Abstract
Kampo formulations are traditional herbal medications used in China and Japan for many centuries to treat diarrheal diseases such as cholera. Our studies were undertaken to identify and verify by chemical synthesis the active components that inhibited cholera toxin (CT), the virulence factor secreted by Vibrio cholerae, the causative agent of cholera. The Kampo formulation, Daio-kanzo-to, inhibited CT activities (i.e., ADP-ribosylation, Chinese hamster ovary cell elongation); in Daio-kanzo-to, Daio (Rhei rhizoma) was responsible for this effect. Among several components purified from Daio extract, rhubarb galloyl-tannin, a compound characterized by a polygallate structure, was the most effective. To define the active component, gallate analogues similar to rhubarb galloyl-tannin were synthesized. These gallate compounds inhibited all CT activities including ADP-ribosylation, elongation of Chinese hamster ovary cells, and importantly, fluid accumulation in ileal loops. Thus, Kampo formulations or their gallate components might be effective adjunctive therapy with oral rehydration solution for the severe diarrhea of cholera.
Keywords: ADP-ribosylation‖diarrheal disease‖alternative medicine‖cAMP‖ guanine nucleotide-binding proteins
Cholera toxin (CT), the major virulence factor produced by Vibrio cholerae, the causative agent of cholera, is an oligomeric protein consisting of a single A subunit and five B subunits (1, 2). The latter form a pentameric ring, with the A subunit attached to the B oligomeric core. Biological action of CT is initiated by the binding of the B subunits to the receptor, ganglioside GM1, on intestinal epithelial cell membranes. Multivalent binding of B subunits to GM1 induces a conformational change in the CT molecule and is followed by insertion of the A subunit into the cell, where it mono-ADP-ribosylates Gsα, a guanine nucleotide-binding regulatory protein, resulting in persistent adenylyl cyclase activation (1, 2). The consequence of this and/or other effects, such as increased synthesis of prostaglandins by the intoxicated cell, is excessive accumulation of salt and water in the intestinal lumen and the copious “rice-water” diarrhea characteristic of the disease.
Oral rehydration salt (ORS) solution (3) is a commonly used therapy for cholera. Although the ORS solution effectively aids recovery from dehydration, the combination of ORS solution with pharmacological agents that suppress the severe diarrhea or inactivate CT would be advantageous. Kampo formulations are traditional medicines used in China for more than 2,000 years and modified by the Japanese in recent centuries. The formulations are usually prepared from so-called “crude drugs” of natural origins, including plant leaves, roots, and mushrooms. For the preparation of Kampo formulations, usually more than two crude drugs are boiled together in water; the resulting water extracts contain the pharmacologically active agents. The type of Kampo formulation prescribed depends on the diagnosis.
Because Kampo formulations have been used for the treatment of diarrhea (4), in particular those of bacterial origin, such as cholera, we examined their effects on CT activities, in particular, the mouse ligated ileal loop, ADP-ribosyltransferase, and Chinese hamster ovary (CHO) cell elongation assays. Rhubarb galloyl (RG)-tannin, which has a heterologous polyphenol/gallate structure, was identified as the active component in the Kampo preparation (5, 6). To verify the activity of this gallate compound, homologues were synthesized chemically and shown to have effects similar to traditional Kampo preparations.
Materials and Methods
Chemicals.
Kampo formulation, Daio-kanzo-to, crude drugs extracts, Rhei Rhizoma, and RG-tannin were prepared by Tsumura & Co. as lyophilized powders. Emodin and sennidine A were purchased from Extrasynthese (Genay, France), and epigallocatechin gallate was from Funakoshi (Tokyo).
The Kampo formulation, crude drug extracts, and RG-tannin were dissolved in distilled water, and emodin and sennidine A were dissolved in 99.5% ethanol. CT, CTA subunit (CTA), and CTB subunit were purchased from List Biological Laboratories (Campbell, CA); [adenine-U-14C]NAD (250–300 mCi/mmol) and [adenylate-32P]NAD were from Amersham Pharmacia; DTT, ovalbumin, NAD, agmatine, GTP, and ADP-ribose were from Sigma; d-glucose, d-glucitol, d-mannitol, maltose, maltotriose, N,N-dimethylformamide, benzyl bromide, NaH, acetic acid, chloroform, methylene chloride, phosphorus pentachloride, pyridine, toluene, acetone, Celite, SiO2, 1,4-dioxan, and magnesium chloride were from Wako Pure Chemical (Osaka); gallic acid methyl ester and tetra-n-butylammonium iodide were from Tokyo Chemical Industry, and 10% palladium-C was from Rare Metallic (Tokyo). All reagents were analytical grade.
Synthesis of Gallate Derivatives.
During synthesis of gallate derivatives, the three hydroxyl groups of gallic acid methyl ester were protected with benzyl groups (7, 8). Hydroxyl groups of d-glucose, d-glucitol, d-mannitol, maltose, cellobiose, lactose or maltotriose were esterified with gallic acid derivatives, then debenzylated quantitatively to yield the indicated compounds. As an example, the synthesis of 1,2,3,6-tetra-O-galloyl-α-d-glucopyranose (4G-G) is described below.
Tetra-n-butylammonium iodide (2.0 g) and benzyl bromide (63.9 ml) were added to gallic acid methyl ester (20.0 g) in N,N-dimethylformamide (400 ml). The mixture was stirred for 30 min in ice-cold water and, after addition of NaH (21.7 g), cooled to room temperature and stirred for 5 h. The mixture was neutralized with acetic acid and dried in vacuo; the residue was partitioned between chloroform and water, and the organic layer was dried with MgSO4. Remaining solvent was evaporated in vacuo and the residue was chromatographed on a SiO2 column (80 × 330 mm) with 40:1 toluene-acetone to separate tri-O-benzyl methyl gallate (34.5 g).
Tri-O-benzyl methyl gallate (29.3 g) in 1,4-dioxan (300 ml) was added dropwise to 12.9% aqueous NaOH (100 ml). The mixture was stirred for 3 h at 80°C, concentrated in vacuo, and neutralized with HCl. The precipitated crystals were collected and recrystallized from chloroform to yield tri-O-benzyl gallic acid (22.8 g).
Phosphorus pentachloride (1.30 g) was added to a solution of tri-O-benzyl gallic acid (2.45 g) in methylene chloride (50 ml), which was stirred overnight at room temperature. The dried in vacuo product was tri-O-benzyl galloyl chloride.
Tri-O-benzyl galloyl chloride (2.55 g) was added to d-glucose (250 mg) in pyridine (100 ml) and stirred for 18 h at room temperature. The solvent was evaporated in vacuo, and the residue was chromatographed on SiO2 (80 × 330 mm column) with 30:1 toluene-acetone to separate 1,2,3,6-tetra-O-(tri-O-benzyl galloyl)-α-d-glucopyranose (362 mg).
Palladium-C (1 g) was added to 1,2,3,6-tetra-O-(tri-O-benzyl galloyl)-α-d-glucopyranose (362 mg) in methanol (20 ml)-ethyl acetate (20 ml)-formic acid (10 ml). The mixture was stirred for 17 h at room temperature under argon, then filtered through Celite. The filtrate was evaporated in vacuo to give 4G-G (151 mg). Structures of the products were confirmed by NMR.
CHO Cell Assay.
CHO cells in a 96-well culture plate (1 × 104 cells/well) were grown for 2 days in MEM/10% FCS (200 μg/well) (9). After washing with MEM/1% FCS, cells were incubated for 12 h at 37°C in MEM/1% FCS, with CT (10 ng) or other additions (200 μl/well) in a CO2 incubator. Cells morphology was evaluated by phase-contrast microscopy. CT (10 ng) caused elongation of 40% of cells vs. 2–3% elongation among controls. Inhibition of CT activity was complete (100%) if the percentage of elongated cells was <5%.
NAD:agmatine ADP-Ribosyltransferase Activity.
The reaction mix contained 50 mM potassium phosphate (pH 7.5), 100 μM GTP, 5 mM MgCl2, 100 μM [adenine-U-14C]NAD (60,000 cpm), 20 mM agmatine, 0.1 mg/ml ovalbumin, 20 mM DTT, and 1 μg CTA (total volume = 300 μl) (10). After incubation for 180 min at 30°C, a 50-μl sample was applied to an AG 1-X2 resin column (5 × 45 mm) (Bio-Rad) and eluted with H2O for quantification of [adenosine-14C]ADP-ribosylagmatine.
Fluid Accumulation Induced by CT.
Fluid accumulation induced by CT was examined with mouse and rabbit ileal loops, as described (11, 12). Female ddYS mice (20–25 g; Japan SLC, Hamamatsu) or male Japanese white rabbits (2 kg; Japan SLC) were starved for 24 and 48 h before operation, respectively, with water available ad libium. Mice and rabbits were anesthetized with pentobarbital sodium and thiopental sodium, respectively, and the intestines were exteriorized through a midline incision. In mice, one intestinal segment (about 4 cm) was ligated. In each rabbit 6–10 segments (about 10–12 cm) were ligated. CT (100 or 250 ng) and/or drug (total volume 200 μl) were simultaneously injected into the loop. At the indicated time thereafter, animals were killed and the loops were excised. The ratio of weight of fluid contained loop to length of the loop (fluid accumulation ratio, mg/cm) was calculated as a reflection of CT activity.
Transferase Assays.
NAD:Gsα ADP-ribosyltransferase and NAD glycohydrolase activities were quantified as described (13, 14).
Ganglioside GM1 ELISA.
ELISA on a ganglioside GM1-coated plate was performed essentially as described (15).
Results
Inhibitory Effects of Kampo Formulations and Its Components on CT-Induced Elongation of CHO Cells and ADP-Ribosylation.
Among traditional Kampo formulations used to treat diarrheal disease such as cholera, Daio-kanzo-to, an aqueous extract from a blend of Daio (R. rhizoma) and Kanzo (Glycyrrhizae radix), inhibited CT activities in the CHO cell and ADP-ribosyltransferase assays (data not shown). When tested individually, Daio, but not Kanzo, was inhibitory.
The major chemical constituents of R. rhizoma (Daio) extracts are glycoside compounds, tannin, epigallocatechin, emodin, and sennidine A, which were tested for CT inhibitory activity in the NAD:agmatine ADP-ribosyltransferase assay. RG-tannin, isolated by extraction with 100% methanol, was the most potent inhibitor, with 50% inhibition by 0.3 μg/300 μl of RG-tannin (Fig. 1A). Epigallocatechin gallate, another tannin compound, was also inhibitory; the IC50 was almost the same as that of R. rhizoma, on a dry weight basis. Emodin and sennidine A extract were less effective by almost 1 order of magnitude. The glycosides, isolated by water extraction and purification over LH-20 resin, were inactive.
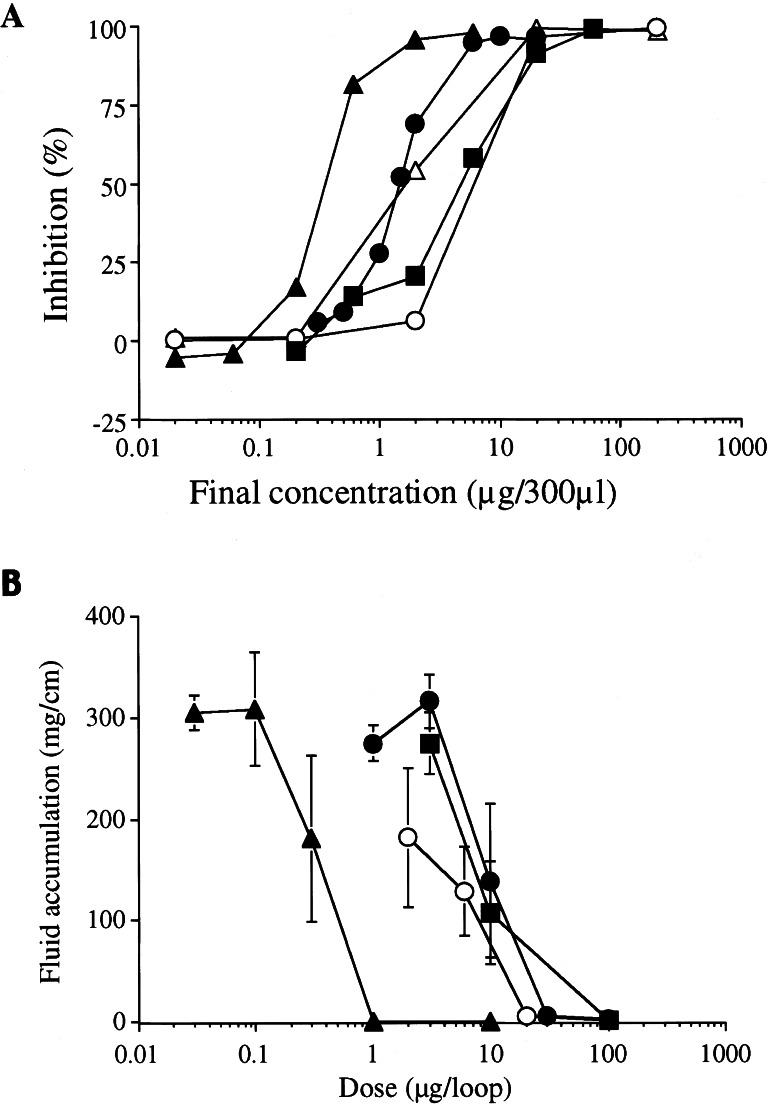
Figure 1.
(A) Inhibitory effects of R. rhizoma on CT-catalyzed ADP-ribosylation. CTA (1 μg) was added with the indicated amounts of R. rhizoma (●), RG-tannin (▴), epigallocatechin gallate (■), emodin (○), and sennidine A (▵). Values represent the average of duplicate tubes from one experiment representative of three. (B) Effects of R. rhizoma on CT-induced fluid accumulation in ileal loops. Each mouse ileal loop was injected with 0.1 ml of a solution containing 100 ng of CT, and the indicated amounts of R. rhizoma (●), RG-tannin (▴), epigallocatechin gallate (■), and sennidine A (○). The mice were killed at 6 h and the fluid accumulation ratio (mg/cm) was calculated. Values represent means ± SD for five determinations.
Inhibition of the Effect of CT on Fluid Accumulation in the Ileum by R. rhizoma and Its Components.
CT-induced fluid accumulation was studied in mouse and rabbit ileal loops. As shown in Fig. 1B, fluid accumulation induced by instillation of CT (100 ng) was inhibited in a dose-dependent manner by simultaneous administration of RG-tannin (1 μg), prepared by the method of Yokozawa et al. (16).
Although epigallocatechin gallate and sennidine A also inhibited fluid accumulation, about 10 times higher concentrations were required. Thus, the inhibitory activity of R. rhizoma can be explained by the presence of RG-tannin.
The inhibitory effect of RG-tannin on CT-induced fluid accumulation was confirmed in the rabbit intestinal loop assay, which is used as an animal model of cholera diarrhea (17) (Fig. 2, Table 1). No intestinal bleeding was observed in rabbit ileal loops injected with up to 10 μg of RG-tannin, with or without CT (Fig. 2, Table 1).
Figure 2.

Inhibitory effects of RG-tannin on CT-induced fluid accumulation in the rabbit ileal loop. Test was carried out as described in Materials and Methods, and the rabbit was killed after 18 h. Loops contained the indicated amounts of CT and RG-tannin. 1, no additions; 2–7, 250 ng of CT; 3 and 8,10 μg of RG-tannin; 4, 3 μg of RG-tannin; 5, 1 μg of RG-tannin; 6, 300 ng of RG-tannin; and 7, 100 ng of RG-tannin. Experiment was replicated five times.
Table 1.
Effect of RG-tannin on cholera toxin-induced fluid accumulation in rabbit ileal loop
| RG-tannin, μg | CT, 250 ng | Ileal fluid, g/cm |
|---|---|---|
| 0 | − | 0.003 ± 0.002* |
| 10 | − | 0.004 ± 0.002* |
| 0 | + | 1.99 ± 0.183 |
| 0.1 | + | 2.20 ± 0.175 |
| 0.3 | + | 1.84 ± 0.189 |
| 1 | + | 1.22 ± 0.441 |
| 3 | + | 0.003 ± 0.002* |
| 10 | + | 0.007 ± 0.002* |
Data are means ± SD (n = 5).
, P < 0.001 vs. CT alone (Student's t test).
Mechanism of Inhibitory Effects of RG-Tannin on CTA-Catalyzed ADP-Ribosylation.
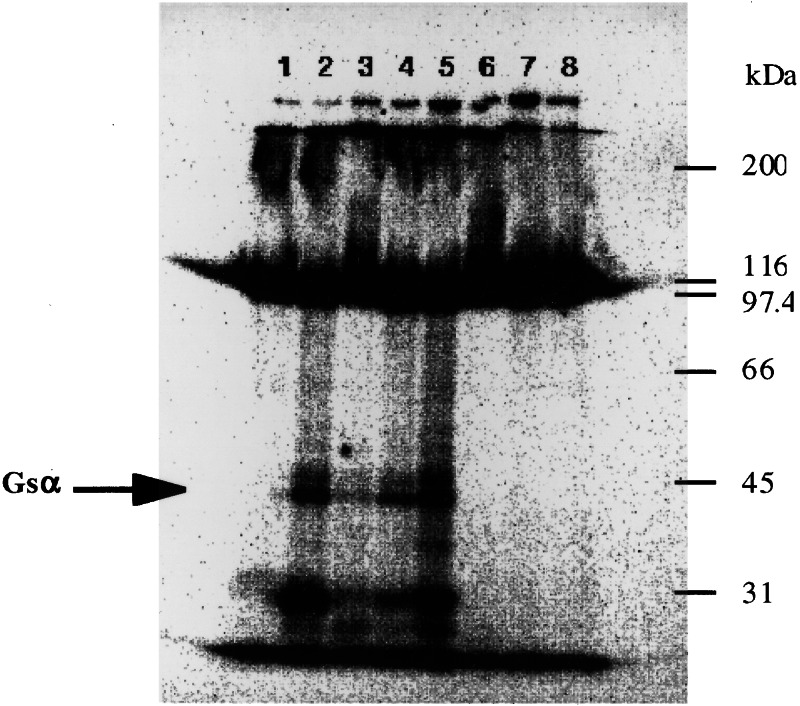
Inhibitory effects of RG-tannin on the CTA-catalyzed ADP-ribosylation of Gsα in membranes from Caco-2 cells, a human intestinal cell line, were concentration-dependent. RG-tannin also inhibited CTA-catalyzed NAD glycohydrolase and NAD:agmatine ADP-ribosyltransferase activities (data not shown). RG-tannin had no effect on the Km for NAD, but significantly reduced the maximal velocity. Unlike the case with NAD, RG-tannin significantly increased the Km for agmatine without altering the maximal velocity (data not shown). RG-tannin had no effect on binding of CTB subunit to the CT receptor ganglioside GM1 or on endogenous ADP-ribosylation of membrane proteins (Fig. 3). We conclude that RG-tannin specifically inhibits the catalytic activity of the CTA. Because there are obvious differences between the mode of action of CT and that of heat-stable enterotoxin (ST) of enterotoxigenic Escherichia coli, the effects of RG-tannin on ST activity were examined. RG-tannin did not inhibit ST-induced fluid accumulation in mouse ileal loops (data not shown). These results also supported the hypothesis that RG-tannin is a specific inhibitor of CT but not of intestinal secretion and absorption systems.
Figure 3.
Effects of RG-tannin on CTA-catalyzed ADP-ribosylation of Caco-2 cell membranes. CT-catalyzed ADP-ribosylation assays as described in Materials and Methods contained the indicated amounts of CTA and RG-tannin. Lane 1, no additions; lanes 2–5, 2.5 μg of CTA; lanes 3 and 6, 15 μg of RG-tannin; lanes 4 and 7, 10 μg of RG-tannin; and lanes 5 and 8, 5 μg of RG-tannin.
Effects of Gallate Derivatives on CT ADP-Ribosyltransferase Activity.
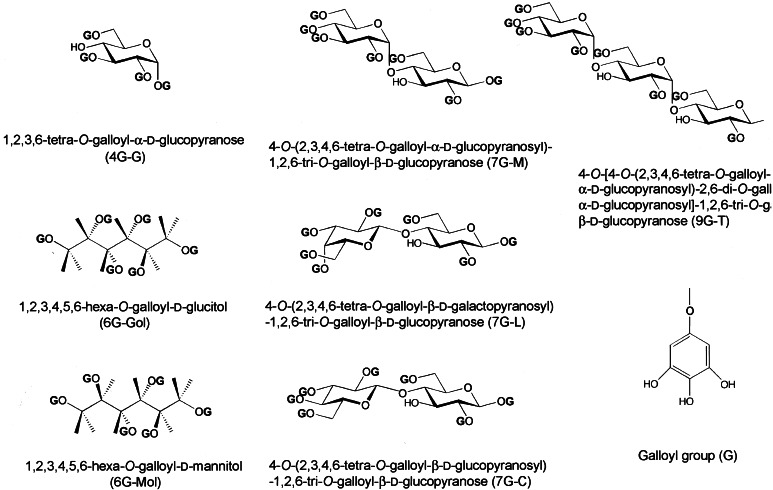
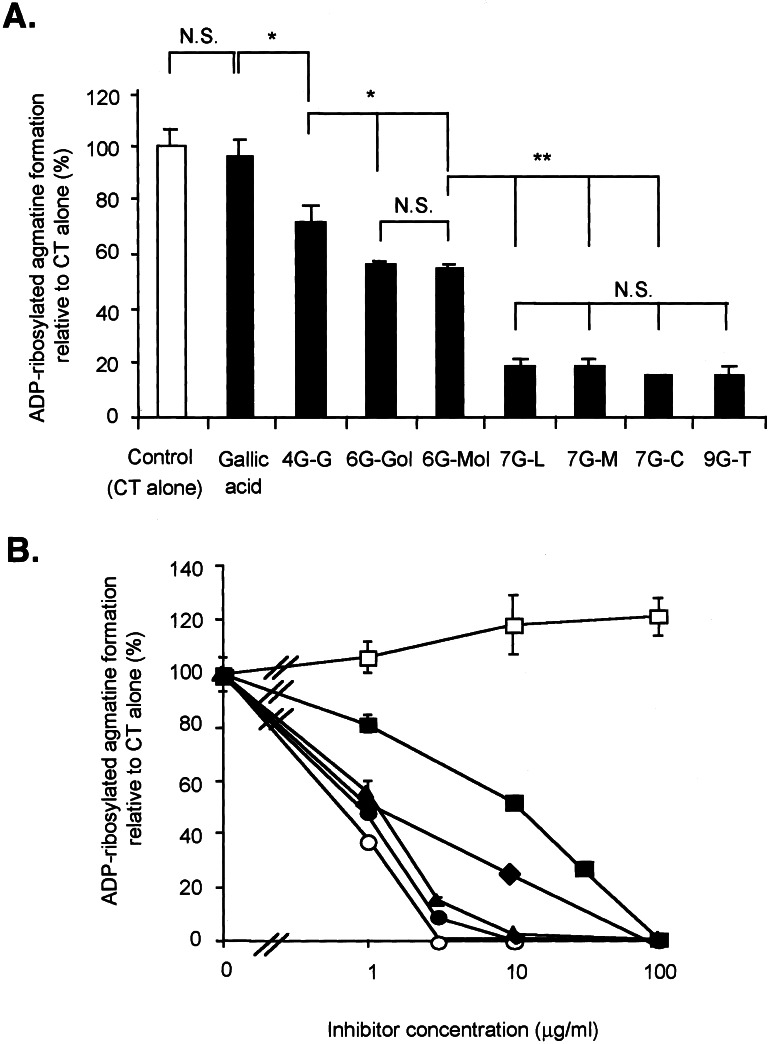
Gallate derivatives (Fig. 4) have different numbers of OH groups that are esterified with galloyl groups, e.g., 4G-G is a compound in which d-glucose is esterified with four galloyl groups. At a concentration of 2 μg/ml, all gallate derivatives inhibited CT ADP-ribosyltransferase activity; 7G-M, 7G-L, 7G-C, and 9G-T were the most potent, 6G-Gol and 6G-Mol were similarly inhibitory, and 4G-G was the least effective (Fig. 5A). 7G-M, 9G-T, and RG-tannin were essentially equally active, 6G-Gol and 4G-G less so (Fig. 5B). Gallic acid, d-glucose, d-glucitol, maltose, and maltotriose had no effect (data not shown).
Figure 4.
Structures of synthetic gallate derivatives.
Figure 5.
(A) Comparison of inhibitory effects of gallate derivatives on CT-catalyzed ADP-ribosylation. Assays contained CT, agmatine, and [14C]NAD, as described in Materials and Methods without or with gallic acid or gallate derivatives (2 μg/ml). Values represent means ± SD for three determinations. *, P < 0.05; **, P < 0.01, by unpaired t test. N.S., not significant. (B) Effect of gallate derivatives on CT-catalyzed ADP-ribosylation of agmatine. ADP-ribosyltransferase activity of CT was determined in the agmatine assay with [14C]NAD as an ADP-ribose donor. CT was incubated with agmatine and [14C]NAD in the presence or absence of gallic acid (□), RG-tannin (○), 4G-G (■), 6G-Gol (⧫), 7G-M (▴), and 9G-T (●). Values represent means ± SD for three determinations.
Inhibitory Effects of Gallate Derivatives in the Mouse Ileal Loop Model.
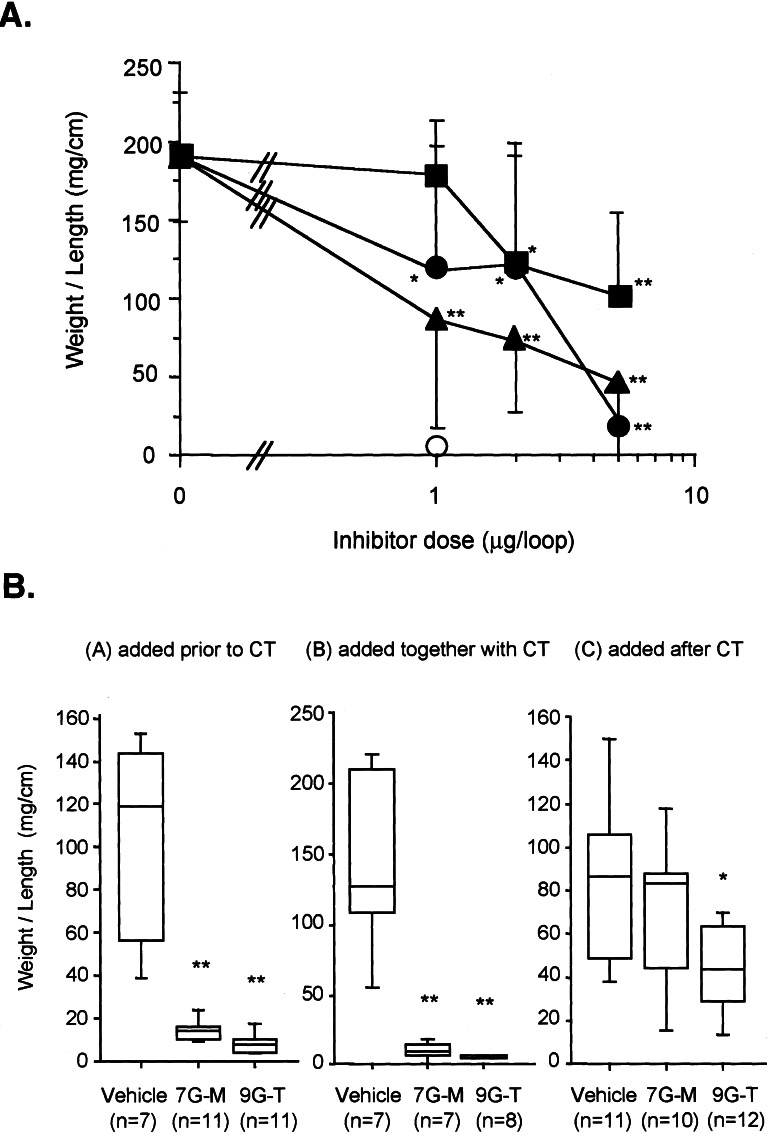
Fluid accumulation induced by CT (0.5 μg) was inhibited in a concentration-dependent manner by 7G-M (ED50 = 0.85 μg) and 9G-T (ED50 = 1.17 μg) (Fig. 5A); 4G-G was the least effective. Under similar conditions, RG-tannin (1 μg) completely inhibited CT-induced fluid accumulation. 7G-M and 9G-T were effective when added 10 min before or together with CT. They were less effective when added 10 min after CT (Fig. 6).
Figure 6.
(A) Effect of gallate derivatives on CT-induced fluid accumulation in ileal loops. CT (500 ng) was added to the mouse ileal loop with a gallate derivative, 4G-G (■), 7G-M (▴), 9G-T (●), and RG-tannin (○). Weight/length ratios were calculated. Values represent means ± SD for a minimum of five animals. *, P < 0.05; **, P < 0.01, vs. CT alone by unpaired t test. (B) Effects of gallate derivatives on CT-induced fluid accumulation in ileal loops. (BA) added before CT. Each loop was injected with 0.1 ml of sample solution (saline, 0.25 mg/loop 7G-M, 0.25 mg/loop 9G-T) and 10 min later CT (600 ng) was added, (BB) added together with CT, and (BC) added 10 min after CT. *, P < 0.05; **, P < 0.01, vs. vehicle, by Mann–Whitney U test.
Discussion
Our results indicate that Daio-kanzo-to, RG-tannin, and synthetic gallate compounds inhibit CT effects, including elongation of CHO cells, ADP-ribose transfer, and accumulation of fluid in intestinal loops. In the case of the synthetic compounds, inhibitory effects increased with the number of galloyl groups in the molecule. Toda et al. (18) recently reported that tea extract and epigallocatechin gallate protected against experimental infection by V. cholerae O1. We confirmed here that epigallocatechin gallate had inhibitory activity toward CT although it was one-tenth the activity of RG-tannin. Hör et al. (19) recently found that Guazuma ulimifolia extract, which is used by the Mixe Indians of Oaxaca (Mexico) to treat diarrhea, completely inhibited CT-induced fluid accumulation. The most active fraction contained procyanidins with a degree of polymerization higher than 8. Based on structure, RG-tannin is most likely a procyanidin polymer (5). These findings are consistent with the hypothesis that the active compound in G. ulimifolia extract is similar to RG-tannin.
We investigated whether gallate derivatives were effective against CT in the mouse ileal loop assay when added after the toxin, which could have implications for treatment of cholera infection during an outbreak. Two of our gallate derivatives, 7G-M and 9G-T, almost completely inhibited CT-induced fluid accumulation in the mouse ileal loop when added after CT, albeit at a much higher concentration than needed when added before CT. The effectiveness of these compounds, when added after CT may be related to the time necessary for internalization of the toxin and release of the catalytically active A subunit.
Although RG-tannin inhibited the NAD:agmatine ADP-ribosyltransferase activity of CT, RG-tannin did not affect endogenous ADP-ribosylation of membrane proteins. In a mutagenicity test of the derivatives by the umu-test (20), 7G-M and 9G-T had no detectable activity at concentrations up to 10 μg/ml, a concentration at which both derivatives completely inhibited the enzymatic activity of CT. Thus, it may be possible to develop nontoxic synthetic gallate derivatives as inhibitors of CT. Daio-kanzo-to, RG-tannin, or other gallates could be added to oral rehydration solutions as an adjunctive therapy for the treatment of cholera patients in epidemic areas.
For preventive strategies, medicines based on gallate derivatives could be taken by tourists when traveling in regions where the risk of cholera is high. With this in mind, it is appropriate to mention that the heat-labile enterotoxin from enterotoxigenic E. coli is a major etiologic agent in travelers' diarrhea (21). The E. coli enterotoxin is structurally and functionally very similar to CT (22, 23). As shown in this article, alternative medicines may provide sources for novel therapeutics; confirmation by chemical synthesis is helpful in defining the molecular basis for their pharmacological efforts.
Acknowledgments
We thank Dr. Iwao Kato and Dr. Yoshifumi Takeda for their helpful discussions. We gratefully acknowledge the helpful technical advice regarding the separation of R. rhizoma and comments of Dr. Minoru Okada, Dr. Yasuhiro Komatsu, and Dr. Makoto Ishimatsu. We thank Dr. Martha Vaughan for thoughtful discussions and critical review of the manuscript. We thank Mrs. Michiko Hatano for her expert secretarial assistance. This work was supported by Grants-in-Aid of Scientific Research (03454180, 04304032, 05271205, 06264204, and 08770184) from the Ministry of Education, Science and Culture of Japan and Grant for International Health Cooperation Research (11A-1–1999, 11A-1–2000) from the Ministry of Health and Welfare of Japan.
Abbreviations
- CT
cholera toxin
- CTA
CTA subunit
- CHO
Chinese hamster ovary
- 4G-G
1,2,3,6-tetra-O-galloyl-α-d-glucopyranose
- RG
rhubarb galloyl
References
- 1.Fishman P H. In: ADP-Ribosylating Toxins and G Proteins: Insights into Signal Transduction. Moss J, Vaughan M, editors. Washington, DC: Am. Soc. Microbiol.; 1990. pp. 127–140. [Google Scholar]
- 2.Moss J, Vaughan M. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- 3.Bhan M K, Chea Woo E, Fontaine O, Maulen-Radovan I, Pierce N F, Ribeiro H., Jr Lancet. 1995;345:282–285. [Google Scholar]
- 4.Kobe Research Association of Chinese Medicine. Explanation of Prescription in Chinese Medicine. Tokyo: Ishiyaku Press; 1985. [Google Scholar]
- 5.Nishioka I. J Trad Sino-Japanese Med. 1990;11:70–75. [Google Scholar]
- 6.Nishioka I. Yakugaku Zasshi. 1983;103:125–142. doi: 10.1248/yakushi1947.103.2_125. [DOI] [PubMed] [Google Scholar]
- 7.Brimacombe J S. Methods Carbohydr Chem. 1972;192:131–132. [Google Scholar]
- 8.Ott E. Org Synth II. 1943;2:528–529. [Google Scholar]
- 9.Honda T, Shimizu M, Takeda Y, Miwatani T. Infect Immun. 1976;14:1028–1033. doi: 10.1128/iai.14.4.1028-1033.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noda M, Tsai S-C, Adamik R, Bobak D A, Moss J, Vaughan M. Biochemistry. 1989;28:7636–7940. doi: 10.1021/bi00445a057. [DOI] [PubMed] [Google Scholar]
- 11.Gorbach S L, Banwell J G, Chatterjee B D, Jacobs B, Sack R B. J Clin Invest. 1971;50:881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitotsubashi S, Fujii Y, Yamanaka H, Okamoto K. Infect Immun. 1992;60:4468–4474. doi: 10.1128/iai.60.11.4468-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morinaga N, Noda M, Kato I. FEBS Lett. 1990;271:211–214. doi: 10.1016/0014-5793(90)80408-b. [DOI] [PubMed] [Google Scholar]
- 14.Noda M, Tsai S-C, Adamik R, Bobak D A, Moss J, Vaughan M. Biochem Biophys Acta. 1990;1034:195–199. doi: 10.1016/0304-4165(90)90076-9. [DOI] [PubMed] [Google Scholar]
- 15.Honda T, Sato M, Miwatani T. J Clin Microbiol. 1984;20:664–667. doi: 10.1128/jcm.20.4.664-667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokozawa T, Fujioka K, Oura H, Nonaka G, Nishioka I. Nephron. 1991;58:155–160. doi: 10.1159/000186406. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Pickett M J. J Bacteriol. 1969;100:1142–1143. doi: 10.1128/jb.100.2.1142-1143.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toda M, Okubo S, Ikigai H, Suzuki T, Suzuki Y, Hara Y, Shimanura T. Microbiol Immunol. 1992;36:999–1001. doi: 10.1111/j.1348-0421.1992.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 19.Hör M, Rimpler H, Heinrich M. Planta Medica. 1995;61:208–212. doi: 10.1055/s-2006-958057. [DOI] [PubMed] [Google Scholar]
- 20.Oda Y, Nakamura S, Oki I, Kato T, Shinagawa H. Mutat Res. 1985;147:219–229. doi: 10.1016/0165-1161(85)90062-7. [DOI] [PubMed] [Google Scholar]
- 21.Nataro J P, Kaper J B. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji T, Inoue A, Miyama A, Noda M. FEBS Lett. 1991;291:319–321. doi: 10.1016/0014-5793(91)81311-u. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Tamura T, Yokota T. J Biol Chem. 1984;259:5037–5044. [PubMed] [Google Scholar]