Abstract
The aim of this study was to test the hypothesis that the dorsal hippocampus plays a critical role in pontine-wave (P-wave) generator activation-dependent memory processing of two-way active avoidance (TWAA) learning. To achieve this objective, rats were given small bilateral lesions in the CA1, dentate gyrus (DG), or CA3 region of the dorsal hippocampus by microinjecting ibotenic acid. After recovery, lesioned and sham-lesioned rats were trained on a TWAA learning paradigm, allowed a 6-hr period of undisturbed sleep, and then were tested on the same TWAA paradigm. It was found that lesions in the CA3 region impaired retention of avoidance learning. Conversely, lesions in the CA1 and DG regions had no effect on TWAA learning retention. None of the groups showed any changes in the baseline sleep–wake cycle or in the acquisition of TWAA learning. All rats showed increased rapid eye movement (REM) sleep and increased REM sleep P-wave density during the subsequent 6-hr recording period. Impaired retention in the CA3 group occurred despite an increase in REM sleep and P-wave density, suggesting that during REM sleep, the P-wave generator interacts with the CA3 region of the dorsal hippocampus to aid in consolidation of TWAA learning. The results of the present study thus demonstrate that P-wave generator activation-dependent consolidation of memory requires an intact CA3 subfield of the dorsal hippocampus. The results also provide evidence that under mnemonic pressure, the dorsal hippocampus may not be involved directly in regulating the sleep–wake cycle.
Keywords: REM sleep, two-way active avoidance, learning and memory, excitotoxic lesion, retention
Many behavioral studies involving learning and memory in humans and animals provide considerable evidence to support the hypothesis that post-training sleep is critical for consolidation and integration of memories (reviewed in Hennevin et al., 1995; Smith, 1995; Graves et al., 2001; Datta and Patterson, 2003; Maquet et al., 2003; Pavlides and Ribeiro, 2003). Although the neurobiological mechanisms of memory processing during sleep are not yet fully understood, it is hypothesized that adaptive information acquired during wakefulness is actively restructured and strengthened by activation of the sleep-generating network during the subsequent sleep period (Datta and Patterson, 2003).
Based on patterns of brain electrical activity, eye movements, and muscle tone, sleep in mammals can be broadly divided into rapid eye movement (REM) sleep, also known as paradoxical sleep (PS), and non-rapid eye movement (non-REM) sleep, also known as slow-wave sleep (SWS; for review, see Datta, 1995). Although there is an ongoing debate concerning the different contribution of REM sleep and SWS to memory consolidation in humans, sleep and learning studies in animals have demonstrated that memory consolidation after task training requires processes selectively active during REM sleep (Smith, 1995; Datta, 2000; Datta and Patterson, 2003; Datta et al., 2004).
A study looking at the possible mechanisms of REM sleep-dependent memory processing found that two-way active avoidance (TWAA) training trials increase post-training REM sleep phasic pontine wave (P-wave) density (Datta, 2000). Furthermore, it was found that improvement of TWAA learning performance on test trials is directly proportional to this increase in P-wave density (Datta, 2000). The P-wave generator’s role in memory processing is demonstrated further by studies showing that after TWAA training trials, immediate supplemental activation of the P-wave generator significantly increases memory retention (Mavanji and Datta, 2003) and that activation of the P-wave generator prevents REM sleep deprivation-induced TWAA learning impairment (Datta et al., 2004). More recently, we have shown that the selective elimination of cell bodies of the P-wave generator prevents retention of TWAA learning memory (Mavanji et al., 2004). Based on the above evidence, we hypothesized that the P-wave generator in the brainstem may act as an on switch to provide activating input to forebrain structures for sleep-dependent memory processing.
The P-wave, field potentials that reflect a phasic activation of a specific group of glutamatergic cells, is generated in the pons during REM sleep and part of SWS (Datta and Hobson, 1994, 1995; Datta, 1997; Datta et al., 1998). It has been shown that the functionally identified P-wave-generating cells project to the hippocampus and the amygdala (Datta et al., 1998). Based on neurobehavioral studies and special architectural features, the dorsal hippocampus (DH) has long been considered an ideal structure for temporally integrating the sequence of events that ultimately forms a memory (O’Keefe and Nadel, 1978; Squire, 1992; Moser et al., 1993; Hock and Bunsey, 1998; Bannerman et al., 1999; Alreja et al., 2000; Morris et al., 2003). A number of physiological and biochemical studies have demonstrated that during post-training REM sleep periods the hippocampus is reactivated (Pavlides and Winson, 1989; Poe et al., 2000; Laureys et al., 2001; Ribeiro et al., 2002). Based on this evidence, it is reasonable to speculate that during REM sleep, the activated P-wave generator reactivates the hippocampal memory system, aiding in the consolidation and retention of memory.
The present study was designed to identify specific dorsal hippocampal subfields that may be involved in P-wave generator activation-dependent memory processing of TWAA learning. To identify this, we microinjected ibotenic acid to specifically lesion cell bodies of the CA1, CA3, or dentate gyrus (DG) subfields of the DH in different animals. This study also sought to determine whether the DH is involved in the regulation of the sleep–wake cycle.
MATERIALS AND METHODS
Subjects and Housing
Adult male Sprague-Dawley rats (n = 32; Charles River, Wilmington, MA) weighing 200–300 g were used as the experimental animals. These rats were housed individually at 24°C with free access to food and water. Lights were on from 7:00 am to 7:00 pm (light cycle) and off from 7:00 pm to 7:00 am (dark cycle). Principles for the care and use of laboratory animals in research, as outlined by the National Institute of Health (1985), were strictly followed.
Surgical Procedures for the Implantation of Electrodes and Guide Tubes
Treatment of the animals and surgical procedures were in accordance with the approved institutional animal welfare protocol (AN-14085). Rats were anesthetized with pentobarbital (40 mg/kg, intraperitoneally), placed in the stereotaxic apparatus (David Kopf, model 1730; Tjunga, CA) and secured using blunt rodent ear bars. Using sterile procedures, cortical electroencephalogram (EEG), dorsal neck muscle electromyogram (EMG), electrooculogram (EOG), and pontine EEG (to record P-waves) electrodes were chronically implanted for recording polygraphic signs of the sleep–wake cycle, as described elsewhere (Datta, 2000). Before the electrode implantation procedure, the rats were divided randomly into four groups: control/sham-lesioned (S-L), CA1-lesioned (CA1-L), CA3-lesioned (CA3-L), and dentate gyrus-lesioned (DG-L). In the CA1-L group of rats (n = 8), bilateral stainless steel guide tubes (26 gauge) with equal-length stylets inside were stereotaxically (Paxinos and Watson, 1997) implanted 2 mm above the CA1 field of the DH (in relation to Bregma [in mm]: posterior, 3.80; lateral, 2; and horizontal/dorsoventral, 2.5). Similarly, in the CA3-L (n = 8) and the DG-L (n = 8) groups, bilateral stainless steel guide tubes were implanted 2 mm above the CA3 (Bregma [mm]: posterior, 3.80; lateral, 3.8; and dorsoventral, 3.2) or the DG (Bregma [mm]: posterior, 3.80; lateral, 2; and dorsoventral, 3.1) fields of the DH. Half of the rats in the S-L group (n = 4) were implanted with bilateral guide tubes in the DG and the other half (n = 4) were implanted with bilateral guide tubes in the CA3. All electrodes and guide tubes were secured to the skull with dental acrylic. After a postsurgical recovery period of 3–7 days, rats were habituated to a sound-attenuated recording cage, a shuttle box, and the freely moving recording conditions for 7 days, as detailed previously (Datta et al., 2004).
Intracerebral Microinjection System
The microinjection system consisted of a 32-gauge stainless steel injector cannula that extended 2.0 mm beyond the implanted guide tube. The injector cannula was fitted with a 26-gauge collar that was attached to a 1.0-μl motor-driven microsyringe with PE 20 tubing. After filling the injection system with ibotenic acid or saline, a small air bubble was introduced into the PE tubing to monitor the movement of the fluid during the injection.
Avoidance Learning
The apparatus was an automated two-way shuttle scan shock-avoidance box (45.7 × 20.3 × 30.5 cm) with sides made of high-grade acrylic (Shuttle-flex test chamber, model SF II; AccuScan Instruments Inc., Columbus, OH). This apparatus has been described in detail previously (Datta, 2000; Mavanji and Datta, 2003, Datta et al., 2004). For training, the rat was placed in one compartment of the apparatus and given 15 min of acclimatization before training trials began. During acclimatization and the training trials, the rat could move freely from one compartment to the other within the shuttle box. Each rat was trained on a massed 30-trial shuttle box TWAA task. The procedure for the conditioned stimulus (CS) and unconditioned stimulus (UCS) was as follows. A tone (3,600 Hz; 65 dB) and a pulsatile light (2.5 Hz) were presented in the compartment with the animal as the CS and were paired 5 sec later with 0.8-mA scrambled foot shock (UCS) delivered through the floor grid. To avoid receiving a foot shock, the rat had 5 sec to move to the opposite compartment. If the animal did not move to the other compartment, UCS was delivered for a maximum of 5 sec. and CS ended with UCS. While receiving UCS, if the animal moved to the other compartment, both CS and UCS ended immediately. The intertrial interval was variable with a mean of 60 sec.
Experimental Design
After postsurgical recovery and habituation sessions for 10–14 days, all rats underwent two sessions of baseline recording for electrode testing and additional habituation with recording setup. Because all 32 rats exhibited good quality recordings, all rats were used for further experimentation. After these two baseline recordings, the rats underwent one session of final baseline recording. On these baseline recordings days, rats were placed in the shuttle box for 45 min (9:00–9:45 am) and then transferred to a recording cage for 6 hr of polygraphic recordings (10:00 am to 4:00 pm). A day after the final baseline recording (hereafter labeled as the first baseline recording), the CA1-L, CA3-L, and DG-L groups were bilaterally microinjected through the chronically implanted guide tubes with ibotenic acid solution (10 mg/ml; 50 nl/side) to produce bilateral lesions selectively in CA1, CA3, or DG field of the DH. Similarly, the S-L group was bilaterally microinjected with saline as a vehicle control (50 nl/side). A week after the lesioning, all rats underwent three more sessions of baseline recording (hereafter labeled as postlesion baseline recording), to see if any changes occurred in the sleep–wake cycle after lesioning the CA1, CA3, or DG area of the DH. On the postlesion baseline recordings days, rats were placed in the shuttle box for 45 min (9:00–9:45 am) and then transferred to a recording cage for 6 hr of polygraphic recordings (10:00 am to 4:00 pm). The day after the completion of postlesion baseline recordings, the rats were placed in the shuttle box at 9:00 am, and after 15 min of acclimatization, CS–UCS trials began (training trials session). After 30 trials, rats were transferred to the polygraphic recording cage. They were then recorded for a 6-hr session (10:00 am to 4:00 pm) of undisturbed sleep-wakefulness (posttraining recording session). At the end of the 6-hr recording, animals were tested on the CS–UCS learning trials (test trials session, between 4:05 and 4:50 pm).
Histologic Localization and Measurement of the Lesion Site
At the end of all experimental recording sessions, the rats were deeply reanesthetized with pentobarbital (60 mg/kg, intraperitoneally) and perfused transcardially with heparinized cold phosphate buffer (0.1 M; pH 7.4) followed by 4% para-formaldehyde in 0.1 M phosphate buffer. The brains were then removed and processed for staining and histologic localization of the lesion sites. The sections were examined under a microscope (Eclipse E800; Nikon Corp., Tokyo, Japan) and images were captured using a color CCD camera (Optronics, Goleta, CA) using Image-Pro Plus v5.1 imaging software (Media Cybernetics, Inc. Silver Spring, MD). From these digitized images, using the mathematical formula of a sphere and Image-Pro Plus software, the total surface area of cell loss by the microinjection of ibotenic acid was calculated.
Determination of Behavioral States and Data Analysis
For the purpose of determining possible effects of lesioning and training on sleep and wakefulness, three behavioral states were distinguished based on the visual scoring of polygraphic records as described earlier (Datta, 2000). Wakefulness (W), slow-wave sleep (SWS), and REM sleep were scored in successive 10-sec epochs. The polygraphic measures provided the following dependent variables that are quantified for each recording session: (1) percentage of recording time spent in W, SWS, and REM sleep; (2) latency to onset of the first REM sleep episode; (3) total number of REM sleep episodes; (4) mean duration of REM sleep episodes; and (5) P-wave density (waves/min) during REM sleep. For latency analysis, the data collection began immediately after connecting the animal to the polygraph. All variables from the first baseline-recording session (before the lesioning) were analyzed using a one-factor analysis of variance (ANOVA) between control and experimental groups, using StatView statistical software (Abacus Concepts, Inc., Berkeley, CA) as described previously (Datta, 2000; Datta et al., 2004). These analyses were carried out to be sure that control (S-L) and experimental (CA1-L, CA3-L, and DG-L) groups were not different before the lesionings. Similarly, all variables from the final postlesion baseline-recording day (before the shuttle box trials) were analyzed using a one-factor ANOVA (between S-L, CA1-L, CA3-L, and DG-L groups). These analyses were carried out to be sure that the control group (S-L) and the lesioned groups were not different before the learning trials. All of the above variables recorded during the first baseline, postlesion baseline, and posttraining sessions were analyzed for a 6-hr recording period using a one-factor ANOVA to determine the effect of avoidance learning and lesioning the CA1, CA3, and DG on sleep–wake variables. After ANOVA, post-hoc Scheffe’s F-tests were done to determine the individual levels of significant difference between the groups.
Analysis of Avoidance-Learning Data
All four groups (S-L, CA1-L, CA3-L, and DG-L) of rats were subjected to two sessions of learning trials. The first (training trials session) occurred before sleep recording and the second (test trials session) occurred after a 6-hr session of undisturbed polygraphic sleep recording. For each of the two sessions, the percentage of conditioned avoidance responses was calculated for each rat in each of the six blocks of trials and then averaged across animals. The percentage of avoidance data from the training trials were subjected to a two-way ANOVA (group × blocks) to test whether learning rates in the initial training period were different between groups. Next, the percentage of avoidance data from the training trials and test trials sessions of the sham-lesioned and lesioned groups was analyzed using a two-way ANOVA (trial × block) with block as a repeated measure. A post-hoc Scheffe’s F-test was then carried out to determine the level of significance in the individual blocks. These analyses were carried out to determine the differences in learning curves between the training trials session and the test trials session. The percentages of avoidance in control and lesioned groups’ test trials were also compared (Scheffe’s F-test) to see if there was any effect on the learning curve after lesioning different subfields of the DH.
RESULTS
Histologic Changes After Microinjection of Ibotenic Acid or Control Saline Into the Different DH Subfields
Bilateral microinjections of ibotenic acid (0.5 μg in 50 nl) into the CA1, CA3, and DG of the DH affected a spherical area in and around the injection sites. This was determined by the disappearance of neurons and the presence of gliosis (Fig. 1). Histologic examination revealed that all microinjection sites were within the targeted subfields (CA1, CA3, and DG) of the DH. The total surface areas (n = 8 sites; mean ± standard deviation [SD] mm2) of lesions on the left side of CA1 (1.50 ± 0.39 mm2), CA3 (1.01 ± 0.37 mm2), and DG (0.79 ± 0.15 mm2) were comparable (Scheffe’s F-tests) to the total surface areas of lesions in the right side of CA1 (1.48 ± 0.41 mm2), CA3 (0.98 ± 0.28 mm2), and DG (0.75 ± 0.19 mm2), respectively. The combined (left and right side) total surface areas of lesions in the CA1 (2.98 ± 0.79 mm2), CA3 (1.99 ± 0.61 mm2), and DG (1.54 ± 0.32 mm2), however, were significantly different from each other (one-way ANOVA; F2,21 = 11.998; P < 0.01). Post-hoc tests (Scheffe’s F-test) revealed that the total area of lesions in the CA1 was significantly larger than was the total area of lesions in the CA3 (F = 5.38; P < 0.05) and the total area of lesions in the DG (F = 11.48; P < 0.001). The total area of lesions in the CA3 was also slightly larger than was the total area of lesions in the DG, but this size difference was not statistically significant (F = 1.14). Bilateral microinjections of equal volume (50 nl) of control saline into the CA3 and the DG of the DH did not cause any apparent changes in the shape of neurons in and around the injection sites.
Fig. 1.
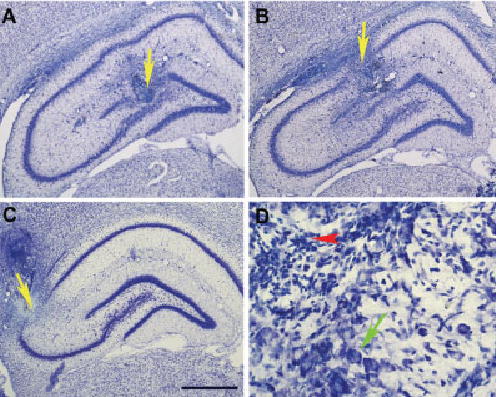
Examples of histologic localization of ibotenic acid microin-jection-induced lesion sites in different subfields of the dorsal hippocampus. A: A representative coronal section from a rat brain showing an ibotenic acid microinjection-induced lesion in the dentate gyrus (yellow arrow). B: A representative coronal section showing an ibotenic acid microinjection-induced lesion in the CA1 (yellow arrow). C: A representative coronal section showing an ibotenic acid micro-injection-induced lesion in the CA3 (yellow arrow). D: A magnified photomicrograph of part of the ibotenic acid diffusion site in the CA3 subfield demonstrating the cell loss caused by ibotenic acid microinjection. The red arrowhead points to the core of the ibotenic acid diffusion site showing the invasion of glial cells caused by the degeneration of neuronal cells. Note the presence of normal intact cell bodies (green arrow in D) about 400 μm away from the center of the lesion site. Scale bar = 1 mm (A–C); 200 μm (D).
Effects of DH Lesions on Wake, SWS, REM Sleep, and P-Wave Density
In the first baseline recording session, before microinjections of ibotenic acid or saline into the three different subfields of DH, the total percentages of time spent in W, SWS, and REM sleep and P-wave density were not significantly different (one-factor ANOVA) between the S-L, CA1-L, CA3-L, and DG-L groups (Fig. 2). Before lesioning, the groups were thus equal in terms of time spent in W, SWS, and REM sleep and P-wave density during the 6-hr baseline recording session. Even after elimination of cells from the different DH subfields, during the 6-hr recording session the total percentages of time spent in W, SWS, and REM sleep and P-wave density were not significantly different between S-L, CA1-L, CA3-L, and DG-L groups (one-factor ANOVA; Fig. 2). Similarly, individual post-hoc comparisons (Scheffe’s F-tests) of the total percentage of time spent in W, SWS, and REM sleep, and REM sleep P-wave density revealed no significant difference between baseline recording sessions before and after lesioning. These results indicate that even after receiving a sham or ibotenic acid lesion in different subfields of the DH, the groups remained equal in terms of time spent in W, SWS, and REM sleep, and P-wave densities (Fig. 2).
Fig. 2.
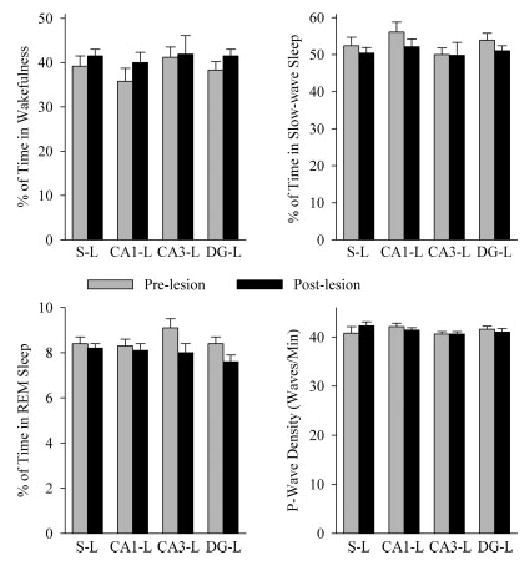
The effects of lesioning different subfields of the dorsal hippocampus on sleep–wake parameters. Bars represent the total percentage (mean and SEM) of time spent in wakefulness, slow-wave sleep, or rapid eye movement (REM) sleep, or the pontine-wave (P-wave) density during REM sleep in the 6-hr sleep–wake recording sessions before (light gray bars) and after (dark gray bars) lesioning. Note that ibotenic acid microinjection-induced lesions and sham-lesions did not change the total percentage of wakefulness, slow-wave sleep, or REM sleep, nor did it change REM sleep P-wave density. S-L, sham lesioned group; CA1-L, CA1 lesioned group; CA3-L, CA3 lesioned group; DG-L, dentate gyrus lesioned group.
Effects of TWAA Learning Training Trials on Sleep–Wake States and REM Sleep P-Wave Density
The effects of TWAA learning training on the percentages of time spent in W, SWS, and REM sleep and REM sleep P-wave density in the S-L, CA1-L, CA3-L, and DG-L groups are summarized in Figure 3 and 4. The sleep–wake parameters before and after lesioning were comparable; thus, for convenience, only the postlesion baseline values were considered for the next steps of statistical analysis. One-factor ANOVA on the total percentages of W, SWS, and REM sleep of eight different conditions (1, S-L baseline; 2, S-L posttraining; 3, CA1-L baseline; 4, CA1-L posttraining; 5, CA3-L baseline; 6, CA3-L posttraining; 7, DG-L baseline; and 8, DG-L posttraining) revealed a significant difference on the total percentage of time spent in REM sleep (F = 16.132; P < 0.001), but not on the total percentage of time spent in W (F = 1.395; P = 0.198) and SWS (F = 1.412; P = 0.191). Post-hoc analysis (Scheffe’s F-test) revealed that compared to the baseline recording session, the total percentage of time spent in REM sleep during the posttraining recording session is significantly higher in the S-L (67.1% higher; F = 10.09; P < 0.001), CA1-L (71.6% higher; F = 11.9; P < 0.001), CA3-L (78.8% higher; F = 12.8; P < 0.001), and DG-L (82.9% higher; F = 13.7; P < 0.001) groups. These results showed that after avoidance-learning training trials, all animals spent more time in REM sleep. Post-hoc analysis also showed that the avoidance training trial-induced increase in the total percentage of REM sleep between S-L, CA1-L, CA3-L, and DG-L groups was not significantly different (Fig. 4). These results indicated that an ibotenic acid lesion in the CA1, CA3, or DG region of the hippocampus did not affect TWAA training trial-induced increases in REM sleep.
Fig. 3.
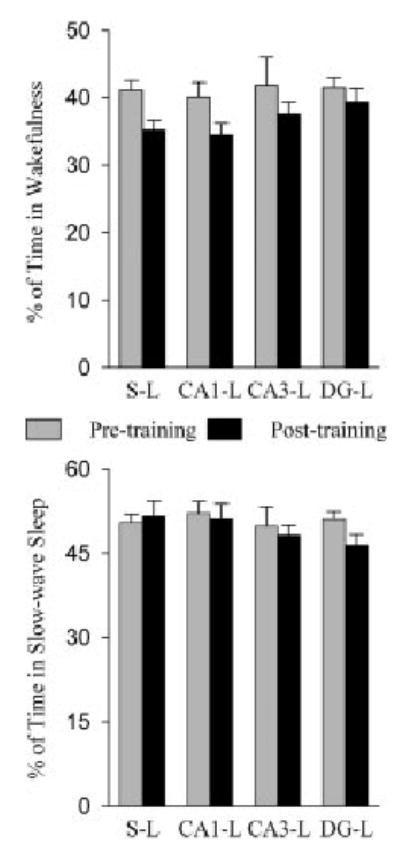
Effects of two-way active avoidance (TWAA) training trials on wakefulness and slow-wave sleep in rats with sham-lesions and lesions in the different subfields of the dorsal hippocampus. Bars represent the total percentage (mean and SEM) of time in wakefulness or slow-wave sleep during the 6-hr sleep recording sessions. Note that the percentages of wakefulness and slow-wave sleep during the 6-hr sleep–wake recording session after training trials are comparable to the percentages of wakefulness and slow-wave sleep during the baseline recording session. Abbreviations are the same as in Figure 2.
Fig. 4.
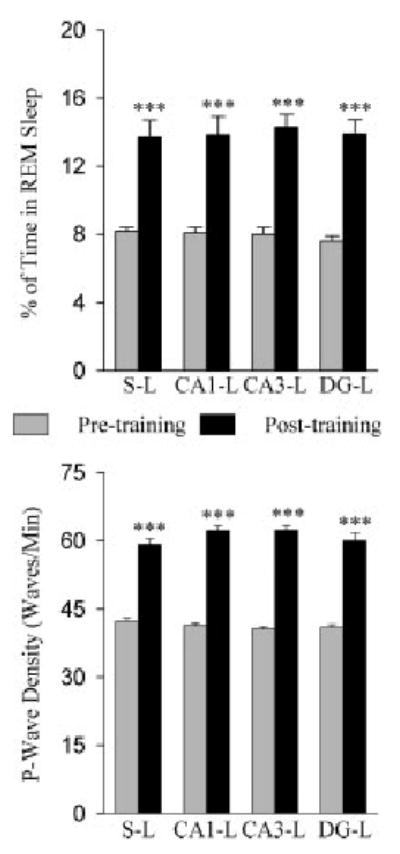
Effects of two-way active avoidance (TWAA) training trials on rapid eye movement (REM) sleep and REM sleep pontine-wave (P-wave) density in animals with sham-lesions and lesions in the different subfields of the dorsal hippocampus. In the upper panel, bars represent the total percentage (mean and SEM) of time spent in REM sleep during the 6-hr sleep recording session. In the lower panel, bars represent the REM sleep P-wave density (mean and SEM) during the 6-hr recording session. Note that the percentage of REM sleep and the percentage of P-wave density are significantly higher in the recording session after learning training trials than they were during the baseline recording session. Post-hoc tests (Scheffe’s F-test); ***P < 0.001 for comparison between baseline and post-training recording sessions.
Having documented an increase in the total percentage of REM sleep after the learning trials, we next quantified the latency, number of episodes, and duration of REM sleep episodes for each recording session. One-factor ANOVA identified a significant effect on the latency (F = 6.428; P < 0.001) and number (F = 5.152; P < 0.01) of REM sleep episodes. No effect was found in the duration of REM sleep episodes (F = 1.038; P = 0.421). Post-hoc analysis (Scheffe’s F-test) showed that compared to the baseline, in the posttraining recording session the latency of the first REM sleep episode was reduced significantly in the S-L (15.8 ± 2.9 min reduced; F = 4.80; P < 0.01), CA1-L (17.3 ± 3.5 min reduced; F = 3.9; P < 0.01), CA3-L (16.4 ± 2.4 min reduced; F = 5.48; P < 0.01), and DG-L (19.7 ± 3.0 min reduced; F = 4.70; P < 0.01) groups. When the posttraining REM sleep latency was compared between the S-L (49.38 ± 1.8 min), CA1-L (54.9 ± 3.0 min), CA3-L (50.8 ± 2.6 min), and DG-L (54.8 ± 3.9 min) groups, the differences were not statistically significant. The mean number of REM sleep episodes between baseline and posttraining recording sessions was significantly different in the S-L (baseline vs. post-training; mean ± standard error of the mean [SEM], 16.3 ± 0.6 vs. 23.9 ±1.5; P < 0.01), CA1-L (15.01 ± 1.0 vs. 24.3 ±1.8; P < 0.01), CA3-L (15.4 ± 0.8 vs. 26.2 ± 0.9; 0.01), and DG-L (14.8 ± 0.9 vs. 24.0 ± 1.2; P < 0.01) groups. Like the ANOVA, post-hoc analysis also showed that the mean duration of REM sleep episodes between baseline recordings and posttraining recordings of the different groups were not significantly different. These results suggest that the increase in the total percentage of time spent in REM sleep after avoidance-learning training trials is due mainly to the early initiation and increased number of REM sleep episodes.
To calculate the P-wave density (waves/min), P-waves were counted during REM sleep episodes of each recording session and then expressed as mean P-wave density (waves/min). These results are summarized in Figure 2 and 4. One-way ANOVA on eight different conditions (1, S-L baseline; 2, S-L posttraining; 3, CA1-L baseline; 4, CA1-L posttraining; 5, CA3-L baseline; 6, CA3-L posttraining; 7, DG-L baseline; and 8, DG-L post-training) shows that there was a significant effect of condition (F = 121.19; P = 0.0001). Post-hoc analysis (Scheffe’s F-test) revealed that in all four groups (S-L, CA1-L, CA3-L, and DG-L), P-wave density during the posttraining recording session was significantly higher than it was during the baseline recording session (Fig. 4). These post-hoc tests also showed that after training trials, P-wave densities of the S-L, CA1-L, CA3-L, and DG-L groups were comparable to each other (Fig. 4). These results indicate that the TWAA training trials equally increased P-wave density in all four groups.
Effects of CA1, CA3, or DG Lesions on Avoidance-Learning
Performance on the shuttle box avoidance task is shown in Figure 5. The percentage of avoidance data during the training session showed no significant variation between S-L, CA1-L, CA3-L, and DG-L rats (two-way ANOVA, group × block). The groups were thus equal in terms of TWAA learning performance during the training trials. Next, avoidance data from the test trial sessions, together with the data from the training trial sessions, were subjected to two-way ANOVA. Two-way ANOVA indicated a significant main effect of session (F7,56 = 10.542; P = 0.0001), block number (F5,280 = 88.98; P = 0.0001), and interaction between session and block number (F7,35 = 1.5; P = 0.0472). A significant effect of session indicated that there was a difference in the percentage of avoidance between the training session and the test session.
Fig. 5.
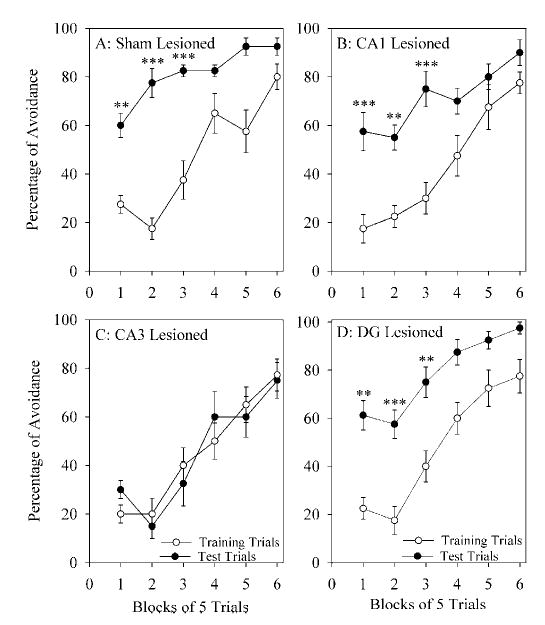
Learning curves of the dorsal hippocampus sham-lesioned and lesioned rats. Learning curves for the two-way active avoidance (TWAA) task before (training trials session; empty circle) and after (test trials session; filled circle) 6 hr of undisturbed sleep in the sham-lesioned (A), CA1 lesioned (B), CA3 lesioned (C), and DG lesioned (D) rats. The percentage of successful avoidance (mean and SEM) is plotted for every consecutive six blocks of five trials. Note that the percentages of avoidance in the training trials are comparable between sham, CA1, CA3, and DG lesioned rats. In the first three blocks of test trails, sham-lesioned, CA1 lesioned, and DG lesioned rats’ percentages of avoidance were significantly higher than was the percentage of avoidance in the first three blocks of training trials. In the CA3 lesioned rats, however, the percentages of avoidance during the test trials were not significantly different from those during the training trials. Post-hoc tests (Scheffe’s F-test); **P < 0.01, ***P < 0.001 for the comparison between training and test trials.
Post-hoc statistical analysis (Scheffe’s F-test) showed that the percentage of avoidance in the test session of the S-L group was significantly higher in the first (118.2% higher; F = 8.51; P < 0.01), second (342.9% higher; F = 20.30; P < 0.001), and third (120.0% higher; F = 9.01; P < 0.01) blocks of trials than it was in the first, second, and third blocks of the training trials session (Fig. 5A). The percentage of avoidance in the control group was also higher in the fourth (26.9% higher), fifth (60.9% higher), and sixth (15.6% higher) blocks of the test session than in the corresponding blocks of the training session; however, these differences did not reach statistical significance. Similarly, in the CA1-L group, the percentage of avoidance in the test trials was significantly higher in the first (228.6% higher, F = 18.10;P < 0.001), second (144.4% higher, F = 11.20; P < 0.01), and third (150.0% higher, F = 13.25; P < 0.01) blocks than it was in the training trials session (Fig. 5B). The percentage of avoidance in the CA1-L group during the fourth (47.4% higher), fifth (18.5% higher), and sixth (16.1% higher) blocks of the test trials session was also higher than were the corresponding blocks of the training trials session, but these differences did not reach statistical significance. In the CA3-L group, the percentage of avoidance between training trials and test trials was not significantly different (Fig. 5C). As in the S-L and CA1-L groups, the percentage of avoidance in the DG-L group was significantly higher in the first (172.2% higher; F = 15.05; P < 0.01), second (228.6% higher; F = 18.35; P < 0.001), and third (87.5% higher; F = 4.28; P < 0.05) blocks of the test trials than it was in the training trials (Fig. 5D). The percentage of avoidance was also higher in the fourth (45.83% higher), fifth (27.6% higher), and sixth (25.81% higher) blocks of the test trials session, but these differences did not reach statistical significance. No significant differences were found in the percentage of avoidance during the test trials between the S-L, CA1-L, and DG-L groups (Scheffe’s F-test). These post-hoc tests also showed that the percentage of avoidance during the test trials was significantly less for the CA3-L group than it was for all other groups across each of the six blocks of trials. These results indicate that lesioning the CA3 region of the dorsal hippocampus (CA3-L) prevents memory retention.
DISCUSSION
The principle findings of the present study are: (1) small bilateral lesions in the CA1, CA3, or DG sub-fields of the DH did not alter the total percentage of time spent in W, SWS, or REM sleep nor did it alter the REM sleep P-wave density during the 6-hr polygraph recording sessions; (2) after a session of TWAA training, both lesioned and sham-lesioned rats spent more time in REM sleep compared to their baseline; (3) training increased P-wave density in all groups of rats, regardless of the site of lesion in the DH; (4) lesions in the CA1, DG, or CA3 regions of the DH did not alter the acquisition of TWAA learning during the training or test session; (5) lesions in the CA3 region of the DH prevented retention of TWAA memory in the test trials session; and (6) similar bilateral lesions in the CA1 and DG of the DH did not alter normal retention of TWAA memory. These results suggest a strong relationship between the P-wave generator in the brainstem and the CA3 region of the DH for P-wave generator activation-dependent memory processing.
In the present study, small bilateral excitotoxic lesions in the different subfields of the DH did not produce any changes in the total percentage of time spent in W, SWS, or REM sleep, nor did it change the REM sleep P-wave density. Although there are no comparable studies that have lesioned specific subfields of the DH to study the role of the hippocampus in modulation of the sleep–wake cycle, the results of this study are consistent with a number of nonspecific lesion studies that have shown that after hippocampal lesions, sleep–wake patterns and behaviors remained intact in the rat. For example, one earlier study demonstrated that surgical ablation of the DH did not affect sleep–wake behaviors in the rat (Vanderwolf et al., 1978). Another study showed that lesioning the hippocampus and entorhinal cortex with ibotenic acid did not affect the diurnal sleep–wake cycle in the rat (Hagan et al., 1992). More recently, it has been demonstrated that injection into the dentate gyrus of the hippocampus of a noradrenergic neurotoxin that lesions noradrenergic terminals does not affect the normal REM sleep rebound effect after sleep deprivation (Charifi et al., 2001). Conversely, some older studies that used an aspiration technique to remove tissue from a large area of the brain including the hippocampus, septum, and surrounding cortical and subcortical areas in the cat, or a knife-cut lesion to damage a large area of the hippocampus and fornix in the rat, have suggested that these lesions could reduce the total time spent in SWS and REM sleep (Parmeggiani and Zanocco, 1963; Jarrard, 1968; Kim et al., 1975; Yamazaki et al., 1977). Because these hippocampal lesion studies used nonspecific lesion techniques and produced large lesions that included many other parts of the brain; these studies cannot conclude definitively that the hippocampus is involved in modulation of the sleep–wake cycle. The results of the present study suggest that the DH is not critically involved in the regulation of the sleep–wake cycle.
The present study demonstrates that TWAA training trials increase the total percentage of time spent in REM sleep in both control and lesioned animals. This increase in total REM sleep was caused by an increase in the number of REM sleep episodes. This REM sleep increase is consistent with earlier studies in which several learning tasks, including TWAA training trials, have been shown to increase the percentage of REM sleep in normal animals (Smith and Rose, 1997; Datta, 2000; Mavanji and Datta, 2003). The posttraining increase in REM sleep in the lesioned rats also indicates that lesioning different subfields of the DH does not affect the homeostatic drive for posttraining REM sleep, which has been shown to facilitate memory consolidation (Karni et al., 1994; Datta, 2000; Mavanji and Datta, 2003; Datta et al., 2004). This need for posttraining REM sleep is demonstrated by studies that show that posttraining REM sleep deprivation can partially or completely block improved performance on subsequent retesting (Smith et al., 1998; Datta et al., 2004). These animal studies provide evidence that the increase in REM sleep after acquisition is critical for learning.
The present study also demonstrates that during the posttraining sleep recording session, P-wave density in REM sleep increased significantly in both control and lesioned animals. This increased P-wave density after training trials is consistent with our earlier studies (Datta, 2000; Mavanji and Datta, 2003; Datta et al., 2004) and provides further support to the hypothesis that activation of the P-wave generator is a critical physiologic process for TWAA memory processing (Mavanji and Datta, 2003; Datta et al., 2004; Mavanji et al., 2004). In the test trials, only CA3 lesioned animals did not show any retention of TWAA memory. This lack of memory retention is seen despite the CA3 lesioned rats exhibiting increases in REM sleep and in REM sleep P-wave density comparable to the increases seen in the sham-lesioned, CA1 lesioned, and DG lesioned rats. To explain this surprising effect, we believe that in addition to the P-wave generator, an intact CA3 region of the DH is necessary for this retention process.
The hippocampus plays an important role in certain forms of memory processing including spatial and contextual learning (O’Keefe and Nadel, 1978; Squire, 1992; Morris et al., 2003). Studies addressing the role of the hippocampus have shown that the DH plays a critical role in learning and memory, whereas the ventral hippocampus modulates anxiety behavior (Moser et al., 1995; Hock and Bunsey, 1998; Bannerman et al., 2003). Recent behavioral studies have demonstrated that the processing of DH-dependent memory formation is critically dependent on REM sleep (Moser et al., 1993, Graves et al., 2001, 2003). It has been suggested that posttraining REM sleep is crucial for hippocampus dependent spatial and contextual memory processing (Graves et al., 2001, 2003). In support of this notion, studies have shown that REM sleep deprivation after training impaired hippocampal-dependent spatial memory (Smith and Rose, 1996), and that the time spent in REM sleep was increased after training in the spatial version of the Morris water maze (Smith and Rose, 1997). In addition, REM sleep deprivation can negatively affect hippocampal synaptic plasticity in the form of reduced hippocampal long-term potentiation (LTP) in rat in vitro and in vivo preparations (Davis et al., 2003; McDermott et al., 2003). Based on these studies, it is reasonable to consider the DH an important anatomic substrate for REM sleep-dependent memory processing.
The present study demonstrates that bilateral excitotoxic lesions in the CA3 subfield of the DH prevent retention, but not acquisition, of TWAA memory. These excitotoxic lesions limited cell loss to a small part of the CA3. This result can be viewed as an elaboration of earlier electrolytic lesion studies, which have shown that lesioning the CA3 area of the hippocampus impaired retention of TWAA memory (Calderazzo Filho et al., 1977, 1979). Two other studies supported this data by demonstrating that the injection of neurotoxin AF64A impaired retention of TWAA memory by damaging the CA3 area of the DH (Bailey et al., 1986; Lermontova et al., 1998). Another recent study has shown that knocking out the N-methyl-D-asparate subunit 1 (NR1) receptor in the CA3 pyramidal cells selectively disrupts LTP in the CA3 region (Kazu et al., 2002). It has also been reported that chronic stress-induced atrophy in the apical dendrites of the CA3 neurons leads to memory impairments in the rat (McEwen, 1999). Similar to the present study, it has been shown that ibotenate-induced bilateral lesions in the CA3 region of the dorsal hippocampus impaired spatial memory as tested in the Morris water maze (Aznar et al., 1998; Brun et al., 2002). The results of the present study and the studies described above support the idea that the CA3 subfield of the DH is an important anatomic site for REM sleep-dependent memory processing.
Contrary to the view that the CA3 subfield of the DH is involved in TWAA memory processing, some studies dealing with hippocampal lesions and avoidance learning behavior have indicated that shuttle box avoidance learning is enhanced after hippocampal damage (summarized in O’Keefe and Nadel, 1978). Some of these studies, however, considered the hippocampus, for-nix, and entorhinal cortex to be a unitary structure and to lesion the hippocampus, many of these studies used electrolytic lesions that damaged a large area, or a suction method that removed a considerable amount of overlying cortical tissue (summarized in O’Keefe and Nadel, 1978). Additionally, both of these techniques damaged the fibers of passage, resulting in hyperactivity and ultimately in increased performance in avoidance learning (Matus-Amat et al., 2004). It is also known that these types of lesions cause rats to overreact to shock, both behaviorally and in terms of heart-rate changes (Jarrard and Korn, 1969). Rats with these large hippocampal lesions exhibited strong somatomotor reactions, particularly running, in reaction to foot shock and hyperresponsiveness to all sensory stimuli. This nonspecific hippocampal lesion-induced facilitation of avoidance performance could thus be due to hyperactivity during intertrial intervals. It has been shown in recent anatomic and functional studies of the hippocampus that there are differences in function between the dorsal and ventral areas of the hippocampus as well as functional differences and different outputs of the three CA fields in the hippocampus (Bannerman et al., 1999, 2003). It thus seems that the conflicting results of these studies can be attributed to the differences between the lesion techniques. Furthermore, nonspecific lesions in the hippocampus of rats cause a significant reduction in the total sleep time (Jarrard, 1968), whereas our cell-specific small lesions do not cause any disturbances in the sleep–wake cycle.
We have demonstrated that bilateral lesions in the CA1 or DG subfields of the DH affected neither acquisition nor retention of TWAA memory. This is consistent with earlier studies, which demonstrated that the electrolytic lesions in the DG (Calderazzo Filho et al., 1979) and CA1 subfield of the DH (Calderazzo Filho et al., 1977; Hagan et al., 1988) did not affect performance of avoidance learning during the test trials. Another study has shown, however, that the application of colchicine, a neurotoxin that blocks mitosis and axoplasmic transport, into the DG enhances memory for TWAA learning (McLamb et al., 1988). The effect of colchicine application in this study could be due to the functional degeneration of axons and passing fibers, rather than the degeneration of granule cells in the DG. It is thus reasonable to suggest that, unlike cell-specific lesions in the CA3, these small cell-specific lesions within the CA1 or DG of the DH were unable to block acquisition and retention of TWAA learning and memory. We also acknowledge that, based on the present study, we are unable to predict whether a bigger lesion in the CA1 or DG would prevent retention of TWAA memory.
To produce a localized lesion in the different sub-fields of the DH, we microinjected a small amount of ibotenic acid directly into the CA1, CA3, or DG of the DH. Although the volume and concentration of ibotenic acid microinjections in the CA1, CA3, and DG were identical, the total areas of the lesions in the CA1 were significantly larger than were those in the CA3 and DG. The fact that these ibotenic acid microinjection-induced lesions were larger in the CA1 was not surprising because it is well known that the CA1 cells are relatively more vulnerable to neurotoxins and many other neuro-degenerative disorders (Mattson and Kater, 1989; Wilde et al., 1997). The CA1 is more vulnerable because the availability of superoxide dismutase and brain-derived neurotrophic factor (BDNF) in the CA1 are relatively less compared to that in the CA3 (Matsuyama et al., 1994; Kato et al., 1995; Yan et al., 1997). Because the total area of the ibotenic acid microinjection-induced lesions were different in different subfields of the DH, is it possible that the deficit in the memory retention of CA3 lesioned rats was mainly due to the differences in lesion size? For the following reason, we believe that this is not likely to be the explanation for the retention deficit observed in the CA3 lesioned rats. Because the total area of the ibotenic acid microinjection-induced lesions in the CA1 were larger than those in the CA3, and lesions in the CA3 were larger than the lesions in the DG were, it is highly unlikely that this CA3 lesion-induced deficit in TWAA memory retention was due mainly to the differences in lesion size.
At present, knowledge of the REM sleep-dependent memory processing neuronal network remains incomplete. Nevertheless, the observation that an intact CA3 subfield of the DH is critical for the retention of P-wave generator activation-dependent TWAA memory is a significant advancement toward a complete understanding of REM sleep-dependent memory processing. Based on the present and earlier findings, we believe that the activation of P-wave generating cells in the brainstem during REM sleep provides a glutamatergic-activating stimulus to the CA3 subfield of the DH causing physiologic reactivation, activation-dependent gene expression, and protein synthesis for long-term neuronal plasticity and long-term memory formation.
Acknowledgments
We thank Drs. J. Luebke and J. Ulloor for helpful discussions and suggestions. We also thank S.M. Datta for statistical analysis and for writing system software.
References
- Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implications for learning and memory. J Neurosci. 2000;20:8103–8110. doi: 10.1523/JNEUROSCI.20-21-08103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Rasmussen T, Zimmer J. Impaired learning correlates with size of excitotoxic hippocampal CA3 lesions in adult rats, but shows no amelioration by CA3 transplants. Restor Neurol Neurosci. 1998;13:141–151. [PubMed] [Google Scholar]
- Bailey EL, Overstreet DH, Crocker AD. Effects of intrahippocam-pal injections of the cholinergic neurotoxin AF64A on open-field activity and avoidance learning in the rat. Behav Neural Biol. 1986;45:263–274. doi: 10.1016/s0163-1047(86)80015-2. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Calderazzo Filho LS, Cavalheiro EA, Izquierdo I. Effect of lesions in hippocampal subareas on rat shuttle behavior. Physiol Behav. 1979;23:989–993. doi: 10.1016/0031-9384(79)90286-5. [DOI] [PubMed] [Google Scholar]
- Calderazzo Filho LS, Moschovakis A, Izquierdo I. Effect of hippo-campal lesions on rat shuttle responses in four different behavioral tests. Physiol Behav. 1977;19:569–572. doi: 10.1016/0031-9384(77)90236-0. [DOI] [PubMed] [Google Scholar]
- Charifi C, Debilly G, Paut-Pagano L, Cespuglio R, Valatx JL. Effect of noradrenergic denervation of medial prefrontal cortex and dentate gyrus on recovery after sleep deprivation in the rat. Neurosci Lett. 2001;311:113–116. doi: 10.1016/s0304-3940(01)02148-6. [DOI] [PubMed] [Google Scholar]
- Datta S. Neuronal activity in the peribrachial area: relationship to behavioral state control. Neurosci Biobehav Rev. 1995;19:67–84. doi: 10.1016/0149-7634(94)00043-z. [DOI] [PubMed] [Google Scholar]
- Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–365. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Hobson AJ. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson AJ. Suppression of ponto-geniculo-occipital waves by neurotoxic lesions of pontine caudo-lateral peribrachial cells. Neurosci. 1995;67:703–712. doi: 10.1016/0306-4522(95)00081-s. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH. 2003. Activation of phasic pontine wave (P-wave): a mechanism of learning and memory processing. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. New York: Oxford University Press. p 135–156.
- Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;30:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Jansen JH, Broekkamp CL. Selective behavioural impairment after acute intoxication with trimethyltin (TMT) in rats. Neurotoxicology. 1988;9:53–74. [PubMed] [Google Scholar]
- Hagan JJ, Verheijck EE, Spigt MH, Ruigt GS. Behavioural and electrophysiological studies of entorhinal cortex lesions in the rat. Physiol Behav. 1992;51:255–266. doi: 10.1016/0031-9384(92)90139-s. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep. Relevance for memory. Behav Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Behavior of hippocampal lesioned rats in home cage and novel situations. Physiol Behav. 1968;3:65–70. [Google Scholar]
- Jarrard LE, Korn JH. Effects of hippocampal lesions on heart rate during habituation and passive avoidance. Commun Behav Biol. 1969;3:141–150. [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Araki T, Liu XH, Kato K, Itoyama Y. Immunohistochemical localization of superoxide dismutase in the hippocampus following ischemia in a gerbil model of ischemic tolerance. J Cereb Blood Flow Metab. 1995;15:60–70. doi: 10.1038/jcbfm.1995.7. [DOI] [PubMed] [Google Scholar]
- Kazu N, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Choi H, Kim CC, Kim JK, Kim MS, Park HJ, Ahn BT. Effect of hippocampectomy on sleep patterns in cats. Electroencephalogr Clin Neurophysiol. 1975;38:235–243. doi: 10.1016/0013-4694(75)90244-8. [DOI] [PubMed] [Google Scholar]
- Laureys S, Peigneux P, Phillips C, Fuchs F, Degueldre C, Aerts J, Del Fiore G, Petiau C, Luxen A, Van Der Linden M, Cleeremans A, Smith C, Maquet P. Experience-dependent changes in cerebral functional connectivity during human rapid eye movement sleep. Neuroscience. 2001;105:521–525. doi: 10.1016/s0306-4522(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Lermontova N, Lukoyanov N, Serkova T, Lukoyanova E, Bachurin S. Effects of tacrine on deficits in active avoidance performance induced by AF64A in rats. Mol Chem Neuropathol. 1998;33:51–61. doi: 10.1007/BF02815859. [DOI] [PubMed] [Google Scholar]
- Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci. 2003;23:1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T, Shimizu S, Nakamura H, Michishita H, Tagaya M, Sugita M. Effects of recombinant superoxide dismutase on manganese superoxide dismutase gene expression in gerbil hippocampus after ischemia. Stroke. 1994;25:1417–1423. doi: 10.1161/01.str.25.7.1417. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–370. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Ulloor J, Saha S, Datta S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of two-way active avoidance memory. Sleep. 2004;27:1282–1292. doi: 10.1093/sleep/27.7.1282. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McLamb RL, Mundy WR, Tilson HA. Intradentate colchicine impairs acquisition of a two-way active avoidance response in a Y-maze. Neurosci Lett. 1988;94:338–342. doi: 10.1016/0304-3940(88)90041-9. [DOI] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford: Oxford University Press.
- Parmeggiani PL, Zanocco G. A study on the bioelectrical rhythms of cortical and subcortical structures during activated sleep. Arch Ital Biol. 1963;101:385–412. [PubMed] [Google Scholar]
- Pavlides C, Ribeiro S. 2003. Recent evidence of memory processing in sleep. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. New York: Oxford University Press. p 327–362.
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1997. The rat brain in stereotaxic coordinates. New York: Academic Press.
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;11:1197–1204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- Smith C, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–217. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Kolb B, Cooley RK. Behavior of the rat after removal of the neocortex and hippocampal formation. J Comp Physiol Psychol. 1978;92:156–175. doi: 10.1037/h0077447. [DOI] [PubMed] [Google Scholar]
- Wilde GJ, Pringle AK, Wright P, Lannotti F. Differential vulnerability of the CA1 and CA3 subfields of the hippocampus to superoxide and hydroxyl radicals in vitro. J Neurochem. 1997;69:883–886. doi: 10.1046/j.1471-4159.1997.69020883.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iwahara S, Yoshida K, Yoshida S. Effects of fornix lesions on waking and sleep patterns in white rats. Physiol Behav. 1977;18:41–46. doi: 10.1016/0031-9384(77)90091-9. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neurosci. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]


