Abstract
The transient receptor potential channel TRPV5 constitutes the apical entry pathway for transepithelial Ca2+ transport. We showed that TRPV5 was inhibited by both physiological intra- and extracellular acid pH. Inhibition of TRPV5 by internal protons was enhanced by extracellular acidification. Similarly, inhibition by external protons was enhanced by intracellular acidification. Mutation of either an extra- or an intracellular pH sensor blunted the crossinhibition by internal and external protons. Both internal and external protons regulated the selectivity filter gate. Using the substituted cysteine accessibility method, we found that intracellular acidification of TRPV5 caused a conformational change of the pore helix consistent with clockwise rotation along its long axis. Thus, rotation of pore helix caused by internal protons facilitates closing of TRPV5 by external protons. This regulation by protons likely contributes to pathogenesis of disturbances of Ca2+ transport in many diseased states. Rotation of pore helix may be a common mechanism for crossregulation of ion channels by extra- and intracellular signals.
Keywords: substituted cysteine-accessibility method, transepithelial Ca2+ transport, transient receptor potential
Introduction
Transient receptor potential (TRP) channels are widespread and play many important functions, ranging from store- and receptor-operated Ca2+ entry, thermal, tactile, taste, osmolar, and fluid flow sensing, embryonic development to epithelial Ca2+ and Mg2+ transport (Hoenderop et al, 2002; Montell et al, 2002; Clapham, 2003; Huang, 2004). TRP channels are classified into TRPC, TRPV, TRPM TRPP, TRPML, TRPN, and TRPA subfamilies. The TRPV subfamily is named after the first mammalian member of the subfamily, vanilloid receptor 1 (VR1), and contains six mammalian members, TRPV1–6 (Jordt et al, 2003). TRPV5 and TRPV6 are the only two highly Ca2+-selective TRP channels and mediate trans-epithelial Ca2+ transport in the kidney and intestine (Hoenderop et al, 2002). Structurally, all TRP channels have six predicted transmembrane (TM) segments and N- and C-terminal cytoplasmic tails similar to the topologies of voltage-gated K+, Na+, and Ca2+ channels, cyclic nucleotide-gated (CNG) channels, and hyperpolarization-activated channels (Montell et al, 2002; Clapham, 2003). The six-TM polypeptide subunits of TRP channels likely assemble as tetramers to form cation-permeable pores (Hoenderop et al, 2003).
The bacterial K+ channel KcsA contains two membrane-spanning segments and an intervening pore (P) loop (Doyle et al, 1998). The crystal structure of KcsA reveals an outer selectivity filter gate stabilized by pore helices and an inner gate formed by four inner helices of four identical subunits packed against each other as a bundle near the intracellular aspect of the membrane, giving an appearance of an inverted teepee (Doyle et al, 1998). This architecture is likely a general structure for all P-loop-containing channels including voltage-gated K+ channels, inward rectifier and Ca2+-activated K+ channels, and CNG cation channels (Flynn et al, 2001). How and which gate is activated by individual extra- and intracellular signals for each channel is under intense investigation. The location of the inner gate suggests its role as an activation gate for intracellular signals. However, signals acting from the intracellular aspect of the membrane also impact on the selectivity filter (Flynn and Zagotta, 2001).
TRPV5 is localized to the apical membrane of polarized epithelia and functions as a gatekeeper for transepithelial Ca2+ transport (Hoenderop et al, 2002). Transepithelial Ca2+ transport is inhibited in conditions associated with overproduction of acids (Sutton et al, 1979). How acid inhibits transepithelial Ca2+ transport is not known. One potential mechanism is by direct inhibition of the TRPV5 channel. Little is known about the structure and mechanism involved in the gating of TRP channels. Mutation of aspartate-542 of TRPV5 greatly reduces Ca2+ permeation, indicating that the aspartate and adjacent amino acids form the selectivity filter in the channel (Nilius et al, 2001). A stretch of ∼20 amino acids in the pre-selectivity filter region of TRPV5 and TRPV6 is highly homologous to that in KcsA and cysteine-substituted mutants of the region exhibit a cyclic pattern of reactivity to MTS reagents (Dodier et al, 2004; Voets et al, 2004). Thus, this region likely forms the pore helix of TRPV5 and TRPV6.
In this study, we show that the selectivity filter gate of TRPV5 is crossregulated by physiological extra- and intracellular pH (pHi). Gating of TRPV5 by intracellular acidification is coupled with a clockwise rotation of the pore helix. This intracellular proton-induced conformational change of the pore helix facilitates gating by extracellular protons. These results provide a novel mechanism for crossregulation of ion channels by intra- and extracellular signals.
Results
Inhibition of TRPV5 by intracellular acidification
We examined pHi regulation of TRPV5 by inside-out patch-clamp recording (Figure 1A). As shown, TRPV5 currents were recorded using voltage ramps (from −100 to 100 mV over 400 ms). In inside-out patches, TRPV5 exhibited inwardly rectifying currents in divalent-free (DVF) cytoplasmic solutions (see Figure 1B ramp I–V curve at pHi 9.0: outward (at 100 mV) and inward currents (at –100 mV) were 197 and −412 pA, respectively). The Mg2+-independent inward rectification has been suggested due to intrinsic gating (Voets et al, 2003). Maximal TRPV5 currents occurred when inside-out membranes were exposed to solutions with pH 9.0–9.5 (Figure 1B and C). Decreasing pH from 9.0 to 4.9 caused a progressive reduction of TRPV5 currents, which reversed upon increasing the pH to 9.0 (Figure 1C). At an extracellular pH (pHe) of 7.2 (illustrated in Figure 1A), the apparent pKa for pHi regulation was estimated at 7.31±0.12 (n=21) (Figure 1D). The Hill coefficient for pHi regulation is ∼2.1, indicating that each channel is gated by more than one proton and with cooperativity. Thus, TRPV5 is steeply gated by internal protons at physiological and pathophysiological pHi's.
Figure 1.
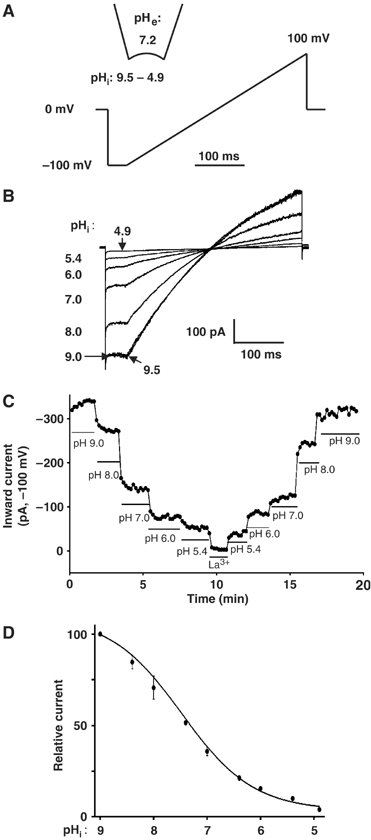
Regulation of TRPV5 by pHi and its modulation by pHe. (A) Inside-out recording and voltage ramp protocol (applied every 10 s). (B) Current–voltage (I–V) relationships of currents (in DVF solution) at different pHi's. The degree of inward rectification in our study appears to be slightly less than that in some study (Voets et al, 2003). Mechanism(s) for the apparent difference awaits further study. (C) Inward currents (pA, at −100 mV) at different pHi's over time. Currents through TRPV5 were inhibited by lanthanum (La3+, 10 mM). In mock-transfected cells, increasing pHi from 4.9 to 9.4 caused a relatively insignificant increase of background inward currents (<50 pA, compared to the mean maximal inward TRPV5 currents at pHi 9.4 (452±18 pA, n=21)). (D) Dose–response curve for inhibition of TRPV5 by intracellular acid. Relative currents (after subtraction of residual currents in 10 mM La3+, normalized to the maximal current, labeled as 100) at any given pHi are shown.
Effects of internal protons on TRPV5 single-channel properties were further studied. As shown in the representative recording with only one active channel in the patch, single-channel open probability (Po) was higher at pHi 8.4 than at 7.0 (Po: 0.86 versus 0.24; Supplementary Figure 1A). In multiple recordings, the average NPo (number of channels in the excised patch [N] × Po) at pHi's 8.4 and 7.0 were 3.05±1.1 and 0.97±0.4, respectively (P<0.05; Supplementary Figure 1B). Single-channel conductance (measured between –25 and –75 mV) at pHi's 8.4 and 7.0 were 78.2±4.6 and 60.4±2.6 pS, respectively (P<0.05; Supplementary Figure 1C). Thus, as for external protons (Yeh et al, 2003), intracellular protons reduce single-channel conductance as well as open probability.
Modulation of pHi regulation of TRPV5 by pHe
Extracellular protons also inhibit TRPV5 (Yeh et al, 2003). We investigated the potential modulation by pHe of pHi regulation of TRPV5. Effects of pHi on TRPV5 at various extracellular (pipette) pH's were studied using inside-out recordings (Figure 2A). Increasing the pHe from 7.2 (indicated by the open triangle curve in Figure 2A and second bar from right in Figure 2B) to 8.4 (black curve and black bar in Figure 2) caused a shift in the pKa for pHi regulation toward more acidic values (see Supplementary Figure 2A and B for original traces). Conversely, decreasing pHe from 7.2 to 6.8 caused a shift in pKa for pHi regulation toward more alkaline pH (Figure 2A and B; Supplementary Figure 2C and D). Thus, opening and closing of the channel by extracellular alkalinization and acidification, respectively, facilitates opening and closing by intracellular alkalinization and acidification. Intra- and pHe could crossregulate TRPV5 by acting on the same gate or by regulating different activation gates. The pHe-induced shift in pKa for pHi regulation based on normalized relative current, nevertheless, suggests that both pH's crossregulate the same gate.
Figure 2.
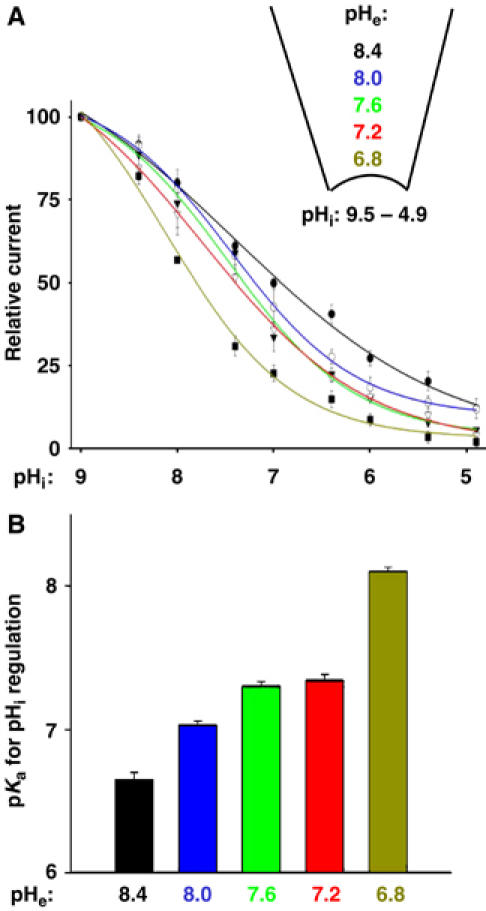
Modulation of pHi regulation of TRPV5 by pHe. (A) Dose–response curves for pHi regulation (pHi from 9.5 to 4.9) at different pHe's. Experimental paradigm is as in Figure 1D. (B) pKa for pHi regulation (Y-axis) at different pHe's (X-axis).
Modulation of pHe regulation of TRPV5 by pHi
If both pH's regulate the same gate, pHi would also modulate pHe regulation of the channel. Using whole-cell recordings (Figure 3A), decreasing intracellular (pipette) pH's from 8.4 to 6.4 caused a shift in pKa for pHe regulation from 5.1±0.1 to 7.3±0.1 (Figure 3A and B), indicating that intracellular acidification increases the sensitivity to extracellular protons. Glutamate-522 in the pre-pore helix region of TRPV5 is an pHe sensor (Yeh et al, 2003). We found that modulation by pHi or pHe regulation was blunted in the E522Q mutant (Figure 3C and D), supporting that pHi's and pHe's crossregulate the same gate.
Figure 3.
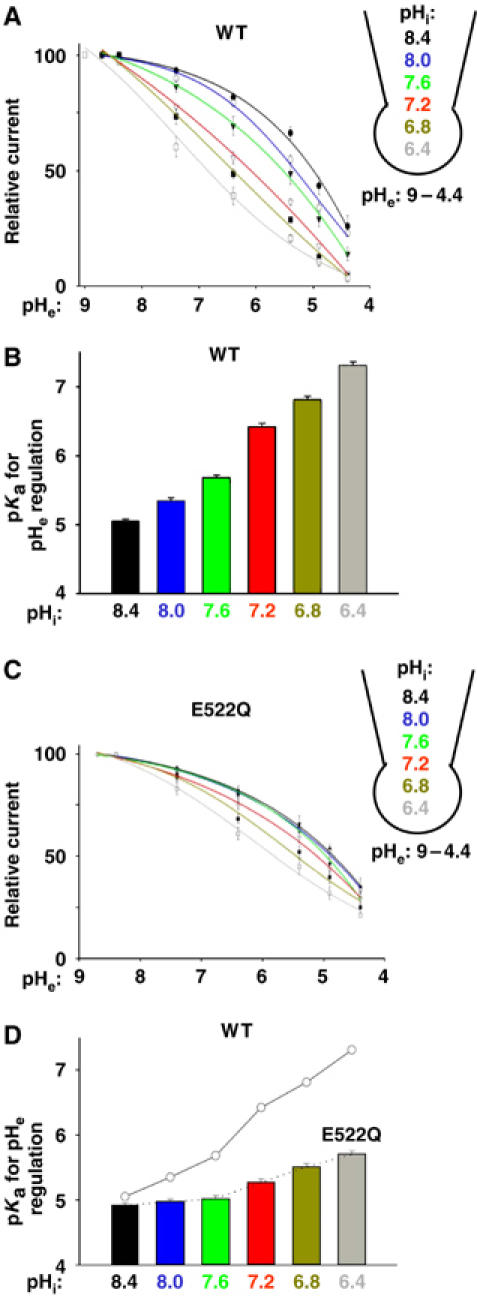
Modulation of pHe regulation of TRPV5 by pHi. (A) Dose–response curves for inhibition of WT TRPV5 by extracellular acidification (pHe from 9.0 to 4.4) at different pHi. Relative currents (normalized to the maximal current, labeled as 100) at any given pHe are shown. (B) pKa for pHe regulation (Y-axis) at different pHi (X-axis) for WT TRPV5. (C) Same as panel A, except for E522Q TRPV5 mutant. The mean inward current densities at pHi 7.2 and pHe 7.4 were 438±29 pA/pF for E522Q mutant and 531±42 pA/pF for WT. (D) Same as panel B, except for E522Q TRPV5 mutant. pKa for WT (from panel B) is shown in circles connected by line for comparison.
Both intra- and extracellular acidification decrease the estimated diameter of TRPV5 pore
To investigate whether internal and external protons crossregulate the selectivity filter gate, we first examined the effects of pHi and pHe on the estimated diameter of ion permeation pore. The pore diameter was estimated by measuring the relative permeability of organic monovalent cations of increasing size (Sabovcik et al, 1995; Voets et al, 2004).
Like many Ca2+-permeating channels, TRPV5 conducts monovalent cations in the absence of Ca2+ ions (Nilius et al, 2001; Yeh et al, 2003). With Na+ as the sole cation in the extra- and intracellular solution, TRPV5 currents reversed at 0 mV (−0.30±0.52 mV, n=18; Figure 4A). Replacing Na+ in the extracellular solution with the same concentration of monomethylammonium (MA+; 3.6 Å), dimethylammonium (DMA+; 4.6 Å), or N-methyl-D-glucamine (NMDG+; 6.8 Å) resulted in reductions of inward currents and shifts of reversal potentials toward negative membrane potentials. The permeability ratios of MA+, DMA+, and NMDG+ relative to Na+ (PX/PNa) were determined from the biionic reversal potential (Hille, 2001). The diameters (Å) of ion permeation pores were estimated (using a modified excluded volume equation (PX/PNa)1/2=dP−dX/dP−dNa, where dP, dX, and dNa are diameters of the pore, cation X+, and Na+, respectively; see Sabovcik et al, 1995) at ∼10.3, 7.5, and 5.6 for pHi's 9.0, 7.4, and 6.0, respectively (Figure 4B and C). These estimated pore diameter values agree with those using another excluded volume equation (Dwyer et al, 1980; Voets et al, 2004) (Supplementary Figure 3). Thus, intracellular acidification reduces the estimated TRPV5 pore diameter. Similarly, extracellular acidification reduces the estimated pore diameter. As shown in Figure 4D and E, the pore diameters (Å) were estimated at ∼11.8, 7.5, and 6.3 for pHe's 9.0, 7.4, and 6.0, respectively.
Figure 4.
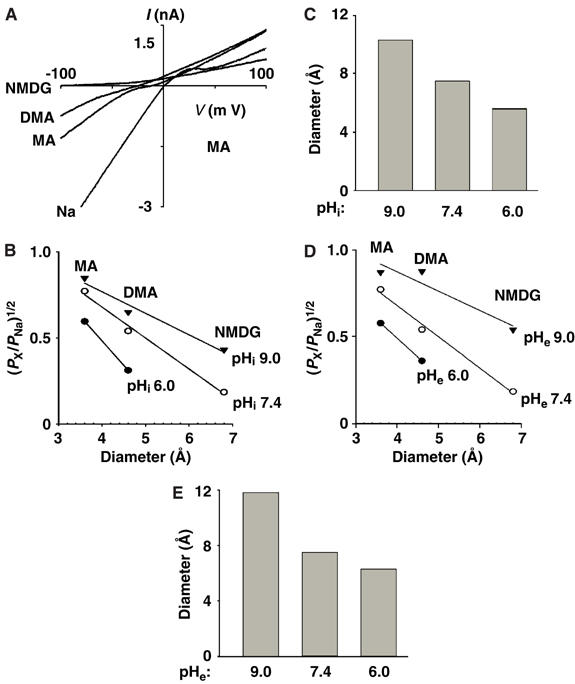
Effects of pHi and pHe on the estimated pore size. (A) Relative permeability of MA+, DMA+, and NMDG+ to Na+ at pHi and pHe 7.4. Whole-cell currents were first recorded in a bath solution containing 130 mM NaAsp, which was subsequently replaced by solutions containing 130 mM X-Asp. X is MA, DMA or NMDG. Shown are I–V curves at these different extracellular cations. The differential rectification in different extracellular cations and the reduction of outward currents in extracellular NMDG+ may be due to pore block by partially permeant cations. (B) Relationships of the relative permeability ratios of permeating ions over Na+ versus diameter of permeating ions at different pHi's. Experiments were performed as in panel A, with pHi at 9.0, 7.4, or 6.0. The relative permeability ratios (PX/PNa) were calculated according to the equation PX/PNa=exp ΔErev × F/RT. ΔErev is the shift of reversal potential upon changing the bath solution from NaAsp to X-Asp. X is MA, DMA, or NMDG with diameters of 3.6, 4.6, and 6.8 Å, respectively. (C) Estimated pore diameter (Å) at pHi's 9.0, 7.4, and 6.0. (D) Relationships of the relative permeability versus diameter of permeating ions at different pHe's. (E) Estimated pore diameter at pHe 9.0, 7.4, and 6.0.
Effects of pHi and pHe on the monovalent cation selectivity of TRPV5
The effects of pH on the selectivity filter of TRPV5 were further investigated by examining the monovalent cation selectivity. Permeability ratios of Li+, K+, and Cs+ relative to Na+ were determined by replacing extracellular Na+ with the respective ions. At pHi 7.4, the relative permeability ratios of Li+, K+, and Cs+ to Na+ were 0.85±0.04, 0.76±0.04, and 0.65±0.05, respectively (Figure 5A and Table I). This selectivity sequence (Na+>Li+>K+>Cs+) follows Eisenman selectivity sequence X, indicating a strong-field-strength-binding site (Hille, 2001; Nilius et al, 2001). An increase in the diameter of the selectivity filter would decrease the electrostatic field strength of the ion-binding site. Indeed, we found that increasing pHi from 7.4 to 9.0 changed the selectivity sequence to Eisenman III or IV, indicating a weak-field-strength-binding site (Figure 5B and Table I). These results support the idea that intracellular protons regulate the selectivity filter gate. Sequence X (at pHi 7.4) represents the second strongest-field-strength-binding sequence among the 11 Eisenman sequences. Accordingly, decreasing pHi from 7.4 to 6.0 did not change the Eisenman sequence (Table I).
Figure 5.
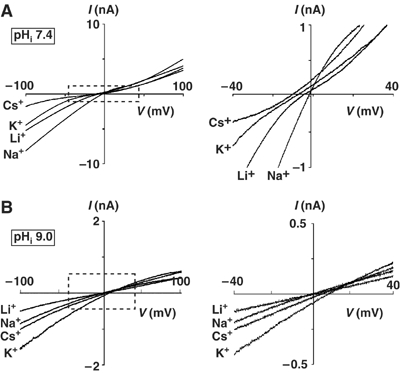
Relative permeability of inorganic monovalent cations to Na+ at pHi 7.4 (A) or 9.0 (B). Whole-cell experiments were performed with extracellular at pH 7.4 and pHi at 7.4 (panel A) or 9.0 (panel B). Currents were first recorded in a bath solution containing 130 mM NaAsp, which was subsequently replaced by solutions containing 130 mM X-Asp. X is Li, Cs, or K. Shown are I–V curves at these different extracellular cations. The two left panels show I–V curves in the full range. The right panels show I–V curves of the boxed regions in an expanded scale.
Table 1.
Effects of pH's on relative permeability of inorganic monovalent cations to Na+
| K+ | Li+ | Cs+ | Permeability sequence | Eisenman sequence | ||
|---|---|---|---|---|---|---|
| pHi (at fixed pHe 7.4) | 6.0 | 0.80±0.09 | 0.92±0.03 | 0.71±0.05 | Na>Li>K>Cs | X |
| pHi (at fixed pHe 7.4) | 7.4 | 0.76±0.04 | 0.85±0.04 | 0.65±0.05 | Na>Li>K>Cs | X |
| pHi (at fixed pHe 7.4) | 9.0 | 1.15±0.13 | 1.01±0.11 | 1.09±0.10 | K>Cs>Na=Li | III or IV |
| pHe (at fixed pHi 7.4) | 7.4 | 0.79±0.04 | 0.91±0.04 | 0.68±0.05 | Na>Li>K>Cs | X |
| pHe (at fixed pHe 7.4) | 9.0 | 1.10±0.07 | 0.88±0.11 | 0.98±0.11 | K>Na=Cs>Li | IV or V |
| Data shown are the relative permeabilities of the respective ions to Na+ (mean±s.e.m.). | ||||||
We further examined the effects of pHe on TRPV5 selectivity profile. Consistent with the results of increasing the pore diameter, extracellular alkalinization from pH 7.4 to 9.0 (at a fixed pHi 7.4) altered the selectivity sequence from Eisenman sequence X to a weaker-field-strength-binding sequence (sequence IV or V; Table I). These results, together with the effects of pH on the pore diameter, indicate that both internal and external protons regulate the selectivity filter gate.
Intracellular acidification causes clockwise rotation of the pore helix
Amino acids 527–538 of TRPV5, likely from the pore helix of TRPV5 (Dodier et al, 2004; Voets et al, 2004), are similar to the pore helix of KcsA (Figure 6A). The selectivity filter of TRPV5 contains aspartate-542 critical for Ca2+ permeation (Nilius et al, 2001). The conformational state of pore helix is critical for the open conformation of selectivity filter (Doyle et al, 1998). We therefore hypothesized that internal and external protons crossregulate the selectivity filter gate via conformational changes in the pore helix.
Figure 6.
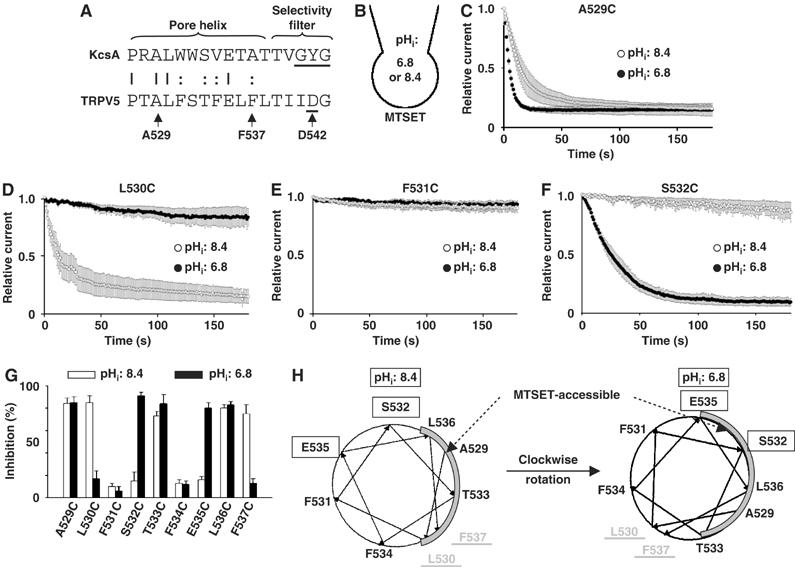
Accessibility of the pore helix to MTSET. (A) Alignment of the pore helix and selectivity filter of TRPV5 versus KcsA. Amino acids 527–543 of TRPV5 are shown. D542 of TRPV5 is critical for permeation of Ca2+ ions. Amino acids A529–F537 were individually mutated to cysteine. (B) Illustration of externally applied MTSET (1 mM) in whole-cell recordings at pHi 8.4 or 6.8. (C–F) Effects of MTSET on A529C (C), L530C (D), F531C (E) or S532C (F) at pHi 8.4 (○) or 6.8 (•). Inward currents (at −100 mV) from voltage ramp applied every second. Cells were transfected with varying amounts of DNA (0.5–5 μg) to produce similar current density for different mutants (ranging from 354±42 to 554±57 pA/pF at −100 mV and pHe 8.4). Currents were normalized to the maximal current prior to application of MTSET. (G) Percent inhibition by MTSET (after 3 min) of cysteine-substituted mutants (from A529 to F537) at pHi 8.4 (white bar) or 6.8 (black bar). (H) Helical wheel projection of amino acids of the pore helix of TRPV5. MTSET accessibility area of the pore helix is shown in gray. E535 and S532 are not accessible at pHi 8.4, but become accessible at 6.8. In contrast, L530 and F537 are accessible at pHi 8.4, but become inaccessible at 6.8. Amino acids L536, A529, and T533 are accessible at both pHi's. Amino acids F531 and F534 are inaccessible at either pHi.
To test this hypothesis, we introduced cysteines into amino acids 529–537 of TRPV5 and examined reactivity of the mutants to extracellular membrane-impermeant cysteine-modifying reagent MTSET at either pHi 8.4 or 6.8 (Figure 6B). Application of MTSET (1 mM) caused a fast inhibition of A529C at both pHi's 6.8 and 8.4 (Figure 6C). The inhibition was not reversible by washout (not shown), indicating covalent modification of cysteine residues by MTSET. The second-order rate constants for inhibition were ∼200 and ∼75 M−1 s−1 at pHi's 6.8 and 8.4, respectively. In contrast, L530C was inhibited by MTSET at pHi 8.4, but barely inhibited after 3 min application of MTSET at pHi 6.8 (Figure 6D). F531C was not inhibited at either pHi (Figure 6E); S532C was inhibited at pHi 6.8, but not at pHi 8.4 (Figure 6F). Figure 6G summarizes the effects of MTSET on cysteine-substituted amino acids from A529C to F537C. A529C, L530C, T533C, L536C, and F537 were >75% inhibited by MTSET at pHi 8.4 in 3 min, whereas F531C, S532C, F534C, and E535C were not. As in helical wheel projection, amino acids sensitive to MTSET are located in the same face of the helix (Figure 6H). In contrast, the MTSET-resistant amino acids are located in a separate face. Assuming that lack of irreversible inhibition reflects inaccessibility to MTSET, these results indicate that the face of pore helix bound by L536 and L530 is exposed to solvents at pHi 8.4. Interestingly, the solvent-exposed face at pHi 6.8 is different from that at pHi 8.4. As shown in Figure 6G and H, E535C and S532C were not accessible at pHi 8.4, but accessible at pHi 6.8. In contrast, F537C and L530C, two of the residues accessible at pHi 8.4, were not accessible at pHi 6.8 (Figure 6G and H). TRPV5 is mostly open at pHi 8.4 (∼90% of the maximal current) and predominantly closed at pHi 6.8 (∼30% of the maximal current). Thus, closing of TRPV5 by internal protons is associated with a clockwise rotation of the pore helix.
Cysteine substitution of pore helix does not affect gating by protons
To exclude that cysteine substitution affects gating by protons, we examined three representative cysteine-substituted mutants (L530C, F531C, and S532C) located in separate faces of the pore helix (see Figure 6H). As shown in Figure 6, L530C is accessible at pHi 8.4, but not at 6.8, whereas F531C is not accessible at either pHi. S532C is accessible at pHi 6.8, but not at 8.4. Here, we found that the sensitivity to pHi for each of L530C, F531C, and S532C mutants was not different from that of wild-type (WT) TRPV5 (Figure 7A). Also, the sensitivity to pHe and its modulation by pHi for the mutants were essentially the same as for the WT (Figure 7B–F). These results indicate that cysteine substitution for L530, F531, and S532 does not affect gating by pH, and support our conclusion that changes in MTSET reactivity for amino acids in the pore helix are due to rotation along its along axis.
Figure 7.
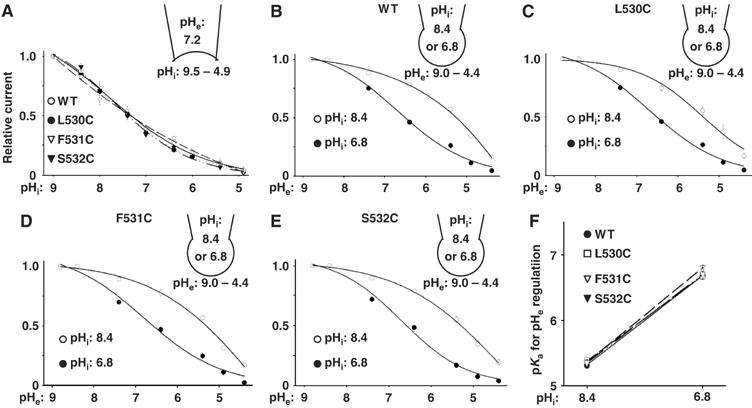
No effects of cysteine substitution on pH gating. (A) pHi regulation of L530C, F531C, and S532C mutants versus WT. Relative currents (maximal current=1) versus pHi's are shown. (B–E) Dose–response curves for pHe regulation at pHi 8.4 or 6.8 for WT (B), L530C (C), F531C (D), and S532C (E). (F) pKa for pHe regulation at pHi 8.4 and 6.8 for WT, L530C, F531C, and S532C. pKa values are from panels B, C, D, and F, respectively.
Amino acid aspartate-542 is important for intracellular acidification-induced changes of the pore diameter
Mutation of aspartate-542 of TRPV5 to alanine (D542A) increases the estimated pore diameter (Voets et al, 2004), suggesting that the side chain of aspartate-542 lines the narrowest part of the ion permeation pathway, forming the molecular sieve for permeating ions. We found that, unlike for WT TRPV5, the relative permeability of MA+, DMA+, and NMDG+ to Na+ for D542A mutant was not altered by intracellular acidification (Figure 8A; see Figure 4B for comparison). The estimated pore diameters (Å) at pHi's 9.0, 7.4, and 6.0 for D542A mutant were ∼10, 9.7, and 9.2, respectively (Figure 8B). For comparison, the estimated pore diameter (Å) at pHi's 9.0, 7.4, and 6.0 for WT TRPV5 were ∼10.3, 7.5, and 5.6, respectively (see Figure 4C). These results support the conclusion that intracellular protons regulate the selectivity gate of TRPV5 formed by aspartate-542.
Figure 8.
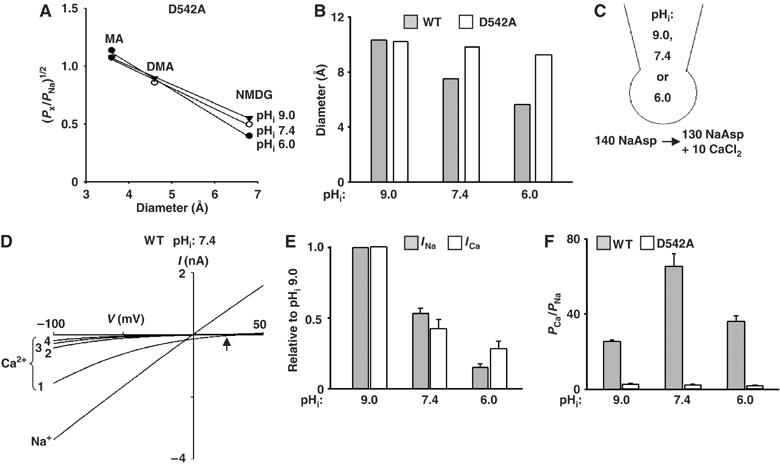
Role of aspartate-542 in pHi-mediated changes of the pore diameter. (A) The relative permeability ratios of permeating ions over Na+ versus diameter of permeating ions at different pHi's for D542A mutant. Experimental paradigm is as in Figure 4B. (B) Estimated pore diameter (Å) at pHi's 9.0, 7.4, and 6.0 for D542A mutant. Experimental paradigm is as in Figure 4C. (C) Solutions and diagram of whole-cell experiments of the relative permeability ratio of Ca2+ versus Na+. (D) I–V curves of WT TRPV5 currents recorded at 140 mM NaAsp bath solution (labeled as Na+) and upon changing to 130 mM NaAsp+10 mM CaCl2 (labeled as Ca2+). Upward arrow indicates reversal potentials in 130 NaAsp+10 CaCl2-containing bath solution. Currents inactivated rapidly in four sweeps (numbered 1, 2, 3, and 4; one voltage ramp sweep per second). (E) Effects of intracellular acidification on Na+ versus Ca2+ currents. Experimental paradigm is as shown in panels C and D. Na+ currents (‘Ina', gray bar) are inward currents (−80 mV) at 130 mM NaAsp-containing bath solution. Ca2+ currents (‘Ica', white bar) are inward currents (at −80 mV) from the first sweep after changing to Ca2+-containing bath solution. Currents at pHi's 7.4 and 6.0 were normalized to pHi 9.0. Mean±s.e.m., n=12–15 for each group. (F) Relative permeability ratio of Ca2+ versus Na+ (PCa/PNa) for WT TRPV5 (gray bar) and D542A mutant (white bar) at different pHi's (9.0, 7.4, 6.0). The permeability ratio of Ca2+ versus Na+ was calculated according to the equation, PCa/PNa=([Na+]i/4[Ca2+]o) exp(ΔErev F/RT) {(1+exp(ΔErev F/RT)}. ΔErev's (shift of reversal potential from Na+ to Ca2+ bath solution) were 7.6±2.5, 22.3±4.5, and 13.5±2.2 mV for pHi's 9.0, 7.4, and 6.0, respectively (see Supplementary Figure 4). Mean±s.e.m., n=8–12 for each group.
The physiological function of TRPV5 is to mediate transepithelial Ca2+ transport. pHe affects TRPV5-mediated Ca2+ entry (Yeh et al, 2003). Here, we further examined the effects of pHi on Ca2+ permeation and selectivity. Whole-cell Na+ current (at pHi 9.0, 7.4, or 6.0) was first recorded in a bath solution containing (in mM) 140 NaAsp (Figure 8C). Subsequently, current was recorded in a bath containing (in mM) 130 NaAsp plus 10 CaCl2. Owing to the anomalous mole fraction effect, inward currents would be predominantly carried by Ca2+ ions in the 10 mM CaCl2-containing solution (Vennekens et al, 2001). The intracellular solution contains 140 mM Na+. As shown in a representative recording at pHi 7.4, currents reversed at ∼0 mV in the 140 mM Na+-containing bath solution, indicating Na+ currents (Figure 8D; labeled ‘Na+'). In the 10 mM Ca2+-containing bath solution, currents reversed at a positive membrane potential (indicated by the upward arrow), indicating inward Ca2+ currents (Figure 8D; labeled ‘Ca2+'). Ca2+ entry through TRPV5 raises the local intracellular Ca2+ concentration and causes feedback inhibition of the channel (Vennekens et al, 2000). As shown, TRPV5 Ca2+ currents decreased over 4 s (Figure 8D; labeled ‘1–4'). The magnitude of the first Ca2+ current (at −100 mV) recorded immediately after changing to the Ca2+ solution (labeled ‘1' in Figure 8D) was rather consistent (about 50–60% relative to that of the Na+ current in all experiments) and thus used for examining effects of pHi on TRPV5-mediated Ca2+ current. Similar to the Na+ current (labeled ‘Ina'), intracellular acidification and alkalinization decreased and increased Ca2+ current, respectively (labeled ‘Ica'; Figure 8E). Thus, the regulation by pHi is likely important for the physiological function of TRPV5 in Ca2+ transport.
The effect of pHi on Ca2+ selectivity was examined by measuring the relative permeability ratio of Ca2+ to Na+ (PCa/PNa). As reported by others (Nilius et al, 2001), we found that TRPV5 was highly Ca2+-selective and D542A mutation markedly decreased the selectivity (PCa/PNa at pHi 7.4: 65±7 and 3.2±0.4 for WT TRPV5 and D542A mutants, respectively; Figure 8F). Interestingly, the relative permeability ratio of Ca2+ to Na+ was decreased by either alkalinization to pHi 9.0 or acidification to pHi 6.0 (P<0.05 versus pHi 7.4; Figure 8F and Supplementary Figure 4). These results are consistent with the idea that the side chains of aspartate-542 of selectivity filter are oriented to provide the most stable interaction with Ca2+ ions at physiological pHi ∼7.4. Displacement of the side chains caused by rotation of the pore helix (either clockwise rotation at pHi 6.0 or counter-clockwise rotation at pHi 9.0) destabilizes the interaction between side chains and Ca2+ ions and decreases the selectivity for Ca2+. The decrease in the relative permeability of Ca2+ to Na+ by intracellular acidification, together with the decrease in single-channel conductance and open probability, leads to reduction of calcium entry. Intracellular alkalinization, however, causes an increase in single-channel conductance and open probability besides its effect on the relative permeability of Ca2+ to Na+. The net effect of alkalinization on TRPV5 is to increase the calcium entry (see Figure 8E above).
Amino acid lysine-607 is important for pHi sensing and crossregulation by intra- and extracellular protons
We further searched for pHi sensor(s). There are more than 140 titratable amino acids in the intracellular portion of TRPV5. We focused on 17 amino acids surrounding the putative intracellular entrance of the pore (i.e., from the fourth TM domain to the proximal C-terminus; see Figure 9A). These include lysine-484 in the intracellular loop between the fourth and fifth TM domains (IC loop; Figure 9A), aspartate-489 and arginine-492 in the fifth TM domain, glutamate-535 and aspartate-542 in the pore region, tyrosine-595 in the sixth TM domain, and aspartate-580, histidine-582, arginine-584, glutamate-588, aspartate-590, glutamate-591, and lysine-607 in the proximal carboxyl-terminus of the channel. We mutated each of these amino acids to a nontitratable amino acid and found that only mutation of lysine-607 to asparagine (K607N) decreased the sensitivity of the channel to inhibition by intracellular acidification. The pKa for inhibition by intracellular acidification were 7.41±0.15 and 6.17±0.13 for WT TRPV5 and K607N mutant, respectively (P<0.05, n=5 each; Figure 9B). We further examined a role of lysine-607 in the crosstalk between pHi and pHe. As in Figure 7F, intracellular acidification from pHi 8.4 to 6.8 increased the pKa for pHe regulation of WT TRPV5 from 5.1±0.07 to 6.8±0.08 (Figure 9C). The increase in the pKa for pHe regulation by intracellular acidification was blunted for K607N mutant (pKa: 4.9±0.06 and 5.6±0.06 at pHi's 8.4 and 6.8, respectively). Thus, lysine-607 is an pHi sensor that contributes to crossregulation by pHi and pHe.
Figure 9.
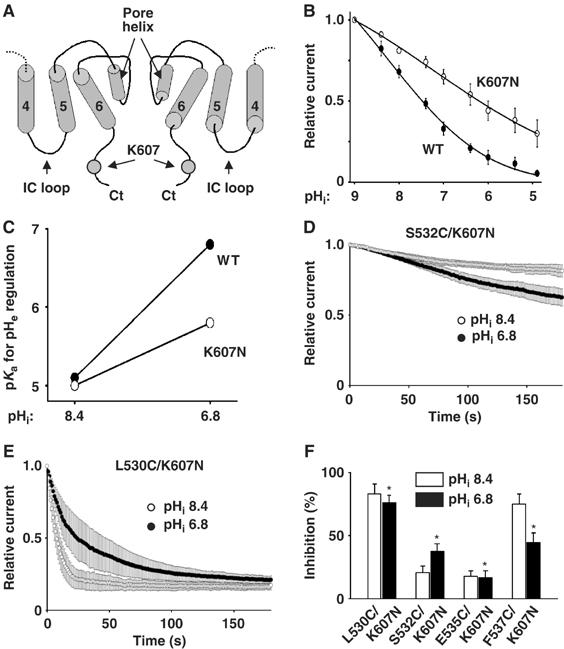
Amino acid lysine-607 is important for pHi sensing and crossregulation by pHi and pHe. (A) Location of lysine-607 (K607) in the intracellular proximal carboxyl terminus (Ct). Illustrated is side-view of two subunits of a tetrameric channel. Only the fourth, fifth, and sixth TM (labeled ‘4', ‘5', and ‘6') and pore helices are shown. (B) Mutation of lysine-607 to asparagine (K607N) decreases intracellular acidification-mediated inhibition. Experimental paradigm is as in Figure 1. WT and K607N mutant channels in inside-out patches were exposed to pHi ranging from pH 9 to 5. (C) pKa for pHe regulation at pHi's 8.4 and 6.8 for WT (•) and K607N (○). Experimental paradigm is as in Figure 7F. pKa's for pHe regulation were studied using whole-cell recording at pHi 8.4 or 6.8. (D–E) Effects of MTSET on double mutants S532C/K607N (D) and L530C/K607N (E) at pHi 8.4 (○) or 6.8 (•). Experimental paradigm is as in Figure 6. (F) Percent inhibition by MTSET (after 3 min) of cysteine-substituted and K607N double mutants (L530C/K607N, S532C/K607N, E535C/K607N, and F537N/K607N) at pHi 8.4 (white bar) or 6.8 (black bar). Experimental paradigm is as in Figure 6. * Indicates P<0.05 by unpaired t-test, double mutants versus single cysteine-substituted mutants at pHi 6.8 taken from Figure 6G (L530C, S532C, E535C, and F537C, respectively).
S532C and E535C were not accessible to MTSET at pHi 8.4, but accessible at pHi 6.8. In contrast, L530C and F537C were accessible at pHi 8.4, but not accessible at pHi 6.8 (see Figure 6). These changes in accessibility are most consistent with the interpretation that intracellular acidification causes a clockwise rotation of the pore helix. We found that double mutants S532C/K607N (Figure 9D and F) and E535C/K607N (Figure 9F) were less sensitive to MTSET at pHi 6.8 than single-cysteine mutants S532C and E535C, respectively (see Figure 6F and G). In contrast, double mutants L530C/K607N (Figure 9E and F) and F537C/K607N (Figure 9F) were more sensitive at pHi 6.8 than single mutant L503C and F537C, respectively. Thus, K607 contributes to the rotation of pore helix associated with gating by internal protons. As controls, the effects of MTSET on double mutants at pHi 8.4 (Figure 9D–F) were not significantly different from that on single mutants (Figure 6G). Also, the pKa for pHi regulation for the double mutants (6.21±0.15, 6.09±0.13, 6.26±0.17, and 6.11±0.14 for L530C/K607, S532C/K607N, E535C/K607N, and F537C/K607N, respectively; n=3–4 for each) were not significantly different from that for K607N mutant (Figure 9B), confirming that cysteine substitution does not affect pHi gating. These results support the conclusion that binding of protons to lysine-607 leads to rotation of the pore helix. Mutation of lysine-607 blunts the rotation caused by internal protons.
Discussion
Using the substituted cysteine accessibility method (SCAM), we report in this study that intracellular acidification causes conformational change(s) of the pore helix of TRPV5. One of the changes likely involves clockwise rotation of the pore helix along its long axis. This conclusion is based on the following assumptions. First, only cysteine residues at a water-accessible surface of the protein will react with MTSET. Second, reaction with MTSET will inhibit TRPV5. These two assumptions are likely valid in our present study given that MTSET is positively charged and membrane-impermeant (Holmgren et al, 1996) and that MTSET is large in size and the pore helix is in close proximity and critical for ion conduction (Doyle et al, 1998). Finally, intracellular acidification could, in theory, alter the local electrostatic environment of the cysteine residues and therefore affect their reactivity to MTSET without causing rotation of the pore helix (Wilson et al, 2000). However, the localization of MTSET-reactive and nonreactive residues to two separate, opposite faces of a predicted α-helix and the pattern of reactivity at pHi 8.4 versus 6.8 (see Figure 6H) provide compelling evidence for a clockwise rotation of the pore helix caused by intracellular acidification. Other types of movements/conformational changes of the pore helix such as translation and/or tilting may also occur in intracellular acidification.
The pore helix of ion channels is critical for ion conduction. Aromatic amino acids of the pore helices of KcsA make contacts with aromatic amino acids in the selectivity filter to hold the filter open (Doyle et al, 1998). Moreover, pore helices point their carboxyl-termini to a water-filled cavity at the membrane center. The partial negative charge of the oriented pore helices provides a favorable electrostatic force to stabilize K+ ions at the membrane center (Roux and MacKinnon, 1999). In the crystal structure of another bacterial K+ channel KirBac1.1 in the closed state, pore helices no longer point directly at the center of the channel cavity (Kuo et al, 2003). It is believed that this misalignment of pore helices contributes to the closed state. For TRPV5, rotation with or without translation and/or tilting of the pore helix caused by intracellular acidification may cause a similar misalignment of the helix dipoles and close the channel. Rotation of the pore helix can also alter conformation of the selectivity filter.
Binding of extracellular protons to glutamate-522 in the pre-pore helix region of TRPV5 causes conformational changes and channel closure (Yeh et al, 2003). We find that pHi modulates pKa for pHe regulation and vice versa. Mutation of glutamate-522 blunts the modulation by pHi of pHe regulation. Together, these results indicate that extra- and pHi crossregulate the same activation gate. Where is this activation gate? The crystal structure of KcsA suggests two potential activation gates: an outer gate formed by the selectivity filter and pore helix and an inner gate formed by bundle crossing of four inner helices near the intracellular aspect of the membrane (Doyle et al, 1998; Flynn et al, 2001). Gating of voltage-dependent K+ channels is associated with structural rearrangements of the inner helices and changes in their accessibility to intracellular Ag+ (del Camino and Yellen, 2001), suggesting that bundle crossing is the activation gate in response to changes in TM voltage. Bundle crossing also serves as activation gate for intracellular signals. Phillips et al (2003) showed that gating of Kir6.2 channels by intracellular ATP involves movement of inner helices at the bundle crossing. Based on the crystal structure, Jiang et al (2002) proposed that opening of the MthK channel by intracellular Ca2+ occurs via bending of the inner helices at a hinge located deep in the membrane to splay open the bundle.
In contrast, studies of CNG channels revealed that, though the bundle crossing undergoes conformational changes associated with gating by intracellular cyclic nucleotide, it does not form a physical barrier for permeating ions in the closed state (Flynn and Zagotta, 2001). The pore helix and selectivity filter of the CNG channels both undergo conformational changes in association with gating by intracellular cyclic nucleotide (Becchetti et al, 1999; Liu and Siegelbaum, 2000). Gating-associated movement of pore helix has also been found for other channels including voltage-dependent K+ channels (Yang et al, 1997) and KcsA (Perozo et al, 1999). Whether the fifth and/or sixth TM helices of TRPV5 form an inner gate and, if so, whether they serve as an activation gate for internal protons is not known. Our results, nevertheless, suggest that the pore helix and selectivity filter of TRPV5 is at least one of the activation gates for intracellular protons (see the model in Figure 10). Gating by intracellular acidification occurs, at least partly, at the selectivity filter via rotation of the pore helix.
Figure 10.
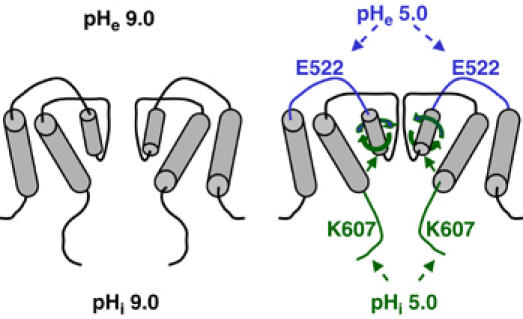
Model for crossregulation of TRPV5 by pHi and pHe. Conformational changes caused by intra- and extracellular acidification are illustrated. See text for details.
How does the pore helix senses intracellular protons? The inner helices of KcsA make direct contacts with pore helices (Doyle et al, 1998). It is believed that rotation of the pore helix of CNG channel in response to cGMP binding to the intracellular binding site occurs indirectly through conformational changes of the sixth TM inner helix (Flynn and Zagotta, 2001; Flynn et al, 2001). We propose that binding of protons to intracellular proton sensor(s) such as lysine-607 causes conformational changes of the sixth (and/or the fifth) TM helix (model in Figure 10). Conformational changes of the sixth TM helix then cause rotation of the pore helix of TRPV5 similarly to that in the CNG channel. Extracellular protons cause conformational changes of the channel by binding to a pH sensor (glutamate-522) located immediately upstream of the pore helix (Yeh et al, 2003). We suggest that both extra- and intracellular protons cause pore helices to rotate in the clockwise direction, leading to closing (narrowing) of the selectivity filter gate. Rotation of the pore helices by intracellular acidification will therefore facilitate closing of the channel by extracellular acidification and vice versa. This model of gating by protons at the level of pore helix and selectivity filter is supported by the results that protons decrease selectivity for Ca2+, pore diameter, and the single-channel conductance, as well as reduction in open probability.
TRPV5 is the apical entry pathway for transepithelial Ca2+ transport in many tissues, including kidney, intestine, placenta, and prostate (Hoenderop et al, 2002). Overproduction of acid, as in metabolic acidosis or high dietary animal protein intake, inhibits renal transepithelial Ca2+ transport, causing increased urinary Ca2+ excretion and kidney stone diseases (Sutton et al, 1979; Huang, 2004). Most conditions of acid overproduction leading to kidney stone diseases cause both extra- and intracellular acidosis (such as high dietary animal protein intake) (Amanzadeh et al, 2003; Nicoletta and Schwartz, 2004). We report in this study that internal and external protons crossinhibit TRPV5-mediated Ca2+ entry. We suggest that inhibition of TRPV5-mediated renal Ca2+ transport by internal and/or external protons contribute to the pathogenesis of disturbances of Ca2+ transport in these diseases. Moreover, crossregulation by internal and external protons will allow the effects of extracellular acidosis on TRPV5 be modulated by the pHi and vice versa. For example, extracellular acidification from pHe 7.4 to 6.4 inhibits TRPV5 by 18% at pHi 7.6, but by 24% at pHi 6.8 (see Figure 3A). Thus, the combined effects of extra- and intracellular acidosis on calcium transport may be amplified or blunted depending on existing pHe and pHi.
Gating of ion channels in response to external and internal signals is a crucial cell function. How signals regulating ion channels are integrated across the cell membrane is poorly understood. The pore helix of ion channels is pivotal for ion conduction through the selectivity filter and accessible to both external and internal signals (Flynn et al, 2001). Thus, conformational changes of the pore helix may be a common mechanism for integrating extra- and intracellular signals in the regulation of ion channels.
Materials and methods
Molecular biology and cell culture
Mutant rabbit TRPV5's in pCDNA3 mammalian expression vector were constructed as described (Yeh et al, 2003). Chinese hamster ovary (CHO) cells (at ∼50% confluence) were cotransfected with cDNA for pEGFP (0.5 μg) plus cDNAs for WT or mutant TRPV5 (0.5–5 μg) using lipofectamine-plus transfection kits (Gibco) as described (Yeh et al, 2003). About 24–48 h after transfection, cells were dissociated by limited trypsin treatment and placed in a chamber for recording. Transfected cells were identified using epifluorescent microscopy for recordings.
Electrophysiological recordings
Inside-out and whole-cell recordings were performed using an Axopatch 200B patch-clamp amplifier (Axon Instruments) (Yeh et al, 2003). For whole-cell recordings, the pipette and bath solution contained (in mM) 140 Na-Asp (sodium aspartate), 10 NaCl, 10 EDTA, 10 HEPES (titrated to pH as specified) and 140 Na-Asp, 10 NaCl, 1 EDTA, and 10 HEPES (pH as specified), respectively. For inside-out recordings, membrane patches were excised into a bath solution containing (in mM) 140 NaAsp, 10 NaCl, 10 EDTA, and 10 HEPES (pH as specified).
To determine the relative permeabilities of MA+, DMA+, and NMDG+ to Na+ in whole-cell recordings, the initial bath and pipette solution contain (in mM) 130 Na-Asp, 10 NaCl, 1 EDTA, 10 HEPES (pH as specified) and 130 Na-Asp, 10 NaCl, 10 EDTA, 10 HEPES (pH as specified), respectively. Na-Asp-containing bath solution was then replaced by one that contains 130 X-Asp, 10 X-Cl, 10 HEPES, and 1 EDTA (X is MA, DMA, or NMDG). X-Asp solutions were prepared by mixing methylamine (Fluka Chemika), dimethylamine (Aldrich), or NMDG (Sigma) (preweighted to a final concentration of 140 mM) with aspartic acid (preweighted to a final concentration 130 mM) and HCl (preweighted to a final concentration 10 mM) and adjusted pH using MA, DMA, or NMDG, respectively. For determination of the relative permeabilities of K+, Li+, and Cs+ to Na+, K-Asp, Li-Asp, and Cs-Asp solutions were prepared similarly.
To test the accessibility of substituted cysteines, MTSET (Toronto Research Chemicals) was added to the bath solution from 100 mM stocks. Stock solutions were prepared daily and kept at 4°C until use. Voltage protocols for each experiment were described in the individual figure. Currents were low-pass filtered at 1 kHz using an eight-pole Bessel filter, sampled every 0.1 ms (10 kHz) with Digidata-1300 interface and stored directly onto a computer hard disk using pCLAMP9 software. Data were transferred to compact discs for long-term storage.
Data analysis
To analyze the sensitivity of the channel to inhibition by extracellular or intracellular protons, relative currents at different pH values were fitted with modified Hill equation using the Sigma-Plot program (Yeh et al, 2003). To calculate the apparent second-order rate constant for inhibition of channels by MTSET, time constant was obtained by fitting the time course of inhibition of channels by MTSET with a single exponential. Rate constant was calculated by dividing the reciprocal of the time constant by concentration of reagent (Yeh et al, 2003). The permeability ratios of different monovalent cations versus Na+ were calculated based on the shifts of reversal potential (ΔErev) on exchanging the extracellular solutions, PX/PNa=exp(ΔErev × F/RT), where R, T, and F have the usual meaning (Hille, 2001). MA+, DMA+, and NMDG+ (pKa 9.25, 10.62, and 9.5, respectively) dissociate significantly at pH 9.0. Thus, concentrations of ionized MA+, DMA+, and NMDG+ at pH 9.0 were calculated using the respective pKa. PX/PNa was calculated as exp(ΔErev × F/RT) × ([Na+]/[ionized X+]). The permeability ratio of Ca2+ versus Na+ was calculated according to the equation, PCa/PNa=([Na+]i/4[Ca2+]o) exp(ΔErevF/RT){(1+exp(ΔErev F/RT)}. The activity coefficients for Na+ and Ca2+ were assumed 0.75 and 0.33, respectively. All potentials were corrected for liquid junction potentials. Liquid junction potentials were calculated and verified experimentally (Neher, 1992; Barry, 1994). The diameter of ion permeation pore was estimated by fitting PX/PNa versus diameter of permeating ions using a modified excluded volume equation: (PX/PNa)1/2=dP−dX/dP−dNa, where dP, dX, and dNa are diameters of the pore, cation X+, and Na+, respectively (Sabovcik et al, 1995). Data are shown as mean±s.e.m. of the number of observations. Statistical comparison was made using unpaired Student's t-test.
Supplementary Material
Supplementary data
Acknowledgments
We thank Dr Michel Baum for critical reading of the manuscript. This study is supported by NIH grants (DK-20543, DK-54368, DK-59530) and an EIA (0440019N) from AHA. CLH holds the Jacob Lemann Professorship in the Calcium Transport of University of Texas Southwestern Medical Center.
References
- Amanzadeh J, Gitomer WL, Zerwekh JE, Preisig PA, Moe OW, Pak CY, Levi M (2003) Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int 64: 2142–2149 [DOI] [PubMed] [Google Scholar]
- Barry PH (1994) JP Calc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurement. J Neurosci Methods 51: 107–116 [DOI] [PubMed] [Google Scholar]
- Becchetti A, Gamel K, Torre V (1999) Cyclic nucleotide-gated channels. Pore topology studied through the accessibility of reporter cysteines. J Gen Physiol 114: 377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE (2003) TRP channels as cellular sensors. Nature 426: 517–524 [DOI] [PubMed] [Google Scholar]
- del Camino D, Yellen G (2001) Tight steric closure at the intracellular activation gate of a voltage-gated K(+) channel. Neuron 32: 649–656 [DOI] [PubMed] [Google Scholar]
- Dodier Y, Banderali U, Klein H, Topalak O, Dafi O, Simoes M, Bernatchez G, Sauve R, Parent L (2004) Outer pore topology of the ECaC-TRPV5 channel by cysteine scan mutagenesis. J Biol Chem 279: 6853–6862 [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais-Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77 [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Adams DJ, Hille B (1980) The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol 75: 469–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn GE, Johnson JP Jr, Zagotta WN (2001) Cyclic nucleotide-gated channels: shedding light on the opening of a channel pore. Nat Rev Neurosci 2: 643–651 [DOI] [PubMed] [Google Scholar]
- Flynn GE, Zagotta WN (2001) Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron 30: 689–698 [DOI] [PubMed] [Google Scholar]
- Hille B (2001) Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ (2002) Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu Rev Physiol 64: 529–549 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M, Liu Y, Xu Y, Yellen G (1996) On the use of the thiol-modifying agents to determine channel topology. Neuropharmacology 7: 797–804 [DOI] [PubMed] [Google Scholar]
- Huang C-L (2004) The transient receptor potential superfamily of ion channels. J Am Soc Nephrol 15: 1690–1699 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) The open pore conformation of potassium channels. Nature 417: 523–526 [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D (2003) Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol 13: 487–492 [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA (2003) Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300: 1922–1926 [DOI] [PubMed] [Google Scholar]
- Liu J, Siegelbaum SA (2000) Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron 28: 899–909 [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX (2002) A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 92: 229–231 [DOI] [PubMed] [Google Scholar]
- Neher E (1992) Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol 207: 123–131 [DOI] [PubMed] [Google Scholar]
- Nicoletta JA, Schwartz GJ (2004) Distal renal tubular acidosis. Curr Opin Pediatr 16: 194–198 [DOI] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ (2001) The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem 276: 1020–1025 [DOI] [PubMed] [Google Scholar]
- Perozo E, Cortes DM, Cuello LG (1999) Structural rearrangements underlying K+-channel activation gate. Science 285: 73–78 [DOI] [PubMed] [Google Scholar]
- Phillips LR, Enkvetchakul D, Nichols CG (2003) Gating dependence of inner pore access in inward rectifier K+ channels. Neuron 37: 953–962 [DOI] [PubMed] [Google Scholar]
- Roux B, MacKinnon R (1999) The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science 285: 100–102 [DOI] [PubMed] [Google Scholar]
- Sabovcik R, Li J, Kucera P, Prod'Hom B (1995) Permeation properties of a Ca2+-blockable monovalent cation channel in the ectoderm of the chick embryo: pore size and multioccupancy probed with organic cations and Ca2+. J Gen Physiol 106: 149–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RA, Wong NL, Dirks JH (1979) Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 15: 520–533 [DOI] [PubMed] [Google Scholar]
- Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ (2000) Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275: 3963–3969 [DOI] [PubMed] [Google Scholar]
- Vennekens R, Prenen J, Hoenderop JGJ, Bindels RJM, Droogmans G, Nilius B (2001) Pore properties and ionic block of the rabbit epithelial calcium channel expressed in HEK 293 cells. J Physiol 530: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Janssens A, Droogmans G, Nilius B (2004) Outer pore architecture of a Ca2+-selective TRP channel. J Biol Chem 279: 15223–15230 [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Prenen J, Droogmans G, Nilius B (2003) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol 121: 245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GG, Pascual JM, Brooijmans N, Murray D, Karlin A (2000) The intrinsic electrostatic potential and the intermediate ring of charge in the acetylcholine receptor channel. J Gen Physiol 115: 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yan Y, Sigworth FJ (1997) How does the W434F mutation block current in Shaker potassium channels? J Gen Physiol 109: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh B-I, Sun T-J, Lee JZ, Chen H-H, Huang C-L (2003) Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem 278: 51044–21052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


