Abstract
The ubiquitin-pathway associated (UBA) domain is a 40-residue polyubiquitin-binding motif. The Schizosaccharomyces pombe protein Mud1 is an ortholog of the Saccharomyces cerevisiae DNA-damage response protein Ddi1 and binds to K48-linked polyubiquitin through its UBA domain. We have solved the crystal structure of Mud1 UBA at 1.8 Å resolution, revealing a canonical three-helical UBA fold. We have probed the interactions of this domain using mutagenesis, surface plasmon resonance, NMR and analytical ultracentrifugation. We show that the ubiquitin-binding surface of Mud1 UBA extends beyond previously recognized motifs and can be functionally dissected into primary and secondary ubiquitin-binding sites. Mutation of Phe330 to alanine, a residue exposed between helices 2 and 3, significantly reduces the affinity of the Mud1 UBA domain for K48-linked polyubiquitin, despite leaving the primary binding surface functionally intact. Moreover, K48-linked diubiquitin binds a single Mud1 UBA domain even in the presence of excess UBA. We therefore propose a mechanism for the recognition of K48-linked polyubiquitin chains by Mud1 in which diubiquitin units are specifically recognized by a single UBA domain.
Keywords: Lys48, Mud1, UBA, ubiquitin
Introduction
The ubiquitin (Ub)-mediated protein degradation pathway regulates many critical eukaryotic cellular functions that include cell cycle control, protein quality control and transcription regulation (Hershko and Ciechanover, 1998). Ub is a 76-residue polypeptide with a well-defined α/β fold and is highly conserved in all eukaryotes (Vijay-Kumar et al, 1987). Proteins targeted for degradation are tagged with a polyUb chain as a result of the sequential action of Ub-activating (E1), Ub-conjugating (E2) and Ub-ligase (E3) enzymes. Chain elongation, carried out by E2 and E3 enzymes, is achieved by ligating the C-terminus of distal Ub moieties to specific lysine side chains on the surface of the proximal moiety. PolyUb chains, linked through one of Ub's several lysines (lysine 6, 11, 27, 29, 33, 48 or 63), carry different, but overlapping functions (Pickart, 2000). A polyUb chain consisting of at least four monomers (Ub4), linked through K48 isopeptide bonds, is thought to be the minimum signal for binding to the 26S proteasome (Thrower et al, 2000). The proteasome is a multisubunit protease composed of a 20S catalytic core and a 19S regulatory particle (RP) that tightly controls access to the 20S proteolytic channel and is involved in substrate recognition (Bochtler et al, 1999). In general, ubiquitinated proteins, once localized to the proteasome, undergo degradation (Janse et al, 2004).
Sequence analysis of genes involved in the Ub pathway identified a novel conserved 40-residue domain, the Ub-pathway associated (UBA) domain (Hofmann and Bucher, 1996). The domain has been shown to interact with Ub (Vadlamudi et al, 1996; Bertolaet et al, 2001b) and more specifically with K48- (Wilkinson et al, 2001; Raasi and Pickart, 2003; Raasi et al, 2004) and K63-linked polyUb (Seibenhener et al, 2004). Structure determination of several UBA domains by NMR spectroscopy revealed a fold composed of a three-helical bundle with a well-defined hydrophobic core (Dieckmann et al, 1998; Mueller and Feigon, 2002; Ciani et al, 2003; Walters et al, 2003; Chim et al, 2004; Ohno et al, 2005). Recently, the CUE domain (coupling of Ub to ER degradation) has been identified as an Ub-interacting polypeptide with structural and sequence homology to the UBA domain (Donaldson et al, 2003; Kang et al, 2003; Prag et al, 2003; Shih et al, 2003). Although determinants of monoUb binding are similar between the CUE (Kang et al, 2003; Prag et al, 2003) and UBA (Ohno et al, 2005) domains, the residues involved are not conserved. In contrast, the UBA-binding site on Ub is an invariant cluster of hydrophobic residues located on Ub's β-sheet. The variation in the Ub-binding surfaces of UBA/CUE domains leads to a wide range of affinities toward monoUb. Reported values range from a Kd of 8 μM for the UBA domain of Ddi1 (Bertolaet et al, 2001b) to a Kd of 500–600 μM for the UBA domains of HHR23A (Mueller et al, 2004).
Proteins that contain a UBA domain and an Ub-like domain (UBL), as well as the Ub-interacting motif (UIM)-containing protein Rpn10/Pus1 (Saccharomyces cerevisiae/Schizosaccharomyces pombe nomenclature), act either as shuttle factors or as alternative Ub receptors for the proteasome (Funakoshi et al, 2002; Elsasser et al, 2004). The ability of these proteins to interact with ubiquitin is crucial to their functions in the proteasome-mediated degradation pathway. The receptor activity of the proteasome depends on its capacity to bind specifically to K48-linked polyUb chains (Deveraux et al, 1994).
When binding to polyUb, the UBA domains of HHR23A demonstrate specificity for K48-linked chains, and an affinity that increases markedly with Ub chain length (Raasi and Pickart, 2003; Raasi et al, 2004). In contrast, the recognition of K63-linked polyUb by the UBA2 domain of HHR23A shows only a modest length dependence, such that the observed macroscopic Kd for K63-linked Ub2 is simply the result of two independent UBA-binding sites (Varadan et al, 2004). Interestingly, the solution structure of K63-linked Ub2 is rather open and linear, while that of K48-linked Ub2 is closed, with the two hydrophobic patches that interact with the UBA domain packed against each other (Cook et al, 1992; Varadan et al, 2002). The affinities and stoichiometries of binding of the two HHR23A UBA domains to polyUb chains of defined lengths were recently reported (Raasi et al, 2004).
Mud1 is the S. pombe ortholog of Ddi1 and is a member of a eukaryotic protein family with a predicted aspartyl protease domain (Krylov and Koonin, 2001). The gene was isolated as a suppressor of a temperature-sensitive mutant of the proteasome base subunit Mts4 (Rpn1 in S. cerevisiae). Preliminary SPR studies of Mud1 showed that it binds Ub4 with an apparent affinity of 30 nM, although binding to monoUb was undetectable (Wilkinson et al, 2001). Here, we present the crystal structure of the Mud1 UBA domain and investigate its Ub-binding properties by employing a number of techniques. The results lead to a model for how K48-linked polyUb is recognized specifically by the UBA domain of Mud1.
Results and discussion
The crystal structure of the Mud1 UBA domain
The crystal structure of the Mud1 UBA domain was solved by sulfur SAD (Table I), and is a bundle of three helices similar to other UBA and CUE domain structures (Figure 1A). The crystal asymmetric unit contains two molecules, termed A and B, that adopt similar conformations (overall backbone r.m.s.d. 0.57Å). The domain is tightly folded, with a well-defined hydrophobic core composed of the side chains of the conserved residues Leu303, Phe308, Ala316, Leu317, Ala327 and Leu331 (Figure 2). The first loop contains the highly conserved MetGlyPhe (MGF) motif (residues 306–308 in Mud1), which has been shown to be involved in Ub binding (Mueller and Feigon, 2003; Ryu et al, 2003; Wang et al, 2003). Residues Phe308 and Asp309 assume a β conformation preceding Pro310 at the beginning of the second helix. A hydrogen bond between the backbone amide group of Asp309 and a side-chain carboxyl oxygen of Glu312 probably contributes to the stability of this β loop conformation. Between helices 2 and 3, the asparagine-rich loop is stabilized by a network of backbone carbonyl-amide hydrogen bonds, and is clearly defined by electron density.
Table 1.
Crystallographic data, phasing, and refinement statistics for Mud1UBA
| Sulfur SAD set | Refinement set | |
|---|---|---|
| Data collection and phasing | ||
| Space group | P6122 | P6122 |
| Unit cell (Å) | a=b=60.62 | a=b=60.60 |
| c=96.52 | c=96.35 | |
| Wavelength (Å) | 1.7712 | 0.931 |
| Resolution (Å) | 32.1–3.0 (3.3–3.0) (2.46–2.34)a | 28.868–1.8 (1.9–1.8) |
| Completeness (%) | 99.7 (99.9) (99.5) | 99.8 (99.8) |
| Multiplicity | 44.2 (47.2) (33.5) | 10.3 (10.6) |
| 〈I〉/〈σ〉 | 12.7 (8.2) (1.8) | 10.1 (1.7) |
| Rsym (%) | 5.1 (8.6) (42.1) | 5.0 (44.4) |
| Anom. Cmpl. (%) | 100.0 (100.0) (97.3) | |
| Solvent flattening | Solvent content=55.8% | |
| Correlation coefficient | 0.8022 (0.510)b | |
| Refinement | ||
| Resolution range (Å) | 21.89–1.80 | |
| No. of reflections | 10248 | |
| Rwork/Rfree (%) | 16.8/19.6 | |
| Average B factor (Å2) | 26.8 | |
| Protein/solvent atoms | 569/95 | |
| Coordinate errorc (Å) | 0.094 | |
| aOuter resolution shells in the phasing and acquisition data sets, respectively. | ||
| bCorrelation coefficient calculated for a P6522 space group in parentheses. | ||
| cDPI based on Rfree. | ||
Figure 1.
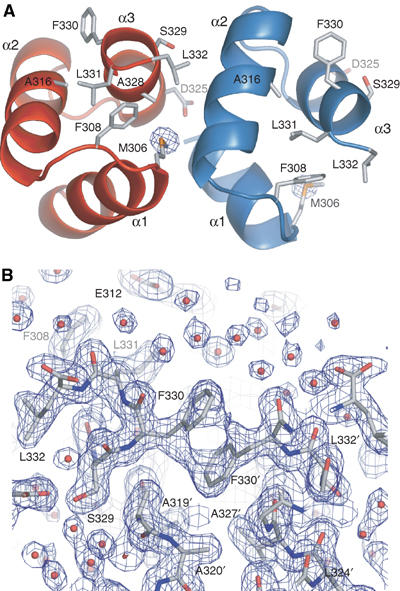
Crystal structure of the UBA domain from Mud1. (A) Cartoon representation of the asymmetric unit of the Mud1 UBA crystal structure formed by chain A (red) and B (blue). Labeled side chains are involved in domain–domain contacts or in Ub binding, and are drawn in stick mode. The sulfur anomalous difference map is shown as a blue contour at 10.0σ. (B) Electron density 2Fo–Fc map of Mud1 UBA crystal structure at a contour level of 1.0σ, showing a crystal contact between two ‘A' monomers in the crystal structure. Note the stacking of the side-chain phenyl groups of two Phe330 residues. Residues in a different symmetry-related molecule are tagged with an apostrophe.
Figure 2.
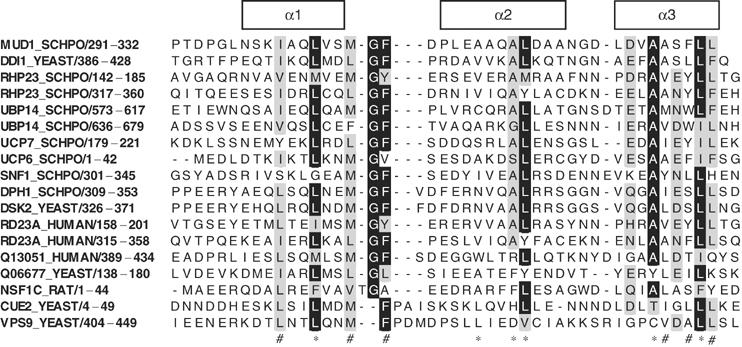
Alignment of UBA and CUE domains found in multiple proteins. The SWISS-PROT ID code and residue numbers of the sequences are shown on the left. The secondary structure of Mud1 UBA is indicated by the boxes above the alignment. Residues with more than 70% conservation are shaded. Below, (*) indicates conserved hydrophobic core residues and (#) indicates conserved hydrophobic residues exposed at the surface of the UBA domains. The SWISS-PROT ID codes for P62, SWA2 and P47 are Q13501, Q06677 and NSF1C, respectively.
Hydrophobic surfaces were identified using the GRID hydrophobicity potential (Goodford, 1985). The side chain of the conserved C-terminal residue Leu332 is well ordered and forms a hydrophobic patch with residues Met306, Phe308 and Ala328 (Supplementary Figure S1). Another hydrophobic site is found on the same side of the domain and is formed by aliphatic carbons of Gln302, Leu303 and Leu324. Finally, a prominent hydrophobic site is detected on the surface formed by helices 2 and 3, and consists of the aromatic ring of Phe330. Interestingly, this side chain in molecule A points outward and stacks against the aromatic ring of Phe330 from a two-fold symmetry-related molecule (Figure 1B).
To determine whether Mud1 UBA dimerizes in solution, sedimentation equilibrium experiments were performed at various concentrations and rotor speeds. The data fitted a monomeric model without self-association (Supplementary Figure S2). Moreover, proton NMR spectra of Mud1 UBA acquired at a wide range of concentration (0.1–1.5 mM) were virtually identical, further supporting the view that the isolated Mud1 UBA domain is strictly monomeric. However, the gel filtration elution profile of full-length Mud1 suggests that the protein is a dimer in solution (data not shown). This result is in agreement with the finding that Ddi1 homodimerization is mediated by the internal region of the protein (Bertolaet et al, 2001a).
Mud1 UBA interactions with K48-linked polyUb chains
We determined affinity constants using surface plasmon resonance to characterize the association of Mud1 UBA and K48-linked polyUb chains of various lengths. Binding of monoUb to immobilized biotin-Mud1UBA was weak and did not appear to saturate even at the highest concentration used (400 μM; Figure 3A). However, extrapolating from the observed binding responses, we can estimate that the Kd value is ca. 390 μM (Table II). This value is comparable to that obtained for CUE2-1 (155 μM) and HHR23 (500–600 μM) UBA domains (Ryu et al, 2003; Mueller et al, 2004). K48-linked Ub2 binding was much tighter, but with fast-on/fast-off kinetics. The estimated Kd of 3 μM represents a >100-fold enhancement in affinity compared to monoUb binding. A similar affinity was obtained for the interaction of Ub2 with full-length Mud1 (Table II and Supplementary Figure S3). We next measured the binding of N–C-linked Ub2, as a mimic of K63-linked Ub2, to the Mud1 UBA domain. A Kd of 140 μM was determined, which is only 2–3-fold greater than the affinity for monoUb, suggesting that the enhancement in affinity for K48-linked Ub2 is linkage-specific.
Figure 3.

Surface plasmon resonance of polyUb chains binding to immobilized biotin-Mud1 UBA. (A) Scaled experimental and fitted binding curves for the binding of polyUb chains to biotin-Mud1 UBA WT (25 RU). (B) Binding curves for the binding of polyUb chains to biotin-Mud1 UBA F330A (25 RU).
Table 2.
SPR equilibrium constants between Mud1UBA and polyubiquitin
| Ligand | Analyte | Kd (μM) | na |
|---|---|---|---|
| Biotin-Mud1UBA WT | Ubiquitin | 390±50 | 1 |
| Biotin-Mud1UBA WT | N-C-Ub2 | 140±15 | 0.96±0.03 |
| Biotin-Mud1UBA WT | K48-Ub2 | 3.1±0.2 | 0.96±0.02 |
| Biotin-Mud1UBA WT | K48-Ub3 | 2.0±0.2 | 0.88±0.02 |
| Biotin-Mud1UBA WT | K48-Ub4 | 1.1±0.4 | 0.61±0.04 |
| Biotin-Mud1UBA F330A | Ubiquitin | 470±70 | 1 |
| Biotin-Mud1UBA F330A | N-C-Ub2 | 200±50 | 0.95±0.04 |
| Biotin-Mud1UBA F330A | K48-Ub2 | 65±15 | 0.97±0.03 |
| Biotin-Mud1UBA F330A | K48-Ub3 | 45±12 | 0.89±0.03 |
| Biotin-Mud1UBA F330A | K48-Ub4 | 38±8 | 0.82±0.04 |
| Amine-coupled Mud1 | K48-Ub2 | 3.7±0.2 | 0.98±0.02 |
| Amine-coupled Mud1 | K48-Ub4 | 0.06±0.02 | 0.96±0.03 |
| Amine-coupled K48-Ub2 | Mud1UBA WT | 6.9±0.3 | 1 |
| Amine-coupled K48-Ub4 | Mud1UBA WT | 6.0±0.3 | 1 |
| Amine-coupled K48-Ub2 | Mud1UBA F330A | 160±12 | 1 |
| Amine-coupled K48-Ub4 | Mud1UBA F330A | 89±8 | 1 |
| aHill coefficient. |
Binding of Ub3 and Ub4 to Mud1 UBA showed similar binding kinetics to that measured for Ub2 at low ligand density (Supplementary Figure S4), and respective Kd values of 2 and 1 μM were determined from the steady-state equilibrium responses (Figure 3 and Table II). However, slow association and dissociation were observed for the binding of Ub4 at high ligand density (Supplementary Figure S5). This experiment was repeated at two different flow rates and the same values were determined, which suggests that the observed slow dissociation does not result from rebinding. The steady-state equilibrium responses for Ub4 binding did not fit to a 1:1 binding model and the associated Hill plot had a slope that was <1 (Table II and Supplementary Figure S6). In contrast, the Hill coefficient for K48-linked Ub2 binding to Mud1 UBA was close to 1.0, supporting a monovalent binding model.
We next carried out the reciprocal experiment and tested the binding of Mud1 UBA as an analyte to amine-coupled Ub2 and Ub4 (Table II and Supplementary Figure S7). Binding to monoUb was weak, in agreement with the previous study (results not shown). A Kd of ∼6 μM was obtained for both Ub2 and Ub4 with fast-on/fast-off kinetics. These results are similar to those obtained in the reverse experiment using Ub2 as the analyte. It is possible that Ub4 immobilization restrains its conformation in a way that precludes it from displaying a fully functional binding epitope to the analyte UBA domain. Alternatively, these results could be explained by a model in which the recognition epitope for the UBA domain is K48-linked Ub2.
Our analysis of the Mud1 UBA crystal structure had identified Phe330 as a conserved residue that is not part of the Mud1 UBA hydrophobic core and is exposed on a surface remote from the previously identified Ub-binding site formed by residues in helices 1 and 3. In the UBA sequence alignment, there is a preponderance of bulky hydrophobic residues at this position, but a notable absence of such residues within the UBA domains of Dsk2, Dph1, P62, SWA2, P47 and the VPS9 CUE domain (Figure 2). To determine whether Phe330 contributes to the interaction between Mud1 UBA and K48-linked polyUb, the residue was mutated to an alanine and affinities of the mutant for monoUb, Ub2, Ub3 and Ub4 were determined (Figure 3B and Table II). The measured Kd for binding of Ub2 to biotinylated Mud1UBA-F330A is 65 μM, a reduction of 20-fold compared to wild-type (WT) biotinylated Mud1UBA. However, binding of monoUb to Mud1UBA-F330A was only slightly reduced compared to binding to WT (Figure 3B and Table II). In the reciprocal experiment, binding of Mud1UBA-F330A to immobilized Ub2 was also reduced by 20-fold. Mud1UBA-F330A binding of Ub3 and Ub4 was also significantly reduced compared to WT Mud1 UBA.
From these results, we conclude that the F330A mutation significantly reduces the affinity of the Mud1 UBA domain for K48-linked polyUb chains without significantly affecting monoUb binding. This reduced affinity could result from either a destabilization of the UBA structure or from modification of a second Ub-binding site that includes Phe330. We next used NMR spectroscopy to distinguish between these two models and investigated the effect of the F330A mutation on the structure of the UBA domain and on its interaction with monoUb and Ub2 in solution.
NMR chemical shift perturbation mapping of Mud1 UBA with monoUb
The 15N–1H HSQC spectrum of Mud1 UBA was first assigned. NOEs and residual dipolar couplings (RDCs) measured in a C12E5:hexanol liquid crystal were in agreement with the crystal structure (Supplementary Figures S8, S9 and Table S1). The 15N-labeled mutant Mud1UBA-F330A was also produced and characterized by NMR. Its HSQC spectrum was very similar to that of the WT UBA (Supplementary Figure S10), and the fit of the RDCs to the crystal structure was as good as for the WT domain (Supplementary Figure S11 and Table S1). This indicates that the reduced affinity of Mud1UBA-F330A for K48-linked polyUb is not caused by a destabilization of its backbone structure.
Specific chemical shift perturbations were observed upon addition of unlabeled monoUb to WT 15N-Mud1 UBA, and the equilibrium between the bound and unbound states was in the fast-exchange regime. The weighted-average chemical shift displacements are shown in Figure 4A. As expected, the conserved residues Leu303, Met306, Gly307, Phe308 and Leu332 showed the largest perturbations. Residues Ala316 and Ala320 are also significantly perturbed. The side chains of these residues are located between helices 2 and 3. The average chemical shift displacements caused by mono Ub binding were mapped on the molecular surface of Mud1 UBA (Figure 4B). Apart from the ‘primary' Ub-binding surface formed by helices 1 and 3, another surface is affected by the addition of Ub that involves residues located between helices 2 and 3. Phe330 and Leu331 exhibit strong perturbations and their amide groups are also located on this surface.
Figure 4.
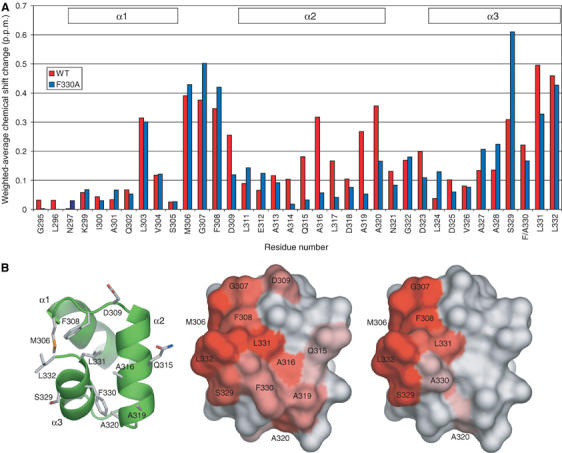
MonoUb-induced chemical shift perturbation studies of 15N-Mud1UBA WT and F330A. (A) Weighted-average chemical shift perturbations in backbone amide HSQC peaks of 15N-Mud1UBA WT (red) and F330A (blue) after addition of monoUb at saturation. For both molecules, the most affected regions are the conserved MGF loop (306–309) and the C-terminus of helix 3. The WT UBA shows additional shifts in helix 2. (B) Molecular surface representation of the chemical shift displacement of the Mud1 UBA backbone amide groups induced by monoUb on WT (center) and F330A (right). The intensity of the red color is proportional to the magnitude of the chemical shift displacement. A visual guide to the structure is shown as a cartoon rendered in ribbon and stick mode (left).
It is possible that the origin of these shift perturbations is a change in the angle between helices 2 and 3. This hypothesis was tested by measuring RDCs on the bound and unbound Mud1 UBA (Supplementary Table S1). The RDC data for the bound UBA fitted well to both chains in the crystal structure. Notably, the fit was significantly better for couplings measured in the presence of monoUb, suggesting that the crystal structure of Mud1 UBA resembles more the bound than the unbound conformation. Closer inspection of the fit of the RDC data collected from the unbound UBA reveals that the discrepancy mostly originates from loop residues. If we select only helical residues, the fit is good for RDCs originating from both the unbound and bound UBA. This suggests that the angles between helices do not change significantly upon Ub binding. Furthermore, the side-chain amide of Gln315, which lies in the middle of helix 2 and does not contact helix 3, also shifts significantly. The most likely explanation, therefore, for the chemical shift perturbations on this second surface is a secondary Ub-binding site located between helices 2 and 3. If the sites are independent, nonlinear fitting of the chemical shifts at different Ub/UBA molar ratios should yield different affinity constants for different sites. We determined dissociation constants using the larger observed 15N and 1H shift changes and found that they are all in the range 225±75 μM, but two distinct sites could not be resolved on the basis of affinity.
Titration of 15N-Mud1UBA-F330A with monoUb (Figure 4) led to shift perturbations similar to those measured for WT UBA for residues 303, 306, 307, 308, 330 and 332. However, perturbations of residues in helix 2 and for Leu331 are significantly less. Thus, the F330A mutation may have disrupted the secondary binding site. Calculation of dissociation constants from shifts mostly yielded values ranging from 150 to 250 μM, but residues 309, 320 and 331 were in the range 430–1000 μM. Residues Asp309 and Ala320 are at either end of helix 2, and together with Leu331 compose part of the proposed secondary binding site (Figure 4B). Taken together, these results support the hypothesis that the F330A mutation weakens the secondary binding site, which could explain the lower affinity of Mud1UBA-F330A for polyUb chains.
We next performed the reverse experiment with 15N-labeled His6-Ub and unlabeled WT UBA (Figure 5A). The chemical shift perturbations observed are very similar to those reported previously for the binding of HHR23A and of HHR23B to monoUb (Ryu et al, 2003; Wang et al, 2003; Mueller et al, 2004), and identify the surface formed by residues Leu8, Arg42, Ile44, Ala46, Gly47, Lys48, Gln49, His68, Val70 and Leu71 (Figure 5B) as the UBA-binding site. There are also significant shifts for residues Ile13, Thr14, Asp32, and the side-chain amide of Gln41 (Figure 5C). The side chain of Gln41 is located in the core of Ub and contacts backbone atoms in helix 1. We therefore suggest that the perturbations in residues 13, 14, 32 and 41 most probably arise from a slight conformational change in monoUb upon UBA binding, but that there is a single UBA-binding surface formed by the cluster of residues enumerated above. Nonlinear fitting of the shifts yielded Kd values ranging from 150 to 350 μM, which are similar to those determined by the reverse titration experiments.
Figure 5.
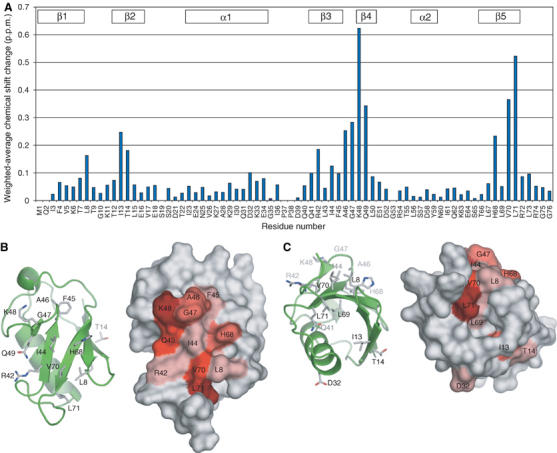
Mud1UBA-induced chemical shift perturbation studies of 15N-monoUb. (A) Weighted-average chemical shift perturbations in backbone amide HSQC peaks of 15N-monoUb after addition of Mud1 UBA at saturation. (B) Molecular surface representation of the chemical shift displacement of the Ub backbone and side-chain amide groups induced by Mud1 UBA on 15N-monoUb. The intensity of the red color is proportional to the magnitude of the chemical shift displacement. (C) The molecule in (B) was rotated by 120° to show shifts not associated with the hydrophobic patch.
Comparison of monoUb binding to UBA domains
Similar to other UBA domains, Ub binding to the Mud1 UBA domain is mediated via a conserved hydrophobic patch located on the surface of Ub's five-stranded β-sheet (Ryu et al, 2003; Wang et al, 2003; Mueller et al, 2004; Ohno et al, 2005). In contrast to the consistency shown in the results of mapping Ub's UBA-binding site, previous studies to identify the monoUb-binding sites on various UBA domains by NMR (Ryu et al, 2003; Wang et al, 2003; Mueller et al, 2004; Ohno et al, 2005) and mutational studies (Bertolaet et al, 2001b; Chen and Madura, 2002; Mueller and Feigon, 2002) have suggested that, although different UBA domains share features of binding, there are also differences. For almost all characterized UBA domains (including the Mud1 UBA in this study), the conserved MGF and dileucine motifs undergo significant chemical shifts on addition of monoUb and define the core of a primary Ub-binding site. The residues of the HHR23A UBA1 (internal) and UBA2 (C-terminal) domains contacted by monoUb contribute 520 and circa 650 Å2, respectively, to the UBA molecular surface (Mueller et al, 2004). A similar calculation carried out for Mud1 UBA yields a value of 975 Å2. In contrast to these earlier studies, we find that Mud1 UBA residues that form the interface between helices 2 and 3 additionally contribute to the monoUb-binding site and extend the monoUb footprint. Notably, a previous mutagenesis study of Ddi1 (the S. cerevisiae ortholog of S. pombe Mud1) in which Lys413 (equivalent to Mud1 Asp318; Figure 2) was mutated to a leucine also showed that a residue in this location within helix 2 could affect the association of Ddi1 UBA with monoUb (Mueller and Feigon, 2002).
NMR mapping of Mud1 UBA interaction with K48-linked Ub2
Addition of Ub2 to WT 15N-Mud1UBA led to the progressive line broadening of the HSQC peaks. Peaks reappeared at different positions after addition of slight excess Ub2 (1.5:1 Ub2:UBA), except for residues Leu311 and Glu312, which rapidly broadened and could not be identified at the end of the titration. This is characteristic of a slow–intermediate exchange regime, and it implies a slower dissociation rate constant and a higher affinity compared to monoUb binding (Kd⩽1 μM). This result is consistent with the SPR results, which showed a marked enhancement in affinity from monoUb to Ub2. Differences in the magnitudes and signs of the shift perturbations were obtained by subtracting the monoUb-induced 1H and 15N shifts from the corresponding values measured upon addition of Ub2. The resulting weighted-average shift differences are shown in Figure 6. The perturbations are remarkably similar upon addition of monoUb and Ub2, which suggests that the UBA domain Ub-binding sites experience similar environments in complex with monoUb or Ub2.
Figure 6.
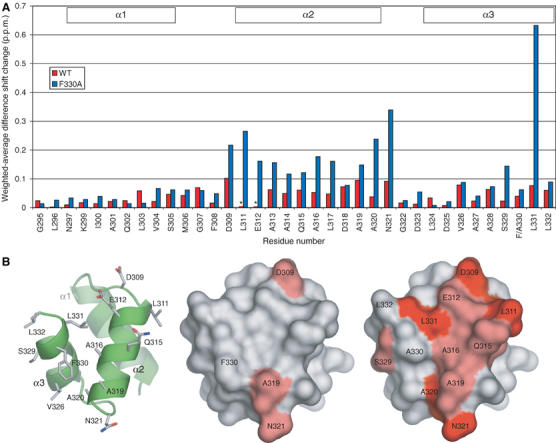
Comparison of Ub2 and monoUb-induced chemical shift perturbations of 15N-Mud1UBA WT and F330A. (A) Weighted average of the difference in chemical shifts induced in backbone amide HSQC peaks of 15N-Mud1UBA WT (red) and F330A (blue) obtained by subtracting 1H and 15N chemical shift changes induced by monoUb from those induced by Ub2. Resonances tagged with an asterisk (*) could not be identified at the end of the titration. (B) Molecular surface representation of the weighted-average shift differences on Mud1 UBA WT (center) and F330A (right). The intensity of the red color is proportional to the magnitude of the chemical shift difference. A visual guide to the structure is shown as a cartoon rendered in ribbon and stick mode (left).
Addition of Ub2 to 15N-Mud1UBA-F330A led to specific chemical shift perturbations in the fast–intermediate exchange regime. Nonlinear fitting of the induced shifts yielded Kd values ranging from 3 to 6 μM, and the data could only be fitted to a 1:1 Ub2:UBA-binding model. The faster exchange regime compared to the WT titration is consistent with Mud1UBA-F330A having a reduced affinity for Ub2, as observed with SPR. Analysis of the difference between monoUb- and Ub2-induced chemical shift perturbations shows a marked increase for most residues in helix 2, as well as Ser329 and Leu331 (Figure 6). This supports the hypothesis that the F330A mutation weakens a secondary Ub-binding site. The secondary binding site of the F330A mutant is no longer able to measurably bind monoUb, but nevertheless forms contacts when the primary site is engaged in the Ub2 complex. Notably, the similarity of the chemical shift changes suggests that the Ub2-bound form of Mud1UBA-F330A closely resembles that of the WT (Supplementary Figure S12).
To obtain direct evidence for our model that there are two Ub-binding sites on Mud1UBA, we carried out a cross-saturation experiment on 2H–15N-labeled Mud1 UBA in complex with unlabeled K48-linked Ub2 (Figure 7A). Cross-peaks displaying large intensity changes correspond to residues in close contact with Ub2. We find that amide groups in both binding sites are in contact with Ub2, namely Phe308, Ala328 and Leu332 in the primary binding site, and Asp318, Ala319, Ala320, Ala327 and Leu331 in the secondary binding site (Figure 7B). We also observed a strong intensity change in the side-chain amino group of Gln315, also located in the secondary binding site. These results confirm that the chemical shift changes observed in the Mud1UBA are due to a direct interaction of the two binding sites with monoUb and Ub2.
Figure 7.
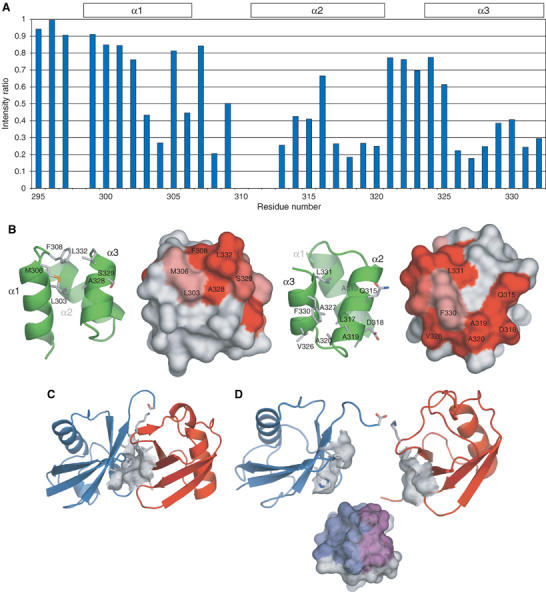
Molecular model for the interaction of Mud1 UBA with K48-linked Ub2. (A) Intensity changes by cross-saturation of the 15N–1H cross-peaks in 2H, 15N-Mud1 UBA in complex with unlabeled K48-Ub2. (B) Primary (left) and secondary (right) binding sites on Mud1UBA as identified by NMR cross-saturation. Resonances showing intensity ratios <0.5 or 0.3 are displayed on the molecular surface of Mud1 UBA in light or dark red, respectively. (C) Closed conformation of Ub2, based on the crystal structure obtained under basic conditions (PDB accession code 1AAR) (Cook et al, 1992). The hydrophobic patches on each Ub moiety interact with each other. The proximal and distal moieties of Ub2 are colored in red and blue, respectively. (D) Open conformation of Ub2, in equilibrium with closed conformation in solution. The two hydrophobic clusters formed by residues Leu8, Ile44, His68 and Val70 are available for binding of a single UBA domain via a primary (purple) and a secondary (blue) Ub-binding sites.
Stoichiometry of the Mud1UBA:Ub2 complex
In order to confirm the 1:1 stoichiometry of UBA binding to Ub2, we analyzed Mud1UBA:Ub2 complex formation by sedimentation equilibrium analytical ultracentrifugation (Supplementary Figure S2). Since the UV absorbance of the Mud1 UBA domain is negligible at 277 nm compared to Ub2, the AUC measurements were assumed to originate only from bound and unbound Ub2. Samples with identical Ub2 concentrations were run in the absence and in the presence of Mud1 UBA. Ub2 was loaded at a concentration at least 20-fold higher than the estimated Kd (3–6 μM by SPR and 1 μM by NMR) to ensure that Ub2 in limiting concentration is completely bound by Mud1 UBA at equilibrium. In the absence of Mud1 UBA, the molecular weight of Ub2 was determined to be 17.4 kDa, in agreement with the predicted molecular weight of 17.1 kDa. In the presence of a two-fold excess of Mud1 UBA (predicted molecular weight 4.5 kDa), an average molecular weight of 21.4 kDa was measured for Ub2. This experiment supports a model where the stoichiometry of the complex is one UBA domain for one Ub2 molecule.
Proposed mechanism of K48-linked polyUb recognition by the Mud1 UBA
The mutation F330A is the first reported UBA mutation that affects polyUb binding without affecting the primary Ub-binding site formed by the conserved MGF and C-terminal dileucine motifs. Since the RDCs of the mutant show that there is no loss of stability or change of structure caused by the mutation, it implies that this aromatic residue is a critical determinant of Mud1 UBA binding to K48-linked Ub2. Cross-saturation experiments confirm that there is a direct interaction between K48-linked Ub2 and the surface formed by helices 2 and 3 of Mud1 UBA. Hence, one possible model is that the Mud1 UBA domain binds Ub2 through the same hydrophobic cluster on each Ub moiety using two hydrophobic pockets, one formed by the MGF and dileucine motifs and the other by residues within helices 2 and 3 and including Phe330 (Figure 7D). Only when Ub2 is K48-linked are the two UBA-binding sites of each Ub correctly disposed to bind to both Mud1 UBA Ub-binding sites. This model accounts for the observed stoichiometry, the difference in affinity between monoUb and Ub2, the linkage-type specificity and the similarity of chemical shift perturbations between monoUb and Ub2.
This model has implications for the binding of UBA domains to longer K48-linked Ub chains. In this model, Ub4 would have three high-affinity binding epitopes (Ub moieties 1–2, 2–3 and 3–4), and could simultaneously bind two UBA domains, whereas Ub3 would have only two mutually exclusive epitopes (1–2 and 2–3). This hypothesis is supported by our SPR results. The Hill plot derived from the binding of Ub4 to Mud1 UBA has a slope <1 (Table II and Supplementary Figure S6). By contrast, Ub2 and Ub3 have Hill coefficients of 1 and 0.9, respectively. This result could be interpreted by a model in which Ub4 is a bivalent analyte that is capable of binding two UBA ligands simultaneously with high affinity (<0.2 μM), providing the two UBA ligands are in close proximity. At lower analyte concentrations, the high-affinity bivalent binding mode predominates. At higher concentrations, the binding curve reflects an affinity that is close to that of Ub2 (Figure 3A). If this model is correct, different Hill coefficients and dissociation rate constants should be obtained for Ub4 by varying the surface density of biotin-Mud1UBA on the SPR chip. Indeed, this was found to be the case (Supplementary Figures S4–S6). Finally, this model is also supported by the binding of Ub4 to full-length Mud1, which is dimeric. Slow association and dissociation kinetics were observed and fitting of the steady-state equilibrium responses yielded a Kd of 60 nM and a Hill coefficient of 1 (Table II and Supplementary Figure S3).
Does our model for the interaction of Mud1 UBA with K48-linked Ub2 apply to other UBA:Ub2 interactions? Both UBA domains of HHR23/Rhp23 contain an aromatic residue at the position equivalent to Phe330 in Mud1. However, the charge distribution of HHR23A UBA2 is similar to that of Mud1 UBA, but quite different from that of HHR23A UBA1. Thus, we can predict that Rad23/HHR23 UBA2 may interact with K48-linked Ub2 using a ‘sandwich' mechanism, as we have proposed for the interaction of Mud1 UBA with K48-linked Ub2. As mentioned above, recent studies to map the binding of Ub to HHR23A UBA domains have revealed quite different chemical shift perturbations in the UBA domains (Ryu et al, 2003; Wang et al, 2003; Mueller et al, 2004). While there are no shifts in helix 2 of UBA1, UBA2 does experience perturbations at the end of helix 2. A recent study has shown that the UBA2 domain of HHR23/Rhp23 specifically recognizes K48-linked Ub2 (Varadan et al, 2005). In this model, Leu8, Ile44 and Val70 from the proximal Ub and residues Val70 and Leu73 from the distal domain bind to a surface formed by helices 2 and 3 of HHR23A UBA, including a Phe residue equivalent to Phe330 in Mud1. Although there is a preponderance of aromatic residues in UBA domains at the position of Phe330 in Mud1 (Figure 2), this residue is not absolutely conserved, suggesting that only a subset of UBA domains may bind to K48-linked polyUb using the mechanism described here.
The recent crystal and NMR structures of two CUE domains bound to monoUb support the hypothesis that different UBA domains may have related but distinct mechanisms for Ub recognition (Kang et al, 2003; Prag et al, 2003). CUE domains do not have an aromatic residue at the position equivalent to Mud1 Phe330, but they do not recognize specifically K48-linked polyUb. The CUE and UBA domains are very similar, the most obvious difference being that the CUE domain has an insertion of four amino acids in the loop between helices 1 and 2. Functionally, CUE domains may be involved in recognizing monoUb, while UBA domains specifically bind to polyUb, although this functional difference has yet to be demonstrated. The CUE2-1 domain binds to monoUb through a complementary hydrophobic surface that includes the conserved MFP and dileucine motifs (structurally equivalent to the UBA MGF and dileucine motifs) and other residues from helices 1 and 3. The VPS9P CUE monomer has a very low affinity for monoUb (∼1 mM), but, through reciprocal exchange of α3, can form an interlocked dimer that wraps around monoUb to contribute two hydrophobic surfaces that simultaneously bind to two different Ub surfaces. This dimerization creates a binding pocket that binds monoUb with high affinity (∼1 μM) by orienting two Ub-interacting surfaces for optimal interaction. By analogy, linkage of two Ub moieties through a K48 isopeptide bond orients the two UBA-binding surfaces to create a single high-affinity UBA-binding pocket. A longer polyUb chain would present multiple copies of this binding site. The slight enhancement of affinity observed for longer K48-linked polyUb chains may result from the combined effect of high-affinity K48-linked Ub2 recognition, avidity and the observed gradual openness of proximal Ub moieties. Indeed, NMR studies of Ub4 in solution have shown that the UBA-binding sites are not all equally available, since the most proximal Ub moieties are in a more ‘open' conformation than the distal ones (Varadan et al, 2002). Furthermore, we can hypothesize that binding of a UBA molecule to a polyUb chain may change the conformation and dynamics of the chain and affect its availability for further interaction with other Ub-interacting proteins and/or the proteasome.
Materials and methods
Cloning and sample preparation
The sequence corresponding to Mud1 UBA (293–332) was amplified by PCR and cloned into pGEX-6P-1 using standard molecular biology techniques. PCR mutagenesis of Mud1 UBA to introduce the F330A mutation was performed directly on the WT pGEX-6P-1-Mud1UBA vectors. Transformed BL21-DE3 Escherichia coli cells were grown in 1L of LB (all constructs), or 15NH4Cl-M9 (Mud1 UBA, His6-tagged human monoUb), or M9 medium supplemented with 15NH4Cl and 2H6-glucose in 99.9% D2O (Mud1 UBA) at 37°C to OD600 ∼0.8, and induced for protein expression using 200 μM IPTG, with overnight agitation at 20°C. Cells were pelleted and resuspended in 25 ml of HBS (10 mM HEPES, pH 7.5, 150 mM NaCl, 0.01% MTG, 0.01% NaN3) with the addition of a cocktail of protease inhibitors. Glutathione-sepharose 4B (Amersham Biosciences) was used for affinity purification of GST-Mud1UBA. The 3C protease-cleaved UBA domain was then isolated on a Superdex 30 (16/60) column (Amersham Biosciences), equilibrated in low-salt HBS (50 mM NaCl) for unlabeled molecules or NMR buffer (20 mM sodium phosphate, pH 7.0, 100 mM NaCl, 0.05% NaN3) for NMR samples. Full-length Mud1 was cloned and purified as described previously (Wilkinson et al, 2001). The fusion protein GST–Mud1 was cleaved with thrombin and further purified on a Superdex 200 (26/60) column. N–C-linked Ub2 was expressed from a pET-21a vector carrying two human Ub sequences linked head-to-tail by a Gly–Ser linker. His6-tagged human monoUb and N–C Ub2 were purified on Ni-NTA agarose (Qiagen) and eluted with imidazole. The proteins were further purified and buffer-exchanged on a Superdex 75 (16/60) column. Unlabeled bovine monoUb was purchased from Sigma-Aldrich. K48-Ub2, Ub3 and Ub4 were synthesized in vitro using ATP, bovine Ub, recombinant human E1 and Cdc34 E2 conjugating enzyme (Piotrowski et al, 1997). The chains were separated by cation-exchange chromatography on a mono-S column (HR 5/5, Amersham Biosciences) as described (Chen and Pickart, 1990), and further purified by gel filtration on a Superdex 75 (16/60) column equilibrated in HBS or NMR buffers (Supplementary Figure S13).
Crystallization, data collection and crystal structure determination of Mud1 UBA
Diffraction data shown in this study were collected from crystals grown in 4.0 M sodium formate and cryoprotected in 6.0 M sodium formate. Sulfur SAD and refinement sets were collected at the ESRF on beamlines BM14 and ID14-3, respectively (Table I; Supplementary Table S2). All data were indexed and integrated using MOSFLM and scaled using SCALA, from the CCP4 program suite (Winn, 2003). The sulfur SAD data set was merged using XPREP, and two strong and weak sites were located by an iterative dual space recycling method using SHELXD (Schneider and Sheldrick, 2002; Uson et al, 2003). Values for sulfur f′ and f″ at 1.771 Å were set to 0.3528 and 0.7188, respectively. The positions and occupancies of the sulfur anomalous scatterer sites were refined and the complete data set was phased by maximum likelihood refinement using SHARP (Fortelle and Bricogne, 1997). The map was readily interpretable and the two strong anomalous scatterer sites corresponded to the methionine side chains (Met306) for the two UBA domains in the asymmetric unit. Models were built for the two molecules (chain A and B) independently. Protein density was observed for residues 295–332 and 298–332 in chains A and B, respectively. Refinement with the high-resolution data set and structural idealization was performed using Refmac5 (CCP4 suite). Solvent atoms were added to the model and the final refinement was performed with anisotropic B-factor refinement.
Surface plasmon resonance
All SPR experiments were conducted at 25°C using the HBS-EP buffer provided by BIAcore (10 mM HEPES, pH 7.4, 150 mM NaCl, 3.4 mM EDTA, 0.005% P20 surfactant). UBA domains (200 μM in PBS pH7) were biotinylated by addition of 6-((biotinoyl)amino)hexanoic acid sulfosuccinimidyl ester to 1.0 mM. Products were purified by Superdex 75 gel filtration in HBS and MALDI-TOF was used to show that a single biotin molecule was attached to each UBA. Biotin-UBA ligands were immobilized at 25 and 60 RU to a streptavidin-coated SA chip (BIAcore). Polyubiquitin chains were injected at 30–40 μl/min for 20–30 s (Supplementary Figures S12 and S13). K48-linked Ub2 and Ub4, and Mud1 (3000, 5000 and 2000 RU respectively) were immobilized to a CM-5 chip using amine coupling in 10 mM sodium acetate, pH 5. For each analyte concentration, a negative control sensorgram was subtracted to account for nonspecific binding of the analyte and bulk refractive index change. Steady-state SPR responses were estimated from the subtracted sensorgrams. Affinities were calculated by fitting steady-state responses (RU) to the Hill equation (Supplementary data).
NMR spectroscopy
15N-Mud1UBA was concentrated to 1.0 mM for assignment and 5% D2O was added. The temperature for all NMR experiments described below was set to 25.0°C, unless indicated otherwise. A sensitivity-enhanced gradient 15N-HSQC-NOESY was recorded on a 14 T (600 MHz) spectrometer, with 36, 132 and 1024 complex points in the 15N, 1Hind and 1HN dimensions, respectively, and a mixing time of 200 ms. A sensitivity-enhanced gradient 15N-HSQC-TOCSY was recorded on a 17.5 T (750 MHz) spectrometer, with 32, 120 and 1024 complex points in the 15N, 1Hind and 1HN dimensions, respectively. A mixing time of 50 ms was used and a delay of 19 ms was introduced to eliminate ROE peaks. All spectra were processed using NMRPipe (Delaglio et al, 1995).
The monoUb chemical shift titration experiments were carried out at 600 MHz 1H frequency. The initial sample consisted of 250 μM 15N-Mud1UBA (WT or F330A) in NMR buffer supplemented with 5% D2O. A sensitivity-enhanced gradient HSQC was acquired after every addition of Ub (7 mM), at Ub:Mud1UBA molar ratios of 0, 0.8, 1.4, 2.9 and 6.2 (Supplementary Figure S14). Similarly, Ub was added to Mud1UBA-F330A at molar ratios of 0, 0.4, 0.9, 1.7, 3.0, 5.0 and 8.0 (Supplementary Figure S15). Ub2 titrations were carried out at 750 MHz, using 200 μM of WT 15N-Mud1UBA (WT or F330A). K48-linked Ub2 was added at Ub2:UBA molar ratios of 0, 0.17, 0.34, 0.51, 0.74, 1.00, 1.25, 1.5 and 2.0 for WT and 0, 0.15, 0.3, 0.5, 0.7, 0.93 and 1.5 for F330A (Supplementary Figure S16). Unlabeled Mud1UBA was added to 15N-Ub (400 μM) at molar ratios of 0, 0.25, 0.5, 1.0, 2.0 and 4.0 (Supplementary Figure S17). Weighted-average chemical shift perturbations were calculated according to the formula 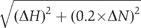 Weighted-average differences in chemical shift perturbations were calculated according to the formula
Weighted-average differences in chemical shift perturbations were calculated according to the formula 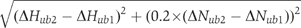 15N–1H RDCs were measured by recording HSQC-IPAP spectra (Ottiger et al, 1998) at 20°C in both isotropic (NMR buffer only) and anisotropic conditions (3–5% C12E5/hexanol, r=1.0) (Otting and Rückert, 2000). RDCs were fitted to the crystal structure using the software Module (Dosset et al, 2001). The cross-saturation experiment was performed on a sample containing 1 mM 2H,15N-Mud1UBAwt, 1.2 mM K48-Ub2 and 90% D2O in the NMR buffer (Takahashi et al, 2000). The saturation frequency of the WURST-2 decoupling scheme was set to 0.9 p.p.m. The recycle delay and saturation time were set to 3.2 and 1.2 s, respectively.
15N–1H RDCs were measured by recording HSQC-IPAP spectra (Ottiger et al, 1998) at 20°C in both isotropic (NMR buffer only) and anisotropic conditions (3–5% C12E5/hexanol, r=1.0) (Otting and Rückert, 2000). RDCs were fitted to the crystal structure using the software Module (Dosset et al, 2001). The cross-saturation experiment was performed on a sample containing 1 mM 2H,15N-Mud1UBAwt, 1.2 mM K48-Ub2 and 90% D2O in the NMR buffer (Takahashi et al, 2000). The saturation frequency of the WURST-2 decoupling scheme was set to 0.9 p.p.m. The recycle delay and saturation time were set to 3.2 and 1.2 s, respectively.
Sedimentation equilibrium analytical ultracentrifugation
Sedimentation equilibrium experiments were all performed in PBS (20 mM sodium phosphate, pH 7.0, 100 mM NaCl) at 20°C on a Beckman Optima XL-I analytical ultracentrifuge. Mud1 UBA was loaded at 0.5 mM and analyzed at rotor speeds of 30 000, 40 000, 45 000 and 50 000 r.p.m. Signal was monitored at 258 nm with a loading absorbance of 0.26. Mixtures of Mud1UBA:K48-linked Ub2 in molar ratios of 0:1, 1:1 and 2:1 were loaded with a constant Ub2 concentration of 125 μM. The three samples were analyzed at 277 nm and rotor speeds of 16 000, 20 000, 24 000 and 28 000 r.p.m. Data were fitted using calculated values for ν1 estimated on amino-acid composition using the software Sednterp (Laue et al, 1992). Data were fitted to a single ideal solute model equation. Details of the analysis are included in the Supplementary data section.
Coordinate deposition
Coordinates and structures factors have been deposited in the Protein Data Bank (accession code IZ96).
Supplementary Material
Supplementary Data
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
We would like to thank Martin Walsh and his colleagues at beamline BM14 at the ESRF for providing excellent facilities for data collection. The E1 construct was a kind gift from Kazuhiro Iwai and Cdc34, and His6-Ub constructs gifts from Randy Poon. We thank Nicole Schueller and Jan Gruber for their assistance in developing the reagents to synthesize polyUb. We are indebted to Louise Johnson and Maria Hoellerer for reading the manuscript, and Melania Strycharska and Jim McDonnell for their many helpful comments. We thank Russell Wallis for his advice and assistance in interpreting AUC data, Irene Taylor, Marc Morgan and Jenny Gibson for general laboratory support, and Simon Colebrooke for the NMR work. J-F Trempe is supported by the Wellcome Trust (Grant Ref: 068990), and NRB and EL by the MRC and Wellcome Trust, respectively.
References
- Bertolaet BL, Clarke DJ, Wolff M, Watson MH, Henze M, Divita G, Reed SI (2001a) UBA domains mediate protein–protein interactions between two DNA damage-inducible proteins. J Mol Biol 313: 955–963 [DOI] [PubMed] [Google Scholar]
- Bertolaet BL, Clarke DJ, Wolff M, Watson MH, Henze M, Divita G, Reed SI (2001b) UBA domains of DNA damage-inducible proteins interact with ubiquitin. Nat Struct Biol 8: 417–422 [DOI] [PubMed] [Google Scholar]
- Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R (1999) The proteasome. Annu Rev Biophys Biomol Struct 28: 295–317 [DOI] [PubMed] [Google Scholar]
- Chen L, Madura K (2002) Rad23 promotes the targeting of proteolytic substrates to the proteasome. Mol Cell Biol 22: 4902–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Pickart CM (1990) A 25-kilodalton ubiquitin carrier protein (E2) catalyzes multi-ubiquitin chain synthesis via lysine 48 of ubiquitin. J Biol Chem 265: 21835–21842 [PubMed] [Google Scholar]
- Chim N, Gall WE, Xiao J, Harris MP, Graham TR, Krezel AM (2004) Solution structure of the ubiquitin-binding domain in Swa2p from Saccharomyces cerevisiae. Proteins 54: 784–793 [DOI] [PubMed] [Google Scholar]
- Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS (2003) Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget's disease of bone. J Biol Chem 278: 37409–37412 [DOI] [PubMed] [Google Scholar]
- Cook WJ, Jeffrey LC, Carson M, Chen Z, Pickart CM (1992) Structure of a diubiquitin conjugate and a model for interaction with ubiquitin conjugating enzyme (E2). J Biol Chem 267: 16467–16471 [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6: 277–293 [DOI] [PubMed] [Google Scholar]
- Deveraux Q, Ustrell V, Pickart C, Rechsteiner M (1994) A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem 269: 7059–7061 [PubMed] [Google Scholar]
- Dieckmann T, Withers-Ward ES, Jarosinski MA, Liu CF, Chen IS, Feigon J (1998) Structure of a human DNA repair protein UBA domain that interacts with HIV-1 Vpr. Nat Struct Biol 5: 1042–1047 [DOI] [PubMed] [Google Scholar]
- Donaldson KM, Yin H, Gekakis N, Supek F, Joazeiro CA (2003) Ubiquitin signals protein trafficking via interaction with a novel ubiquitin binding domain in the membrane fusion regulator, Vps9p. Curr Biol 13: 258–262 [DOI] [PubMed] [Google Scholar]
- Dosset P, Hus JC, Marion D, Blackledge M (2001) A novel interactive tool for rigid-body modeling of multi-domain macromolecules using residual dipolar couplings. J Biomol NMR 20: 223–231 [DOI] [PubMed] [Google Scholar]
- Elsasser S, Chandler-Militello D, Mueller B, Hanna J, Finley D (2004) Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J Biol Chem 279: 26817–26822 [DOI] [PubMed] [Google Scholar]
- Fortelle EL, Bricogne G (1997) Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol 276: 472–494 [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H (2002) Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc Natl Acad Sci USA 99: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem 28: 849–857 [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P (1996) The UBA domain: a sequence motif present in multiple enzyme classes of the ubiquitination pathway. Trends Biochem Sci 21: 172–173 [PubMed] [Google Scholar]
- Janse DM, Crosas B, Finley D, Church GM (2004) Localization to the proteasome is sufficient for degradation. J Biol Chem 279: 21415–21420 [DOI] [PubMed] [Google Scholar]
- Kang RS, Daniels CM, Francis SA, Shih SC, Salerno WJ, Hicke L, Radhakrishnan I (2003) Solution structure of a CUE–ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell 113: 621–630 [DOI] [PubMed] [Google Scholar]
- Krylov DM, Koonin EV (2001) A novel family of predicted retroviral-like aspartyl proteases with a possible key role in eukaryotic cell cycle control. Curr Biol 11: R584–R587 [DOI] [PubMed] [Google Scholar]
- Laue T, Shah X, Ridgeway TM, Pelletier SL (1992) Computer-aided interpretation of analytical sedimentation data for proteins. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science, Harding SE, Rowe AJ, Horton JC (eds) pp 90–125. Cambridge, UK: Royal Society of Chemistry [Google Scholar]
- Mueller TD, Feigon J (2002) Solution structures of UBA domains reveal a conserved hydrophobic surface for protein–protein interactions. J Mol Biol 319: 1243–1255 [DOI] [PubMed] [Google Scholar]
- Mueller TD, Feigon J (2003) Structural determinants for the binding of ubiquitin-like domains to the proteasome. EMBO J 22: 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Kamionka M, Feigon J (2004) Specificity of the interaction between ubiquitin-associated domains and ubiquitin. J Biol Chem 279: 11926–11936 [DOI] [PubMed] [Google Scholar]
- Ohno A, Jee J, Fujiwara K, Tenno T, Goda N, Tochio H, Kobayashi H, Hiroaki H, Shirakawa M (2005) Structure of the UBA domain of Dsk2p in complex with ubiquitin: molecular determinants for ubiquitin recognition. Structure 13: 521–532 [DOI] [PubMed] [Google Scholar]
- Ottiger M, Delaglio F, Bax A (1998) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson 131: 373–378 [DOI] [PubMed] [Google Scholar]
- Otting G, Rückert M (2000) Alignment of biological macromolecules in novel nonionic liquid crystalline media for NMR experiments. J Am Chem Soc 122: 7793–7797 [Google Scholar]
- Pickart CM (2000) Ubiquitin in chains. Trends Biochem Sci 25: 544–548 [DOI] [PubMed] [Google Scholar]
- Piotrowski J, Beal R, Hoffman L, Wilkinson KD, Cohen RE, Pickart CM (1997) Inhibition of the 26S proteasome by polyubiquitin chains synthesized to have defined lengths. J Biol Chem 272: 23712–23721 [DOI] [PubMed] [Google Scholar]
- Prag G, Misra S, Jones EA, Ghirlando R, Davies BA, Horazdovsky BF, Hurley JH (2003) Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell 113: 609–620 [DOI] [PubMed] [Google Scholar]
- Raasi S, Orlov I, Fleming KG, Pickart CM (2004) Binding of polyubiquitin chains to ubiquitin-associated (UBA) domains of HHR23A. J Mol Biol 341: 1367–1379 [DOI] [PubMed] [Google Scholar]
- Raasi S, Pickart CM (2003) Rad23 ubiquitin-associated domains (UBA) inhibit 26S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains. J Biol Chem 278: 8951–8959 [DOI] [PubMed] [Google Scholar]
- Ryu KS, Lee KJ, Bae SH, Kim BK, Kim KA, Choi BS (2003) Binding surface mapping of intra- and interdomain interactions among hHR23B, ubiquitin, and polyubiquitin binding site 2 of S5a. J Biol Chem 278: 36621–36627 [DOI] [PubMed] [Google Scholar]
- Schneider TR, Sheldrick GM (2002) Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr 58: 1772–1779 [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW (2004) Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24: 8055–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Prag G, Francis SA, Sutanto MA, Hurley JH, Hicke L (2003) A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J 22: 1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Nakanishi T, Kami K, Arata Y, Shimada I (2000) A novel NMR method for determining the interfaces of large protein–protein complexes. Nat Struct Biol 7: 220–223 [DOI] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J 19: 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uson I, Schmidt B, von Bulow R, Grimme S, von Figura K, Dauter M, Rajashankar KR, Dauter Z, Sheldrick GM (2003) Locating the anomalous scatterer substructures in halide and sulfur phasing. Acta Crystallogr D Biol Crystallogr 59: 57–66 [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Joung I, Strominger JL, Shin J (1996) p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem 271: 20235–20237 [DOI] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D (2004) Solution conformation of Lys63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem 279: 7055–7063 [DOI] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D (2005) Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain. Mol Cell 18: 687–698 [DOI] [PubMed] [Google Scholar]
- Varadan R, Walker O, Pickart C, Fushman D (2002) Structural properties of polyubiquitin chains in solution. J Mol Biol 324: 637–647 [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar S, Bugg CE, Cook WJ (1987) Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol 194: 531–544 [DOI] [PubMed] [Google Scholar]
- Walters KJ, Lech PJ, Goh AM, Wang Q, Howley PM (2003) DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proc Natl Acad Sci USA 100: 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Goh AM, Howley PM, Walters KJ (2003) Ubiquitin recognition by the DNA repair protein hHR23a. Biochemistry 42: 13529–13535 [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C (2001) Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol 3: 939–943 [DOI] [PubMed] [Google Scholar]
- Winn MD (2003) An overview of the CCP4 project in protein crystallography: an example of a collaborative project. J Synchrotron Radiat 10: 23–25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Supplementary Figure 1
Supplementary Figure 2


