Abstract
Beta-sitosterol (BS) and pteropodine (PT) are constituents of various plants with pharmacological activities potentially useful to man. The chemicals themselves possess biomedical properties related to the modulation of the immune and the nervous systems, as well as to the inflammatory process. Therefore, safety evaluation of the compounds is necessary in regard to their probable beneficial use in human health. The present study evaluates their genotoxic and cytotoxic potential by determining the capacity of the compounds to induce sister chromatid exchanges (SCE), or to alter cellular proliferation kinetics (CPK) and the mitotic index (MI) in mouse bone marrow cells. Besides, it also determines their capacity to increase the rate of micronucleated polychromatic erythrocytes (MNPE) in peripheral mouse blood, and the relationship polychromatic erythrocytes/normochromatic erythrocytes (PE/NE) as an index of cytotoxicity. For the first assay, four doses of each compound were tested: 200, 400, 600, and 1000 mg/kg in case of BS, and 100, 200, 300, and 600 mg/kg for PT. The results in regard to both agents showed no SCE increase induced by any of the tested doses, as well as no alteration in the CPK, or in the MI. With respect to the second assay, the results obtained with the two agents were also negative for both the MNPE and the PE/NE index along the daily evaluation made for four days. In the present study, the highest tested dose corresponded to 80% of the LD50 obtained for BS and to 78% in the case of PT. The results obtained establish that the studied agents have neither genotoxic nor cytotoxic effect on the model used, and therefore they encourage studies on their pharmacological properties.
INTRODUCTION
The isolation and chemical characterization of plant-derived compounds with therapeutic or other beneficial health properties are increasing, in many cases as a consequence of primary information obtained through the use of plant extracts in traditional medicine. However, the possible use of these compounds for human well-being requires solid and lengthy research which includes a number of basic and applied phases; one of the determinations in this complex evaluation concerns the toxic potential of the studied agent.
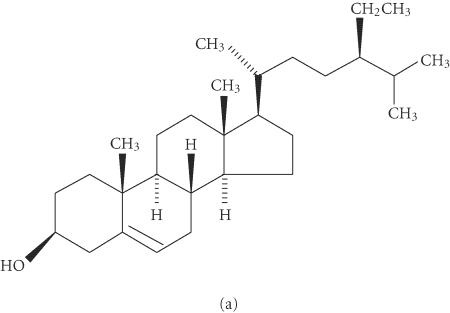
Beta-sitosterol (BS) (Figure 1) is one of the most prevalent vegetable-derived phytosterols in the diet. It is structurally related to cholesterol, but since it is slowly absorbed in the intestinal tract, it may interfere with the cholesterol absorption preventing its rise in serum. BS also appears to modulate the immune function, inflammation, and the pain levels by controlling the production of inflammatory cytokines [1, 2]. This last effect may help to control allergies and reduce prostate enlargement [3, 4]. The compound can affect the structure of cell membranes and alters the signaling pathways that regulate tumor growth and apoptosis [5]. Moreover, BS has shown a decrease in proliferative changes and tumor yields when added to diets of mice and rats treated with colon carcinogens [6, 7]. The compound is found in numerous plants, including rice, wheat, corn, nut, peanut, and particularly in the Peruvian borne Rubiaceae plant cat's claw (Uncaria tomentosa) [8, 9], where it seems to be involved in the curative properties suggested for the vegetable, such as anti-inflammatory and antiviral activities, enhancer of the immune system, and inhibitor of arthritis, ulcer, and cancer [10, 11, 12, 13].
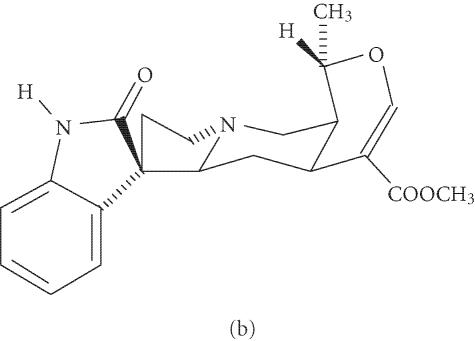
Figure 1.
Stereochemical structure of beta-sitosterol (a) and pteropodine (b).
Pteropodine or uncarine C (PT) (Figure 1) is a chemical specifically isolated from cat's claw. It is a heteroyohimbine-type oxindole whose biological properties have been studied less than those of BS. However, it is probably the most immunologically active of the six oxindole alkaloids identified in the plant, showing a pronounced enhancement of phagocytosis, as well as a modulatory effect on the muscarinic M1 and 5-hydroxytryptamine receptors [14, 15, 16]. These data suggest that it may act in synergism with other components of the plant to induce its biomedical actions.
The compounds selected for this study are constituents of cat's claw, a plant claimed to have therapeutic properties for various diseases, mainly related to inflammatory, immunological, and cancer processes; with respect to the last illness, aqueous extracts of the plant have shown significant antiproliferative effect in leukemia, lymphoma, and breast human cancer cell lines [17, 18]; results that point to the need for in vivo assays of the extracts and their constituents. Therefore, the evaluation of the BS and PT toxicity in a mammalian organism seems mandatory in order to continue with pharmacological and anthropogenic research that may be applicable to humans. In regard to genotoxic potential, insufficient information on the compounds have been reported; it is known that Uncaria tomentosa extracts have no metallic capacity, and a negative result was reported in a study that measured the micronuclei produced by BS, although a single low dose was used in the assay [19, 20]. In regard to PT no information on the matter have been reported.
In view of the knowledge that the determination of damage to genetic material is a useful biomarker of xenobiotic exposure, as well as the fact that our laboratory is evaluating the anthropogenic potential of both compounds in mouse, we found it pertinent to determine the genotoxicity of the compounds. In this report we show that the two substances are not capable of increasing the rate of sister chromatid exchanges (SCE) and micronuclei (MN) in mouse, nor of inducing toxicity in the same model.
MATERIALS AND METHODS
Chemicals and animals
Pteropodine (99% pure) and beta-sitosterol (97% pure) were obtained from Profiqua Chemicals (Mexico City); 5-bromodeoxyuridine (BrdU), doxorubicin, and colchicine were purchased from Sigma Chemicals (St Louis, Mo, USA). The Giemsa stain was obtained from Merck (Mexico City), and sodium citrate, sodium chloride, potassium phosphate, and sodium phosphate from Baker S. A. (Mexico City).
Eight-week-old male mice (NIH) with 25 g of weight were obtained from the National Institute of Hygiene; they were kept in metallic cages at a mean temperature of 23°C and at a 12 hours dark-light cycle, and permitted to freely consume food (Purina) and water.
Genotoxicity protocols
Lethal dose 50
The first step for the genotoxic evaluation was the determination of the acute toxicity of both compounds. For this purpose we applied the method of Lorke, which uses 3 animals and 3 doses in a first step, and 1 animal and four doses in a second step [21]. Mortality among mice was observed for as long as 7 days; the LD50 was obtained as the geometric mean between the minimal lethal dose and the maximal sublethal dose. The test showed an LD50 of 1250 mg/kg for BS and of 771 mg/kg for PT.
Sister-chromatid exchanges, cellular proliferation kinetics, and mitotic index
Six experimental groups per chemical (five mice per group) were used for these determinations. The compounds were intraperitoneally (IP) administered. In the case of BS the groups were as follows: a negative control group of animals administered with mineral oil (0.4 mL/mouse), a positive control group treated with 10 mg/kg of doxorubicin, and four groups administered with 200, 400, 600, and 1000 mg/kg of the tested chemical. With respect to PT, this was dissolved in distilled water (in a water bath at 37°C for a few minutes); thus, water was administered to the animals of the negative control group (0.4 mL/mouse). We also included a positive control group (doxorubicin, 10 mg/kg), as well as four more groups treated with 100, 200, 300, and 600 mg/kg of the tested agent.
To begin the procedure, a BrdU tablet of 50 mg was partially coated with paraffin and subcutaneously implanted to each animal [22]; 1 hour afterwards the chemicals were injected to mice. Twenty-one hours after the tablet implantation, 5 mg/kg of colchicine was IP injected to mice and permitted to act for 3 hours. Then, the femurs of each mouse were dissected and the bone marrow obtained in a solution of KCl (0.075 M) at 37°C after which it was incubated for 30 minutes at the same temperature. The cell suspension was centrifuged for 10 minutes at 1500 rpm, the supernatant was discarded, and the cells were fixed in a solution of methanol-acetic acid (3 : 1). This fixation process was repeated at least twice. To carry out the staining process, two drops of each cell suspension were deposited onto ethanol-cleaned slides and stained with the Hoechst-Giemsa method to differentiate the sister chromatids [22, 23].
Cytogenetic analysis per mouse was made as follows: (a) the rate of SCE was determined in 60-second division metaphases, (b) the cellular proliferation kinetics (CPK) was determined in 100 metaphases, identifying the cells in first (M1), second (M2), and third (M3) cellular division. With these data, the average generation time (AGT) was obtained using the formula AGT = 21/(M1 + 2M2 + 3M3) (100), and (c) the mitotic index (MI) was determined in 1000 cells. Statistical analysis of the obtained data was made with ANOVA test followed by the Student t test.
Micronucleated polychromatic erythrocytes (MNPE)
Six groups with 5 individuals each per chemical were organized for this assay. The compounds were IP inoculated. The groups for BS were a negative control group treated with mineral oil, a positive control group administered with 10 mg/kg of doxorubicin, and four more groups treated with 200, 400, 600, and 1000 mg/kg of BS. In the case of PT, four groups were administered with 100, 200, 300, and 600 mg/kg of the chemical. The results obtained in these animals were compared to the obtained results in the negative control group (distilled water) and the positive control group (10 mg/kg doxorubicin).
Initially, we obtained two drops of blood from the tail of each mouse and smeared them on ethanol-cleaned slides; then, the cells were fixed in methanol for 10 minutes and stained for 15 minutes with Giemsa solution made in PBS (pH = 6.8) [24]. Afterward, the tested chemicals were administered to mice and their blood was obtained at 24, 48, 72, and 96 hours and then it was stained as indicated earlier.
To determine the genotoxic potential of the chemicals, the rate of MNPE in 1000 polychromatic erythrocytes (PE) per mouse was quantified; to evaluate the probable cytotoxic damage, the number of PE and of normochromatic erythrocytes (NE) in 1000 cells per mouse was determined. The statistical significance of the obtained data was determined with the ANOVA and the Student t tests.
RESULTS
Beta-sitosterol
The acute toxicity assay made to this compound showed a lower lethal potential (38%) than the obtained for PT. The results for SCE, AGT, and the MI are indicated in Table 1. With respect to the rate of SCE, we found a similar level (with the four tested doses) to the one observed in the negative control group; however, the positive control agent (doxorubicin) revealed an increase of about six times that level. The same table shows a homogeneous AGT value of the four tested doses of BS, with a variability of no more than 30 minutes among them; these values are very close to those obtained in the controls. A similar effect, that is, no modification by BS was also determined with respect to the cell division rate, as shown by the similar MI values observed in BS-treated mice and the control animals.
Table 1.
Sister chromatid exchanges (SCE), average generation time (AGT), and mitotic index (MI) induced by beta-sitosterol (BS) and pteropodine (PT) in mouse bone marrow cells. MO = mineral oil (0.4 mL added to each mouse). DW = distilled water (0.4 mL added to each mouse). DR = doxorubicin. M1, M2, M3 = cells in first, second, and third cellular division. AGT = 21 (M1+2M2+3M3) (100).
| Agent | Dose | SCE | M1 | M2 | M3 | AGT | MI | ||
| mg/kg |
|
% | % | % |
|
|
|||
| MO | — | 1.88 ± 0.12 | 38 | 48 | 14 | 12.15 ± 0.23 | 5.73 ± 0.34 | ||
| BS | 200 | 2.12 ± 0.28 | 32 | 53 | 15 | 12.14 ± 0.17 | 5.66 ± 0.29 | ||
| BS | 400 | 1.54 ± 0.21 | 36 | 50 | 14 | 12.45 ± 0.12 | 5.70 ± 0.18 | ||
| BS | 600 | 1.34 ± 0.24 | 34 | 55 | 11 | 12.55 ± 0.10 | 5.58 ± 0.33 | ||
| BS | 1000 | 1.0 ± 0.34 | 33 | 52 | 15 | 12.35 ± 0.18 | 6.03 ± 0.22 | ||
| DR | 10 | *16.83 ± 0.42 | 33 | 58 | 9 | 12.37 ± 0.31 | 6.43 ± 0.41 | ||
| DW | — | 2.51 ± 0.12 | 34 | 51 | 15 | 12.45 ± 0.23 | 5.94 ± 0.19 | ||
| PT | 100 | 1.15 ± 0.16 | 35 | 51 | 14 | 12.36 ± 0.14 | 5.97 ± 0.26 | ||
| PT | 200 | 2.03± 0.27 | 37 | 50 | 13 | 12.46 ± 0.21 | 5.80 ± 0.38 | ||
| PT | 300 | 1.08 ± 0.57 | 34 | 51 | 15 | 12.25 ± 0.33 | 6.70 ± 0.18 | ||
| PT | 600 | 1.44 ± 0.22 | 34 | 49 | 17 | 12.63 ± 0.16 | 7.13 ± 0.17 | ||
| DR | 10 | *14.06 ± 0.68 | 39 | 50 | 11 | 12.47 ± 0.26 | 6.16 ± 0.68 | ||
*Statistically significant difference with respect to the result obtained in the negative control group. ANOVA and Student t tests, P ≤ .05.
The rate of micronuclei induced by this chemical is shown in Table 2. The mean number of MNPE/1000 cells determined in the negative control group along the 96 hours was 1.62; the mean values observed in the BS-treated groups were 0.94, 1.17, 1.28, and 1.35 corresponding to the doses of 200, 400, 600, and 1000 mg/kg, respectively, clearly showing an absence of genotoxicity. On the contrary, the MNPE mean determined for doxorubicin was high as expected, particularly at 72 hours (25.4 MNPE/1000 cells). The innocuous capacity of BS for micronuclei induction paralleled the absence of its cytotoxic potential shown by the frequency of polychromatic erythrocytes (Table 3). The table shows PE daily values similar to those determined in the negative control animals and contrary to those in doxorubicin-treated mice, in which a significant PE rate decrease (74% at 72 hours) was found in comparison with the mean of the other groups.
Table 2.
Frequency of micronucleated polychromatic erythrocytes (MNPE) induced by beta-sitosterol (BS) and pteropodine (PT) in mouse blood cells. MO = mineral oil (0.4 mL added to each mouse). DW = distilled water (0.4 mL added to each mouse). DR = doxorubicin.
| Agent | Dose | MNPE ± SD | |||
|---|---|---|---|---|---|
| mg/kg | 24 h | 48 h | 72 h | 96 h | |
| MO | — | 1.6 ± 0.26 | 2.05 ± 0.18 | 1.74 ± 0.21 | 1.1 ± 0.32 |
| BS | 200 | 1.13 ± 0.15 | 0.83 ± 0.31 | 0.83 ± 0.15 | 1.0 ± 0.42 |
| BS | 400 | 1.33 ± 0.21 | 1.03 ± 0.33 | 1.0 ± 0.43 | 1.33 ± 0.32 |
| BS | 600 | 1.40 ± 0.19 | 1.53 ± 0.18 | 1.20 ± 0.23 | 1.0 ± 0.17 |
| BS | 1000 | 1.7 ± 0.17 | 1.3 ± 0.24 | 1.4 ± 0.24 | 1.0 ± 0.26 |
| DR | 10 | *18.26 ± 0.26 | *19.3 ± 0.29 | *25.4 ± 0.31 | *20.5 ± 0.22 |
| DW | — | 1.4 ± 0.31 | 1.35 ± 0.46 | 1.3 ± 0.33 | 1.45 ± 0.17 |
| PT | 100 | 1.3 ± 0.44 | 1.4 ± 0.31 | 1.5 ± 0.25 | 1.5 ± 0.27 |
| PT | 200 | 1.4 ± 0.26 | 1.5 ± 0.16 | 1.6 ± 0.13 | 1.61 ± 0.51 |
| PT | 300 | 1.4 ± 0.39 | 1.55 ± 0.51 | 1.7 ± 0.41 | 1.71 ± 0.28 |
| PT | 600 | 1.5 ± 0.19 | 1.64 ± 0.31 | 1.6 ± 0.32 | 1.65 ± 0.36 |
| DR | 10 | *19.15 ± 0.3 | *21.72 ± 0.17 | *24.3 ± 0.34 | *22.82 ± 0.17 |
*Statistically significant difference with respect to the result obtained in the negative control group. ANOVA and Student t tests, P ≤ .05.
Table 3.
Effect of beta-sitosterol (BS) and pteropodine (PT) in the index polychromatic erythrocytes/normochromatic erythrocytes (PE/NE) in mouse blood cells. MO = mineral oil (0.4 mL added to each mouse). DW = distilled water (0.4 mL added to each mouse). DR = doxorubicin.
| Agent | Dose | PE/NE ± SD | |||
|---|---|---|---|---|---|
| mg/kg | 24 h | 48 h | 72 h | 96 h | |
| MO | — | 20.13 ± 0.23 | 21.10 ± 0.14 | 20.5 ± 0.08 | 21.13 ± 0.26 |
| BS | 200 | 19.8 ± 0.46 | 18.8 ± 0.17 | 17.0 ± 0.42 | 19.5 ± 0.28 |
| BS | 400 | 20.3 ± 0.43 | 19.7 ± 0.52 | 19.16 ± 0.11 | 20.0 ± 0.48 |
| BS | 600 | 20.0 ± 0.19 | 19.5 ± 0.44 | 19.83 ± 0.61 | 21.0 ± 0.12 |
| BS | 1000 | 22.0 ± 0.43 | 20.3 ± 0.19 | 20.14 ± 0.05 | 21.3 ± 0.57 |
| DR | 10 | *9.1 ± 0.37 | *7.33 ± 0.61 | *5.33 ± 0.33 | *6.36 ± 0.42 |
| DW | — | 19.5 ± 0.12 | 20.33 ± 0.21 | 20.16 ± 0.29 | 21.4 ± 0.18 |
| PT | 100 | 16.33 ± 0.38 | 19.66 ± 0.18 | 20.6 ± 0.19 | 21.8 ± 0.49 |
| PT | 200 | 14.9 ± 0.61 | 21.16 ± 0.37 | 20.36 ± 0.10 | 22.9 ± 0.35 |
| PT | 300 | 16.51 ± 0.32 | 21.5 ± 0.23 | 20.83 ± 0.08 | 20.8 ± 0.67 |
| PT | 600 | 17.62 ± 0.29 | 22.0 ± 0.55 | 19.86 ± 0.52 | 21.0 ± 0.47 |
| DR | 10 | *5.33 ± 0.22 | *8.1 ± 0.16 | *9.6 ± 0.27 | *8.06 ± 0.39 |
*Statistically significant difference with respect to the result obtained in the negative control group. ANOVA and Student t tests, P ≤ .05.
Pteropodine
The LD50 of this compound was higher than the determined for BS. However, the results obtained for SCE, AGT, and mitotic index (Table 1) are similar to the described ones for the previous chemical. No increase in any of the four parameters was found with respect to the negative control value, but as expected, a significant SCE rate was determined with doxorubicin which was 5.6 times higher than the control value. The AGT and the mitotic index also showed no modification in relation to the control level.
With respect to the rate of MNPE, the results indicated in Table 2 are in the same line as the described ones before for BS. The frequency obtained with the four tested doses of PT were found in the same range as the determined ones in the negative control mice; nevertheless, a statistically significant MN increase was induced by doxorubicin. In relation to the PE rate, no change induced by PT was observed in comparison with the control animals, but a significant decrease in doxorubicin-treated mice was determined (Table 3).
DISCUSSION
Genotoxic studies are useful to identify the level of DNA damage induced by xenobiotics, as well as to give a clue about the possible clinical consequences of human exposure. In vivo acute studies in mammalian organisms, in particular, are usually considered the last type of assays in a hierarchical test battery designed to determine the genotoxic potential of an agent; in this step, high doses of the tested compound are usually included; however, new studies are needed to analyze risk assessment, that is, incorporating variables such as sex, age, time, dose, or route of administration, to better understand the toxic potential as well as to have more data for a reasonable extrapolation to human exposure. In our study, the use of doses 20% low than the LD50 obtained for the agents did not induce an increase in the rate of SCE nor in the MNPE of mouse, indicating no in vivo genotoxic potential of the chemicals even at a high dose.
Micronuclei represent chromosome fragments or whole chromosomes abnormally segregated to daughter cells during mitosis, and although the SCE formation is not clearly understood, it is usually related to DNA break and rejoining at the replication fork [25, 26]. Thus, the present study demonstrates that two different cytogenetic endpoints agree in their results giving more reliability to a negative genotoxic potential of the tested compounds. This is also important because the evaluated chromosomal endpoints have been suggested as biomarkers for malignant transformation [27, 28], indicating a low potential for this kind of damage by the chemicals. However, tests to measure genic damage, as well as chronic exposure to the compounds, should be performed to have a better conclusion on the agent's genotoxicity.
Our results concerning the absence of genotoxicity by the tested agents are in line with the in vitro studies made in other sterols where low or no genetic alterations have been reported; although cytotoxicity indicated by increased apoptosis and decrease in cell viability have been observed, probably because of the inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A by the production of free radicals [29]. With respect to PT and other oxindoles, a weak cytotoxic effect have also been observed [14]. These data, determined in cultured cells, confirm the importance of studying the effect of mutagens in in vivo models because of the influence of biotransformation and detoxification processes.
The negative data obtained in the present study on the genotoxic and cytotoxic potential of the tested compounds encourage, with more certainty, the development of research with the following aim: to determine the pharmacological and chemopreventive activity of BS and PT, as well as that of plants carrying them, leading to a possible extrapolation to humans. In the antigenotoxicity area in particular, our laboratory has observed a high inhibitory effect of both chemicals on the SCE and MN damage induced by doxorubicin in mouse (data are not shown), a result that agrees with the few reports made for BS on the matter, and with its antioxidant effect [30]. With respect to PT however, no previous data for this activity are known.
References
- 1.Tilvis R.S, Miettinen T.A. Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr. 1986;43:92–97. doi: 10.1093/ajcn/43.1.92. [DOI] [PubMed] [Google Scholar]
- 2.Gupta M.B, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava K.P. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 1980;39(2):157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya A.K, Connor W.E, Lin D.S. The origin of plant sterols in the skin surface lipids in humans: from diet to plasma to skin. J Invest Dermatol. 1983;80(4):294–296. doi: 10.1111/1523-1747.ep12534670. [DOI] [PubMed] [Google Scholar]
- 4.Klippel K.F, Hiltl D.M, Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study Group. Br J Urol. 1997;80(3):427–432. [PubMed] [Google Scholar]
- 5.von Holtz R.L, Fink C.S, Awad A.B. Beta-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr Cancer. 1998;32(1):8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- 6.Awad A.B, Downie A, Fink C.S, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res. 2000;20(2A):821–824. [PubMed] [Google Scholar]
- 7.Raicht R.F, Cohen B.I, Fazzini E.P, Sarwal A.N, Takahashi M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980;40(2):403–405. [PubMed] [Google Scholar]
- 8.Awad A.B, Chan K.C, Downie A.C, Fink C.S. Peanuts as a source of beta-sitosterol, a sterol with anticancer properties. Nutr Cancer. 2000;36(2):238–241. doi: 10.1207/S15327914NC3602_14. [DOI] [PubMed] [Google Scholar]
- 9.Keplinger K, Laus G, Wurm M, Dierich M.P, Teppner H. Uncaria tomentosa (Willd.) DC.—ethnomedicinal use and new pharmacological, toxicological and botanical results. J Ethnopharmacol. 1999;64(1):23–34. doi: 10.1016/s0378-8741(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval-Chacon M, Thompson J.H, Zhang X.J. Antiinflammatory actions of cat's claw: the role of NF-kappaB. Aliment Pharmacol Ther. 1998;12(12):1279–1289. doi: 10.1046/j.1365-2036.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- 11.Mur E, Hartig F, Eibl G, Schirmer M. Randomized double blind trial of an extract from the pentacyclic alkaloid-chemotype of uncaria tomentosa for the treatment of rheumatoid arthritis. J Rheumatol. 2002;29(4):678–681. [PubMed] [Google Scholar]
- 12.Riva L, Coradini D, Di Fronzo G, et al. The antiproliferative effects of Uncaria tomentosa extracts and fractions on the growth of breast cancer cell line. Anticancer Res. 2001;21(4A):2457–2461. [PubMed] [Google Scholar]
- 13.Williams J.E. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on Uña de Gato and Sangre de Grado. Altern Med Rev. 2001;6(6):567–579. [PubMed] [Google Scholar]
- 14.Muhammad I, Dunbar D.C, Khan R.A, Ganzera M, Khan I.A. Investigation of Uña De Gato I. 7-Deoxyloganic acid and 15N NMR spectroscopic studies on pentacyclic oxindole alkaloids from Uncaria tomentosa. Phytochemistry. 2001;57(5):781–785. doi: 10.1016/s0031-9422(01)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Laus G, Brossner D, Keplinger K. Alkaloids of Peruvian Uncaria tomentosa. Phytochemistry. 1997;45:855–860. [Google Scholar]
- 16.Kang T.H, Matsumoto K, Tohda M, et al. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M(1) and 5-HT(2) receptors expressed in Xenopus oocyte. Eur J Pharmacol. 2002;444(1-2):39–45. doi: 10.1016/s0014-2999(02)01608-4. [DOI] [PubMed] [Google Scholar]
- 17.Sheng Y, Pero R.W, Amiri A, Bryngelsson C. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998;18(5A):3363–3368. [PubMed] [Google Scholar]
- 18.Riva L, Coradini D, Di Fronzo G, et al. The antiproliferative effects of Uncaria tomentosa extracts and fractions on the growth of breast cancer cell line. Anticancer Res. 2001;21(4A):2457–2461. [PubMed] [Google Scholar]
- 19.Park K.Y, Jung K.O, Rhee S.H, Choi Y.H. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res. 2003;523–524:43–53. doi: 10.1016/s0027-5107(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 20.Villaseñor I.M, Angelada J, Canlas A.P, Echegoyen D. Bioactivity studies on β-sitosterol and its glucoside. Phytother Res. 2002;16(5):417–421. doi: 10.1002/ptr.910. [DOI] [PubMed] [Google Scholar]
- 21.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54(4):275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 22.Paniagua-Pérez R, Madrigal-Bujaidar E, Reyes C.S, Pérez G.J, Velasco M.O, Molina D. Sister chromatid exchanges produced by imipramine and desipramine in mouse bone marrow cells treated in vivo. Toxicol Lett. 2002;132(2):123–129. doi: 10.1016/s0378-4274(02)00057-7. [DOI] [PubMed] [Google Scholar]
- 23.Wolff S, Perry P. Differential Giemsa staining of sister chromatids and the study of chromatid exchanges without autoradiography. Chromosoma. 1974;48(4):341–353. doi: 10.1007/BF00290991. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-González I, Madrigal-Bujaidar E, Dorado V, Espinosa-Aguirre J.J. Inhibitory effect of naringin on the micronuclei induced by ifosfamide in mouse, and evaluation of its modulatory effect on the Cyp3a subfamily. Mutat Res. 2001;480–481:171–178. doi: 10.1016/s0027-5107(01)00197-x. [DOI] [PubMed] [Google Scholar]
- 25.Heddle J.A, Hite M, Kirkhart B, et al. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983;123(1):61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- 26.Tucker J.D, Auletta A, Cimino M.C, et al. Sister-chromatid exchange: second report of the Gene-Tox Program. Mutat Res. 1993;297(2):101–180. doi: 10.1016/0165-1110(93)90001-4. [DOI] [PubMed] [Google Scholar]
- 27.Bolognesi C, Filiberti R, Neri M, et al. High frequency of micronuclei in peripheral blood lymphocytes as index of susceptibility to pleural malignant mesothelioma. Cancer Res. 2002;62(19):5418–5419. [PubMed] [Google Scholar]
- 28.Kim M.K, Zitzmann S, Westermann F, et al. Increased rates of spontaneous sister chromatid exchange in lymphocytes of BRCA2+/− carriers of familial breast cancer clusters. Cancer Lett. 2004;210(1):85–94. doi: 10.1016/j.canlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien N.M, Callaghan C.O, Lyons N.M, Woods J.A. Biological effects of dietary cholesterol oxidation products. Irish J Agric Food Res. 2000;39:265–273. [Google Scholar]
- 30.Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol (Tokyo) 2003;49(4):277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]