginine as substrate and produces l-citrulline as the second reaction product. l-arginine can be synthesized from l-citrulline in endothelial and other cell types, thereby providing a recycling pathway for the conversion of l-citrulline to NO via l-arginine (16-19).
The oral administration of l-arginine to animals (7, 12, 20-26) and humans (5, 8, 27-29) has been demonstrated to slow the progression of atherosclerosis or its component processes. Likewise, antioxidants can elicit antiatherosclerotic effects (13, 30-33). Coadministration of antioxidants with l-arginine produced an enhanced antiatherosclerotic response (7, 13). The mechanism of action of l-arginine appears to be increased production of NO, whereas antioxidants likely work by protecting the newly formed NO against destruction by reactive oxygen species. The principal explanation for the therapeutic response to l-arginine has been increased substrate availability to eNOS, for example by competing with asymmetric dimethylarginine, an endogenous competitive inhibitor of eNOS that is prevalent in states of atherosclerosis (33-36). In two recent studies, however, chronic administration of l-arginine to low-density lipoprotein receptor-deficient mice produced a marked increase in expression of eNOS protein (25, 26). Thus, up-regulation of eNOS could explain the antiatherosclerotic response to l-arginine. The oral administration of l-citrulline, as a precursor to l-arginine and NO, was reported to be beneficial in sickle cell disease in humans (37). Studies indicate that the l-citrulline to l-arginine recycling pathway in endothelial cells may be the principal mechanism for sustaining localized l-arginine availability for eNOS-catalyzed NO production (17-19). The objective of the present study was to examine the actions of l-arginine, l-citrulline, and antioxidants administered orally to rabbits with atherosclerosis.
Materials and Methods
Animals, Protocols, and Metabolic Treatments. A total of 49 New Zealand White male rabbits, aged 3-4 months and weighing 2.0 to 2.4 kg, were housed individually at 20 ± 3°C with free access to water. Rabbits were divided into the following seven groups (six rabbits per group), depending on diet; amino acid, vitamin, and test agents were administered for 12 weeks: Gp1-HCD, high-cholesterol diet (HCD) (standard diet plus 0.5% cholesterol); Gp2-Arg, HCD plus l-arginine (2.5% in drinking water); Gp3-Cit, HCD plus l-citrulline (2.0% in drinking water); Gp4-Arg+Cit, HCD plus l-arginine and l-citrulline; Gp5-Vit, HCD plus vitamin C (sodium ascorbate; 0.25% in drinking water) and vitamin E (dl-α-tocopherol; 150 mg/kg per day by oral gavage); Gp6-Arg+Vit, HCD plus l-arginine and vitamins C and E; and Gp7-Mix, HCD plus l-arginine, l-citrulline and vitamins C and E. In some experiments, an additional group was studied, Gp8-C (control; standard diet; n = 6). Feeding was restricted per rabbit to 120 g per day. Blood was sampled 24 h after the last feeding. All experiments were conducted in accordance with the institutional guidelines for animal research.
Determination of Plasma Lipids, NO Metabolites, and cGMP. Total cholesterol and triglyceride levels were measured as described (38). HDL-cholesterol was determined after precipitation with phosphotungstate-MgCl2 (39). Plasma 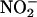 plus
plus 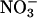 (NOX) was measured by using an NO detector-HPLC system (ENO10; Eicom, Kyoto, Japan), as described (40). Plasma cGMP concentration was determined by specific RIA (RPN226, Amersham Pharmacia) (41).
(NOX) was measured by using an NO detector-HPLC system (ENO10; Eicom, Kyoto, Japan), as described (40). Plasma cGMP concentration was determined by specific RIA (RPN226, Amersham Pharmacia) (41).
Isometric Tension Measurements. After 12 weeks of treatment, rabbits were killed by exsanguination after anesthesia with pentobarbital (50 mg/kg i.v.). The thoracic aorta was excised from the orifice of the left first costal artery down to the diaphragm, cleaned, and sliced into 2-mm-wide transverse rings. Aortic rings were mounted in organ chambers, and isometric tension was measured exactly as described (6). Rings were contracted submaximally with prostaglandin F2α, and endothelium-dependent relaxation elicited by acetylcholine chloride and endothelium-independent relaxation by nitroglycerin (Nihon Kayaku, Tokyo) was determined. In some experiments, indomethacin (5 × 10-6 M) was added 60 min before the experiment to rule out any influence of prostanoids on smooth muscle tone. All indicated concentrations are final concentrations in the bath medium.
Tissue Blood Flow Determined by Laser Doppler Perfusion Imaging. To investigate blood flow near the right central ear artery, we used a laser Doppler imaging system (laser Doppler perfusion imager PIMII, Perimed AB, Linköping, Sweden), as described (42, 43). This method provides a 2D mapping of blood flow in tissues and is based on the principles of laser Doppler flowmetry (42).
Histological Evaluation of Atherosclerosis in Rabbit Aorta. Cross sections of the thoracic aorta used for the evaluation of endothelium-dependent relaxation were stained with van Gieson's elastic stain to determine intimal thickness. Morphometric analysis was performed as described by Weiner et al. (44) and modified by this laboratory (6). Six samples from each rabbit aorta were analyzed.
Detection of Superoxide Anion (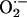 ) in Rabbit Aorta.
) in Rabbit Aorta. 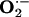 in each aortic segment was assayed by measuring the intensity of chemiluminescent probes in the presence of a Cypridina luciferin analog, 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo1, 2-apyrazine-3-one (45). Briefly, the generation of
in each aortic segment was assayed by measuring the intensity of chemiluminescent probes in the presence of a Cypridina luciferin analog, 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo1, 2-apyrazine-3-one (45). Briefly, the generation of 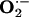 from a 2-mm length of aorta was detected by using a luminescence reader (BLR-201, Aloka, Tokyo), in the absence and presence of superoxide dismutase to verify specificity of the assay for
from a 2-mm length of aorta was detected by using a luminescence reader (BLR-201, Aloka, Tokyo), in the absence and presence of superoxide dismutase to verify specificity of the assay for 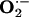
Detection of eNOS, ets-Like Gene-1 (Elk-1), and Phosphorylated cAMP Response Element-Binding Protein (p-CREB). Tissue sections (5 mm) from arterial segments were homogenized (46), and Western blot analysis was performed (47). Gels were transblotted onto nitrocellulose membranes and blocked with milk powder overnight, and samples were incubated with monoclonal antibodies (1:500 dilution for 1.5 h at room temperature) against Elk-1, p-CREB, and eNOS (epitope of NOS-III, no crossreactivity with NOS-I or -II; Santa Cruz Biotechnology) (46-48). Proteins were detected by chemiluminescence (Amersham Pharmacia Biotech enhanced chemiluminescence kit). All other details have been described (46-48).
Data Analysis. Results were expressed as mean ± SEM and represent unpaired data. Data were compared by analysis of variance with repeated measurements. When a significant F value was obtained, Scheffé's test for multiple comparisons was used to identify the differences among groups. P values of <0.05 were considered to be statistically significant.
Results
Influence of Various Treatments on Blood Chemistry. All rabbits appeared to be healthy throughout the study. No significant differences in body weight or plasma triglycerides were observed among groups. Treatment with l-arginine plus l-citrulline with (Gp7-Mix) or without (Gp4-Arg+Cit) antioxidants showed a significant increase in plasma NOX and cGMP levels, as compared with those of the HCD group (Gp1-HCD). However, antioxidant or l-arginine treatment alone had no such effect. Therefore, the combination of l-arginine and l-citrulline possesses the capacity to elevate both NO and cGMP. Presumably, cGMP is elevated because the increased NO production results in activation of soluble guanylate cyclase (Table 1).
Table 1. Plasma levels of lipid, NO products, and cGMP.
| Gp1-HCD | Gp2-Arg | Gp3-Cit | Gp4-Arg + Cit | Gp5-Vit | Gp6-Vit + Arg | Gp7-Mix | |
|---|---|---|---|---|---|---|---|
| T.Chol., mg/dl | 1,758 ± 162 | 1,584 ± 104 | 1,460 ± 210 | 1,350 ± 178 | 1,473 ± 171 | 1,662 ± 162 | 1,257 ± 172 |
| Triglycerides, mg/dl | 104.2 ± 7.1 | 120.7 ± 10.5 | 89.8 ± 4.4 | 88.8 ± 4.5 | 98.4 ± 7.1 | 120.5 ± 8.3 | 72.3 ± 6.9 |
| HDL-C, mg/dl | 29.1 ± 4.8 | 29.4 ± 3.3 | 26.0 ± 3.2 | 34.4 ± 4.1 | 33.5 ± 5.3 | 33.9 ± 4.5 | 35.8 ± 4.1 |
| NO2− + NO3−, nM | 25.1 ± 2.6 | 27.3 ± 4.1 | 25.2 ± 4.0 | 35.1 ± 5.2* | 17.2 ± 3.6 | 26.8 ± 1.8 | 29.1 ± 2.1* |
| cGMP, pg/ml | 19.1 ± 3.3 | 18.9 ± 2.8 | 32.3 ± 3.7 | 36.7 ± 5.2* | 19.3 ± 4.5 | 32.9 ± 6.3 | 41.0 ± 6.1* |
| Body weight, kg | 3.12 ± 0.31 | 2.96 ± 0.40 | 3.11 ± 0.31 | 3.02 ± 0.21 | 3.23 ± 0.34 | 2.97 ± 0.23 | 3.02 ± 0.32 |
Refer to Materials and Methods for the definitions of the treatment groups. Data are expressed as the mean ± SEM from six rabbits per group. T. Chol, total cholesterol; HDL-C, high-density lipoprotein-cholesterol.
Significant difference (P < 0.05) vs. GpI-HCD.
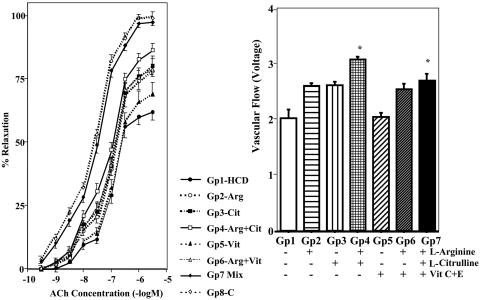
Influence of Fatty Diet and NO-Boosting Supplements on Endothelium-Dependent Vasorelaxation in Thoracic Aorta and Tissue Blood Flow. Acetylcholine elicited endothelium-dependent relaxation of aortic rings (Fig. 1), and the magnitude of relaxation in hypercholesterolemic animals (Gp1-HCD) was markedly diminished when compared with that of the regular diet group (Gp8-C). However, endothelium-dependent relaxation of the aorta from Gp7-Mix animals was remarkably greater than that from Gp1-HCD and was similar to that for Gp8-C animals. Aortic relaxation in Gp4-Arg+Cit, Gp2-Arg, Gp3-Cit, and Gp6-Arg+Vit tended to increase compared with that of the Gp1-HCD group. The endothelium-independent vasorelaxant, nitroglycerin, produced equivalent magnitudes of relaxation in all groups (data not shown). Indomethacin did not appreciably affect endothelium-dependent relaxation (data not shown). Blood flow near the right central ear artery showed a significant improvement in the Gp4-Arg+Cit and Gp7-Mix groups compared with Gp1-HCD (Fig. 1), and these findings are in agreement with the in vitro data from isolated aortic rings. Therefore, ingestion of NO-boosting supplements can improve endothelium-dependent relaxation in atherosclerotic rabbits.
Fig. 1.
Vascular responses of thoracic aortas of rabbits. (Left) Cumulative concentration-response curves to acetylcholine (ACh) during contraction evoked by prostaglandin F2α (2.6 × 10-6 M) in thoracic aortas from eight groups of rabbits (n = 6 per group). (Right) Rate of blood flow near the right central ear artery in seven groups of rabbits (n = 6 per group). Refer to Materials and Methods for definitions of treatment groups. Data are illustrated as the mean ± SEM from six rabbits per group. *, Significant difference (P < 0.05) vs. Gp1-HCD.
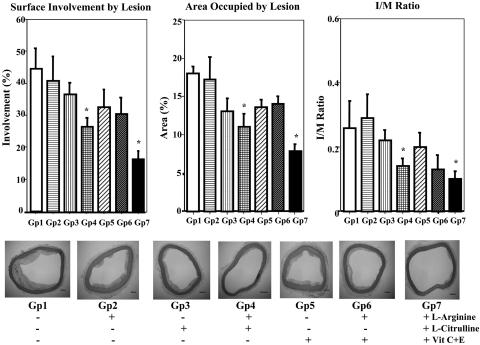
Influence of Fatty Diet and NO-Boosting Supplements on Atheromatous Lesions in Thoracic Aorta. Histological examination of the thoracic aorta revealed markedly smaller atheromatous lesions in the l-arginine plus l-citrulline treatment groups (Gp4-Arg-Cit and Gp7-Mix) than in the hypercholesterolemic group (Gp1) (Fig. 2). The atherosclerotic area was reduced by >50% in animals that received l-arginine, l-citrulline, and antioxidants. Note that the effective treatments also reduced the surface involvement of atherosclerosis and intima/media ratios (Fig. 2). Thus, the initial stages of atherosclerosis and plaque formation were both inhibited by treatment with l-arginine, l-citrulline, and antioxidants.
Fig. 2.
Histological evaluation of atherosclerotic areas of thoracic aortas from seven groups of rabbits. (Upper Left) Surface involvement of atherosclerotic areas in thoracic aorta. (Upper Center) Area occupied by atherosclerotic lesions of the aortic arch and thoracic aorta, Gp1. (Upper Right) The intima/media (I/M) ratio of the aortic arch and thoracic aorta. In each of the above, data are illustrated as the mean ± SEM from six rabbits per group, and * signifies significant difference (P < 0.05) vs. Gp1. (Lower) Representative elastica van-Gieson (EVG)-staining photographic images of cross sections of thoracic aortas from seven groups of rabbits. Original magnification. The scale bar in the lower right corner of each image signifies 200 μm.
Influence of Fatty Diet and NO-Boosting Supplements on Protein Expression of eNOS in Thoracic Aorta. Treatment of rabbits with l-citrulline alone (Gp3) and with the combination of l-arginine and l-citrulline (Gp4) and the combination of l-arginine, l-citrulline, and antioxidants (Gp7) caused a significant increase in eNOS protein expression as compared with Gp1 (Fig. 3). Interestingly, l-citrulline was more effective than l-arginine, and antioxidants tended to increase the effect of l-arginine (Gp6). These data indicate that NO-boosting supplements can up-regulate eNOS gene expression.
Fig. 3.
NO and superoxide production in thoracic aortas from seven groups of rabbits. (Left) Quantification of eNOS protein in thoracic aorta using Western blotting. The Western blot represents a single typical experiment. The bar graph illustrates data from six experiments. Relative amounts of eNOS protein are shown. (Right) Superoxide production in thoracic aortas of seven groups of rabbits (n = 6 per group). Kcpm, multiply numbers shown by 1,000. Data are illustrated as the mean ± SEM from six rabbits per group. *, Significant difference (P < 0.05) vs. Gp1.
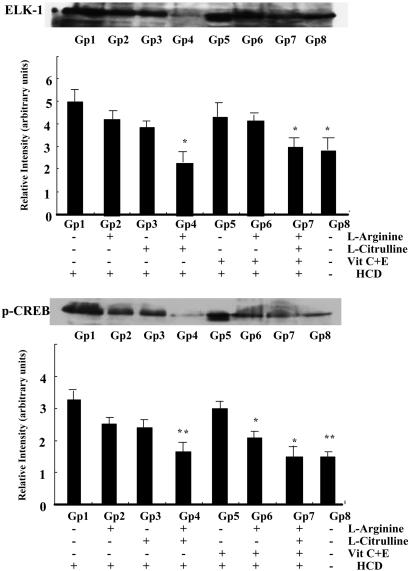
Inhibitory Action of NO-Boosting Supplements on the Increased Production of Superoxide Anion and Elk-1 and p-CREB Protein Expression in Aorta from Hypercholesterolemic Rabbits. 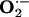 production was ≈3-fold greater in aorta from hypercholesterolemic (Gp1-HCD) rabbits than from control (Gp8-C) rabbits (data not shown). Ingestion of l-arginine and/or l-citrulline tended to decrease
production was ≈3-fold greater in aorta from hypercholesterolemic (Gp1-HCD) rabbits than from control (Gp8-C) rabbits (data not shown). Ingestion of l-arginine and/or l-citrulline tended to decrease 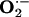 production, although the differences were not statistically significant (Fig. 3). However, the combination of antioxidants with l-arginine (Gp6-Arg+Vit) or l-arginine plus l-citrulline (Gp7-Mix) produced a marked reduction in
production, although the differences were not statistically significant (Fig. 3). However, the combination of antioxidants with l-arginine (Gp6-Arg+Vit) or l-arginine plus l-citrulline (Gp7-Mix) produced a marked reduction in 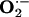 production (Fig. 3). In accord with the well known association between hypercholesterolemia and oxidative stress in tissues, a cholesterol-rich diet caused an increase in oxidation-sensitive Elk-1 and p-CREB expression (compare Gp1 and Gp8; Fig. 4). l-arginine plus l-citrulline, with (Gp7) or without (Gp4) antioxidants completely prevented cholesterol diet-induced increase in Elk-1 and p-CREB expression (Fig. 4). The combination of l-arginine plus antioxidants (Gp6) produced the same effect on p-CREB.
production (Fig. 3). In accord with the well known association between hypercholesterolemia and oxidative stress in tissues, a cholesterol-rich diet caused an increase in oxidation-sensitive Elk-1 and p-CREB expression (compare Gp1 and Gp8; Fig. 4). l-arginine plus l-citrulline, with (Gp7) or without (Gp4) antioxidants completely prevented cholesterol diet-induced increase in Elk-1 and p-CREB expression (Fig. 4). The combination of l-arginine plus antioxidants (Gp6) produced the same effect on p-CREB.
Fig. 4.
Quantification of Elk-1 and p-CREB protein in thoracic aortas from seven groups of rabbits using Western blotting. Western blots represent a single typical experiment. The bar graphs illustrate data from six experiments. Data are illustrated as the mean ± SEM from six rabbits per group. *, Significant difference (P < 0.05) vs. Gp1.
Discussion
The objective of the present study was to evaluate the influence of ingested l-arginine, l-citrulline, and antioxidants (vitamins C and E) on the progression of atherosclerosis in rabbits fed a HCD. The hallmark of diet-induced atherosclerosis in rabbits is vascular endothelial cell dysfunction, which is characterized by marked impairment of endothelium-dependent vasorelaxation in isolated arteries as well as blood flow in vivo and atherosclerosis with distinct atheromatous lesions (3, 6, 30, 40, 45). Moreover, atherosclerosis is characterized by the progressive development of oxidative stress, as evidenced by the increased production of 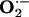 in arteries and increased expression of oxidation-sensitive genes such as Elk-1 and p-CREB (12, 13, 15, 25). The systemic administration of l-arginine and antioxidants to atherosclerotic animals has been demonstrated to slow the progression of disease (7, 12, 20-26). However, the effects of l-citrulline alone or in combination with l-arginine or l-arginine plus antioxidants have not been reported.
in arteries and increased expression of oxidation-sensitive genes such as Elk-1 and p-CREB (12, 13, 15, 25). The systemic administration of l-arginine and antioxidants to atherosclerotic animals has been demonstrated to slow the progression of disease (7, 12, 20-26). However, the effects of l-citrulline alone or in combination with l-arginine or l-arginine plus antioxidants have not been reported.
l-arginine plus l-citrulline, alone or with antioxidants, caused a marked improvement in endothelium-dependent vasorelaxation and rabbit ear blood flow, dramatic regression in atheromatous lesions, and decrease in 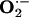 production. These therapeutic effects were associated with concomitant increases in aortic eNOS expression and plasma levels of nitrite plus nitrate (NOX) and cGMP. The data reveal that chronic ingestion of the dietary supplements used in this study promotes an increase in NO production and action. NOX are stable oxidation products of NO and represent markers of NO production. cGMP is the intracellular second messenger that mediates many physiological actions of NO, and its formation is stimulated by NO. In view of the evidence that NO improves endothelial dysfunction, causes vasorelaxation in vitro and vasodilation in vivo, and slows the progression of atherosclerosis (1, 7, 8), it is reasonable to conclude that the pharmacological effects observed in rabbits after dietary supplementation with l-arginine, l-citrulline, and antioxidants are attributed to increased production and action of NO.
production. These therapeutic effects were associated with concomitant increases in aortic eNOS expression and plasma levels of nitrite plus nitrate (NOX) and cGMP. The data reveal that chronic ingestion of the dietary supplements used in this study promotes an increase in NO production and action. NOX are stable oxidation products of NO and represent markers of NO production. cGMP is the intracellular second messenger that mediates many physiological actions of NO, and its formation is stimulated by NO. In view of the evidence that NO improves endothelial dysfunction, causes vasorelaxation in vitro and vasodilation in vivo, and slows the progression of atherosclerosis (1, 7, 8), it is reasonable to conclude that the pharmacological effects observed in rabbits after dietary supplementation with l-arginine, l-citrulline, and antioxidants are attributed to increased production and action of NO.
The chronic oral administration of l-arginine with or without antioxidants to mice was shown to increase the protein expression of eNOS in the aorta (25, 26). Similarly, in the present study, NO-boosting supplements caused a marked up-regulation of eNOS in rabbit aorta. In addition, l-citrulline was tested and found to increase eNOS protein expression. The combined administration of l-citrulline plus l-arginine, with or without antioxidants produced an even greater up-regulation of eNOS. eNOS up-regulation was accompanied by elevated plasma NOX and cGMP, thereby indicating indirectly that the up-regulated eNOS was functionally active. There are at least two possible mechanisms by which l-arginine could have increased NO production. One is up-regulation of eNOS, and a second is increased availability of substrate (l-arginine) to eNOS. The latter mechanism might appear to be less likely than the first, because Km for l-arginine as a substrate for eNOS is 2-15 μM, whereas plasma l-arginine levels in mammals are 100-200 μM, thereby suggesting that eNOS may already be saturated with substrate. This enigma has been termed the “arginine paradox.” However, current evidence suggests that the bulk of intracellular endothelial l-arginine may not be available for NO production. Plasmalemmal caveolae may be the principal source of l-arginine available to eNOS (18, 19). Moreover, the l-citrulline to l-arginine recycling pathway is localized to caveolae and may be the principal source of available l-arginine (17-19). Cytosolic l-arginine availability for eNOS may be limited by uptake into plasmalemmal caveolae (49), and administration of excess l-arginine may create a sufficient concentration gradient to make more l-arginine available to eNOS. Alternatively, the “arginine paradox” has been explained by the presence during atherosclerosis of elevated levels of asymmetric dimethylarginine (ADMA), a competitive inhibitor of eNOS (5, 33-36, 50). Excess l-arginine could effectively compete with ADMA for binding sites on eNOS. Our observations both here and previously in mice (25, 26) that l-arginine can up-regulate eNOS offers a previously undescribed explanation of the “arginine paradox.”
l-citrulline, the second product of the NOS reaction, was reported to elicit endothelium-dependent relaxation of rat aorta accompanied by increases in tissue nitrite and cGMP (51). This is consistent with the knowledge that l-citrulline is converted to l-arginine by mammalian cells, including endothelial cells (16-19, 52, 53). This recycling pathway might be important in sustaining the production of NO in endothelial cells, especially when l-arginine becomes limiting, as is possible in atherosclerosis. In the present study, l-citrulline produced pharmacological effects that closely resembled those of l-arginine administration and NO action. l-citrulline caused a marked improvement in endothelium-dependent vasorelaxation in response to acetylcholine, and the combination of l-citrulline and l-arginine produced a synergistic response in elevating plasma NOX and cGMP, improving rabbit ear artery blood flow and slowing the progression of atherosclerosis. A key observation was the marked up-regulation of eNOS on chronic administration of l-citrulline, and this response might explain, in part or entirely, the NO-like pharmacological effects of l-citrulline.
Atherosclerosis is an inflammatory disease characterized by endothelial dysfunction and impairment of NO production (1, 2, 8). Herein lies a plausible explanation for atherogenesis, because it is well appreciated that NO elicits a multifaceted array of pharmacological actions, all of which are protective against the progression of atherosclerosis (7, 8). A common feature of inflammation and atherosclerosis is oxidative stress (15), which can lead not only to cell membrane injury but also the destruction of NO. Thus, the natural antioxidant properties of NO are lost, and oxidative stress continues unabated. In the present study, fatty diet-induced atherosclerosis and oxidative stress were reversed upon oral administration of l-arginine, l-citrulline, and antioxidants. These observations suggest that NO is the active species in reducing both the markers for oxidative stress and the progression of atherosclerosis.
Cardiovascular disease is the leading cause of morbidity and untimely death both in men and women in the U.S. and may be largely avoidable and even reversible by adopting more sensible programs involving a healthy diet and moderate exercise. A diet low in saturated fat and rich in antioxidants could counter the development of oxidative stress and boost NO production and action (11, 13, 15, 31, 33). Likewise, moderate exercise would boost NO production and decrease the expression of oxidation-sensitive genes (26). The present study demonstrates, at least in rabbits, that chronic ingestion of l-arginine, l-citrulline, and antioxidants can reverse the progression of atherosclerosis. Similar observations were made in humans with l-arginine and antioxidants (5, 8, 27-29). Therefore, taken together, embarking on a low-fat and high-antioxidant-diet moderate exercise program and regimen of NO-boosting dietary supplements might result in a lower incidence of deaths attributed to cardiovascular disease.
Acknowledgments
This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan, and the Japan Society for Promotion of Science [Grant in Aid for Science Research No. 16406001 (to T.H.), Awards for Eminent Scientists 2002-2004 (to L.J.I.), and Postdoctoral Fellowship for Foreign Researchers (to P.A.R.J.)].
Abbreviations: NOX, 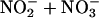 ; eNOS, endothelial NO synthase; p-CREB, phosphorylated cAMP response element-binding protein; HCD, high-cholesterol diet; Gpn, group n.
; eNOS, endothelial NO synthase; p-CREB, phosphorylated cAMP response element-binding protein; HCD, high-cholesterol diet; Gpn, group n.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115-126. [DOI] [PubMed] [Google Scholar]
- 2.Cooke, J. P. (1998) J. Invest. Med. 46, 377-380. [PubMed] [Google Scholar]
- 3.Hayashi, T., Ishikawa, T., Naito, M., Kuzuya, M., Funaki, C., Asai, K. & Kuzuya, F. (1991) Atherosclerosis 87, 23-38. [DOI] [PubMed] [Google Scholar]
- 4.Kauser, K., da Cunha, V., Fitch, R., Mallari, C. & Rubanyi, G. M. (2000) Am. J. Physiol. 278, H1679-H1685. [DOI] [PubMed] [Google Scholar]
- 5.Boger, R. H. & Bode-Boger, S. M. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 79-99. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi, T., Sumi, D., Juliet, P. A., Matsui-Hirai, H., Asai-Tanaka, Y., Kano, H., Fukatsu, A., Tsunekawa, T., Miyazaki, A., Iguchi, A., et al. (2004) Cardiovasc. Res. 61, 339-351. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro, L. J. & Napoli, C. (2004) Curr. Atherosclerosis Rep. 6, 281-287. [DOI] [PubMed] [Google Scholar]
- 8.Cooke, J. P. (2003) Proc. Natl. Acad. Sci. USA 100, 768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro, L. J., Cirino, G., Casini, A. & Napoli, C. (1999) J. Cardiovasc. Pharmacol. 34, 879-886. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro, L. J., Buga, G. M., Wei, L.-H., Bauer, P. M., Wu, G. & del Soldato, P. (2001) Proc. Natl. Acad. Sci. USA 98, 4202-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz, M. N., Frei, B., Vita, J. A. & Keaney, J. F., Jr. (1997) N. Engl. J. Med. 337, 408-416. [DOI] [PubMed] [Google Scholar]
- 12.Boger, R. H., Bode-Boger, S. M., Mugge, A., Kienke, S., Brandes, R., Dwenger, A. & Frolich, J. C. (1995) Atherosclerosis 117, 273-284. [DOI] [PubMed] [Google Scholar]
- 13.Boger, R. H., Bode-Boger, S. M., Phivthong-ngam, L., Brandes, R. P., Schwedhelm, E., Mugge, A., Bohme, M., Tsikas, D. & Frolich, J. C. (1998) Atherosclerosis 141, 31-43. [DOI] [PubMed] [Google Scholar]
- 14.Napoli, C., Ackah, E., de Nigris, F., del Soldato, P., D'Armiento, F. P., Crimi, E., Condorelli, M. & Sessa, W. C. (2002) Proc. Natl. Acad. Sci. USA 99, 12467-12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Nigris, F., Lerman, A., Ignarro, L. J., Williams-Ignarro, S., Sica, V., Baker, A. H., Lerman, L. O., Geng, Y. J. & Napoli, C. (2003) Trends Mol. Med. 9, 351-359. [DOI] [PubMed] [Google Scholar]
- 16.Hecker, M., Sessa, W. C., Harris, H. J., Anggard, E. E. & Vane, J. R. (1990) Proc. Natl. Acad. Sci. USA 87, 8612-8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin, B. L., Solomonson, L. P. & Eichler, D. C. (2004) J. Biol. Chem. 279, 18353-18360. [DOI] [PubMed] [Google Scholar]
- 18.Flam, B. R., Hartmann, P. J., Harrell-Booth, M., Solomonson, L. P. & Eichler, D. C. (2001) Nitric Oxide 5, 187-197. [DOI] [PubMed] [Google Scholar]
- 19.Solomonson, L. P., Flam, B. R., Pendleton, L. C., Goodwin, B. L. & Eichler, D. C. (2003) J. Exp. Biol. 206, 2083-2087. [DOI] [PubMed] [Google Scholar]
- 20.McNamara, D. B., Bedi, B., Aurora, H., Tena, L., Ignarro, L. J., Kadowitz, P. J. & Akers, D. L. (1993) Biochem. Biophys. Res. Commun. 193, 291-296. [DOI] [PubMed] [Google Scholar]
- 21.Cooke, J. P., Singer, A. H., Tsao, P., Zera, P., Rowan, R. A. & Billingham, M. E. (1992) J. Clin. Invest. 90, 1168-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, B.-Y., Singer, A. H., Tsao, P. S., Drexler, H., Kosek, J. & Cooke, J. P. (1994) J. Am. Coll. Cardiol. 23, 452-458. [DOI] [PubMed] [Google Scholar]
- 23.Boger, R. H., Bode-Boger, S. M., Brandes, R. P., Phivthong-ngam, L., Bohme, M., Nafe, R., Mugge, A. & Frolich, J. C. (1997) Circulation 96, 1282-1290. [DOI] [PubMed] [Google Scholar]
- 24.Boger, R. H., Bode-Boger, S. M., Kienke, S., Stan, A. C., Nafe, R. & Frolich, J. C. (1998) Atherosclerosis 136, 67-77. [DOI] [PubMed] [Google Scholar]
- 25.de Nigris, F., Lerman, L. O., Williams-Ignarro, S., Sica, G., Lerman, A., Palinski, W., Ignarro, L. J. & Napoli, C. (2003) Proc. Natl. Acad. Sci. USA 100, 1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napoli, C., Williams-Ignarro, S., de Nigris, F., Lerman, L. O., Rossi, L., Guarino, C., Mansueto, G., Di Tuoro, F., Pignalosa, O., De Rosa, G., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8797-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drexler, H., Zeiher, A. M., Meinzer, K. & Hanjorg, J. (1991) Lancet 338, 1546-1550. [DOI] [PubMed] [Google Scholar]
- 28.Bode-Boger, S. M., Boger, R. H., Alfke, H., Heinzel, D., Tsikas, D., Creutzig, A., Alexander, K. & Frolich, J. C. (1996) Circulation 93, 85-90. [DOI] [PubMed] [Google Scholar]
- 29.Boger, R. H., Bode-Boger, S. M., Thiele, W., Creutzig, A., Alexander, K. & Frolich, J. C. (1998) J. Am. Coll. Cardiol. 32, 1336-1344. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi, T., Sumi, D., Matsui-Hirai, H., Fukatsu, A., Arockia, Rani P. J., Kano, H., Tsunekawa, T. & Iguchi, A. (2003) Atherosclerosis. 168, 23-31. [DOI] [PubMed] [Google Scholar]
- 31.Kleinveld, H. A., Demacker, P. N. M. & Stalenhoef, A. F. H. (1994) Arterioscler. Thromb. 14, 1386-1391. [DOI] [PubMed] [Google Scholar]
- 32.Esterbauer, H., Dieber-Rotheneder, M., Striegl, G. & Waeg, G. (1991) Am. J. Clin. Nutr. 53, 314S-321S. [DOI] [PubMed] [Google Scholar]
- 33.Sydow, K., Schwedhelm, E., Arakawa, N., Bose-Boger, S. M., Tsikas, D., Hornig, B., Frolich, J. C. & Boger, R. H. (2003) Cardiovasc. Res. 57, 244-252. [DOI] [PubMed] [Google Scholar]
- 34.Bode-Boger, S. M., Boger, R. H., Kienke, S., Junker, W. & Frolich, J. C. (1996) Biochem. Biophys. Res. Commun. 219, 598-603. [DOI] [PubMed] [Google Scholar]
- 35.Boger, R. H. & Bode-Boger, S. M. (2000) Semin. Thromb. Hemostasis 26, 539-545. [DOI] [PubMed] [Google Scholar]
- 36.Boger, R. H., Bode-Boger, S. M., Szuba, A., Tsao, P. S., Chan, J. R., Tangphao, O., Blaschke, T. F. & Cooke, J. P. (1998) Circulation 98, 1842-1847. [DOI] [PubMed] [Google Scholar]
- 37.Waugh, W. H., Doeschner, C. W., Files, B. A., McConnell, M. E. & Strandjord, S. E. (2001) J. Natl. Med. Assoc. 93, 363-371. [PMC free article] [PubMed] [Google Scholar]
- 38.Allain, C. C., Poon, L. S., Chan, C. S., Richmond, W. & Fu, P. C. (1974) Clin. Chem. 20, 470-475. [PubMed] [Google Scholar]
- 39.Department of Health, Education, and Welfare (1982) in Lipid Research Clinics Program Manual of Laboratory Operations (U.S. Govt. Printing Office), Vol. 1, Ed. 2, pp. 76-628. [Google Scholar]
- 40.Hayashi, T., Esaki, T., Muto, E., Kano, H., Asai, Y., Thakur, N.K., Sumi, D., Jayachandran, M. & Iguchi, A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 782-792. [DOI] [PubMed] [Google Scholar]
- 41.Okano, K., Takahashi, S., Yasuda, K., Tokinaga, D., Imai, K. & Koga, M. (1992) Anal. Biochem. 202, 120-125. [DOI] [PubMed] [Google Scholar]
- 42.Wardell, K., Jakobsson, A. & Nilsson, G. E. (1993) IEEE Trans. Biomed. Eng. 40, 309-316. [DOI] [PubMed] [Google Scholar]
- 43.Caballero, A. E., Arora, S., Saouaf, R., Lim, S. C., Smakowski, P., Park, J. Y., King G. L., LoGerfo, F. W., Horton, E. S. & Veves, A. (1999) Diabetes 48, 1856-1862. [DOI] [PubMed] [Google Scholar]
- 44.Weiner, B. H., Ockene, I. S., Levine, P. H., Cuenoud, H. F., Fisher, M., Johnson, B. F., Daoud, A. S., Jarmolych, J., Hosmer, D. & Johnson, M. H. (1986) N. Engl. J. Med. 315, 841-846. [DOI] [PubMed] [Google Scholar]
- 45.Thakur, N. K., Hayashi, T., Sumi, D., Kano, H., Tsunekawa, T. & Iguchi, A. (2001) Am. J. Physiol. 281, H75-H83. [DOI] [PubMed] [Google Scholar]
- 46.Napoli, C., de Nigris, F., Welch, J. S., Calara, F. B., Stuart, R. O., Glass, C. K. & Palinski W. (2002) Circulation 105, 1360-1367. [DOI] [PubMed] [Google Scholar]
- 47.de Nigris, F., Franconi, F., Maida, I., Palumbo, G., Anania, V. & Napoli, C. (2000) Biochem. Pharmacol. 59, 1477-1487. [DOI] [PubMed] [Google Scholar]
- 48.Jeremy, R. W., McCarron, H. & Sullivan, D. (1996) Circulation 94, 498-506. [DOI] [PubMed] [Google Scholar]
- 49.Hardy, T. A. & May, J. M. (2002) Free Radic. Biol. Med. 32, 122-131. [DOI] [PubMed] [Google Scholar]
- 50.Tsikas, D., Boger, R. H., Sandmann, J., Bode-Boger, S. M. & Frolich, J. C. (2000) FEBS Lett. 478, 1-3. [DOI] [PubMed] [Google Scholar]
- 51.Santhanam, A. V. & Dikshit, M. (2001) Eur. J. Pharmacol. 431, 61-69. [DOI] [PubMed] [Google Scholar]
- 52.Shuttleworth, C. W. R., Burns, A. J., Ward, S. M., O'Brien, W. E. & Sanders, K. M. (1995) Neuroscience 68, 1295-1304. [DOI] [PubMed] [Google Scholar]
- 53.Xie, L., Hattori, Y., Tume, N. & Gross, S. S. (2000) Semin. Perinatol. 24, 42-45. [DOI] [PubMed] [Google Scholar]