Abstract
Clear links have been established between occupational or therapeutic radiation exposure and breast cancer. Tamoxifen chemoprevention following radiation exposure may be able to reduce the risk of developing breast cancer later in life. In order to model carcinogenesis in this setting, an in vivo model of tamoxifen chemoprevention and tamoxifen failure in a radiation-induced rat mammary carcinoma model was characterized. Two hundred twenty-seven 60 day old female rats received whole body or sham exposure to ionizing radiation. Thirty days later long-term, continuous, tamoxifen chemoprevention was initiated in half the population and all animals were monitored over three and a half years for the development of mammary tumors. Mammary tumors were surgically removed and carcinomas were histologically identified and characterized. Results showed that tamoxifen chemoprevention decreased the incidence and prolonged the latency of radiation-induced mammary carcinomas. However, many individuals receiving tamoxifen chemoprevention developed their first carcinoma very late in life. These carcinomas shared morphological features distinct from the majority of carcinomas that developed in the absence of tamoxifen chemoprevention. Analyses of cell lines established from these carcinomas and immunohistochemisty of tumor sections revealed that the highest levels of Her2/neu expression were associated with in vivo tamoxifen exposure. Treatment of rat mammary carcinoma cells with an anti-rat Her2/neu monoclonal antibody (MAb 7.16.4) inhibited cell growth and this effect was more pronounced in the presence of tamoxifen. These studies suggest that carcinoma growth driven by the Her2/neu pathway may be associated with tamoxifen chemoprevention failure in the rat mammary carcinoma model. Additionally, strategies combining targeted Her2/neu antibodies, vaccines, or drugs with estrogen pathway modification may be more effective in reducing breast cancer chemoprevention failures.
Keywords: Cancer Treatment, Animal Model, Estrogen, Tyrosine Kinase, Herceptin
Introduction
Women who receive radiotherapy for Hodgkins disease between ten and twenty five years of age are at particular risk of developing breast cancer as a second malignancy much later (>20 years) in life[1,2]. Hormonal factors such as early menopause or reduced exposure to hormonal therapy appear to result in a reduced risk of breast cancer following radiotherapy and these findings suggest that tamoxifen chemoprevention should be considered following certain radiation exposures[3]. Selective estrogen receptor modulators (SERMs) such as tamoxifen, significantly reduce the risk of breast cancer in women whose primary tumors are estrogen receptor positive by approximately 40% [4] [5].
Recent findings suggest that inhibition of ErbB family receptor signaling may help to reduce tamoxifen chemoprevention failures. Retrospective analyses of tumor metastases and adjuvant clinical studies suggest that Her2/neu over-expression is associated with tamoxifen resistance [6] [7] [8], and this is further supported by in vitro studies in which Her2/neu transfected mammary cancer cells are rendered hormone independent [9] [10]. Recent evidence also suggests that an estrogen receptor co-activator AIB1, which is phosphorylated by Her2/neu, reduces the antagonistic activity of tamoxifen [11]. In light of these findings, patients who are both Her2/neu and estrogen-receptor positive may benefit from combined SERM-anti-Her2/neu therapeutic and/or chemopreventative approaches for the treatment and prevention of breast cancer [5]. A humanized anti-Her2/neu receptor monoclonal antibody, Herceptin, is currently being used in the treatment of Her2/neu receptor positive breast cancer patients [12]. Several newly developed compounds such as tyrosine kinase inhibitors and antibody-based small molecules exhibit similar, or greater, tumor growth inhibitory effects as Herceptin and may also have potential as chemopreventative agents[13] [14]. Their clinical application and toxic side effects are currently under investigation in preclinical and phase I clinical trials [15] [16].
No model system has been characterized in which to examine the relationship between tamoxifen chemoprevention failure and Her2/neu expression. Systems most commonly used to study HER2/neu targeted approaches involve the use of genetically engineered mice that over-express either the human or rat receptor [17] [18]. However, mammary tumors that result from the forced over-expression of Her2/neu in the mouse may not adequately reflect the natural progression of breast cancer and are of limited use in SERM treatment studies. Rat models of Her2/neu expression in the mammary gland have been extensively studied using retroviral vectors to deliver a mutated Her2/neu gene to the mammary epithelium [19] [20] [21] and rat lines have been developed that express Her2/neu as a transgene [22] [23]. However, studies of the natural expression of Her2/neu in rodent mammary carcinomas have been limited. The female rat is now re-emerging as a breast cancer model because of its long-established predictive value in developing interventions against human breast cancer[24,25] combined with recent advances in rat genomics [23,26]).
Rats exposed to ionizing radiation have a high incidence of mammary tumors, and like breast cancer in humans, some are tamoxifen responsive and/or express HER2/neu [27] [28] [29]. We have characterized a rat mammary carcinoma model to examine the relationship between tamoxifen chemoprevention failure and Her2/neu expression for radiation-induced breast cancer. We found that tamoxifen chemoprevention was effective at reducing the incidence and prolonging the latency of mammary carcinomas that arise following radiation exposure. However, this effectiveness is diminished later in life and numerous tamoxifen resistant carcinomas arise. Her2/neu was expressed at varying levels in all of the carcinomas with the highest levels of expression being found in tamoxifen treated rats. Mammary carcinoma cells obtained from irradiated, tamoxifen treated Sprague Dawley rats are also analogously growth inhibited when treated with anti-Her2/neu MAbs Our findings in characterizing this relevant model provide new insights into the relationship between the estrogen pathway and Her2/neu targeting strategies for a combinatorial approach to the treatment and prevention of breast cancer.
Materials & Methods
Animals
Sixty-day old female Sprague-Dawley rats (Charles River, CD1) were sham exposed or exposed to 300cGy, 500 cGy, or 900 cGy of whole body, photon radiation. At 90 days of age, half of the rats at each radiation dose received subcutaneous sustained-release tamoxifen pellets (Innovative Research, Sarasota, FL) (TAM) that were designed to deliver 200ug of tamoxifen per day for 60 days. At 150 days of age and every 6 months thereafter for life, each TAM rat received a subcutaneous sustained release pellet designed to deliver 20ug of tamoxifen per day for 180 days. The pellets were placed subcutaneously on the dorsum of the neck. Animals were palpated and weighed regularly in order to detect new tumors and in order to control the weight gain of each group since decreased dietary intake while on tamoxifen has an independent effect on reducing incidence of mammary carcinomas in rats [30]. Dietary restriction was used to minimize the indirect effects that tamoxifen has on mammary tumor incidence through reduced weight gain. Rats were fed ad libidum if individual body weight remained at or below the average for the TAM treated rats. Diet was restricted by 13% for individuals that were 0–5% over the average weight of the TAM rats and by 25% if rats weighed over 5% more than the average of the TAM rats. Diet returned to ad libidum once individuals were brought within 5 % of the average for TAM rats. This method of mild dietary restriction in the no TAM rats significantly decreased the incidence of mammary carcinomas compared to matched ad libidum fed groups that were irradiated with 0 or 500cGy photons (unpublished). Palpable tumors were surgically excised under 1% isofluorane anesthesia and immediately fixed in formalin or frozen for later analysis. All rats were housed in a single room of a pathogen free facility that was maintained at 70° F and 30–70 % relative humidity on a 12 hour light-dark cycle. They were in kept in solid bottom cages with filter tops and corn cob bedding. Animal protocols were designed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication 86-23, revised 1996) and were approved by our Institutional Animal Care and Use Committee. A paired t test was used to obtain p values and determine the statistical significance of differences between groups.
Cell Lines
Mammary tumors were sterilely minced in Dulbecco s Modified Essential Medium (DMEM) (Life Technologies) and enzymatically dissociated with collagenase and Dnase treatment for 30–60 minutes. The cells were then centrifuged and resuspended in media containing 10% fetal bovine serum in DMEM supplemented with L-glutamine and penicillin/streptomyocin (growth media) (Life Technologies) and grown in a humidified incubator at 37° C with 5%CO2. Cells had been passaged at least 20 times before experiments were initiated. The MCF-7 human mammary cancer cell line is available from American Type Tissue Culture Collection (Manassas, VA).
Histology
Tumor tissues were fixed for at least 48 hours in 10% buffered formalin, and transverse sections from the middle of each tumor were embedded in paraffin, sectioned at 5um, and stained with hematoxylin and eosin for histopathological evaluation. A histological grading pattern was applied in a blinded fashion to mammary carcinomas. A grading score of 1–3 was used with 1= >95% papillary, tubular, or papillary-cystic pattern; 2= 5–50% solid pattern including cribiform, comedone, solid, and undifferentiated, and 3= > 50% solid pattern.
Immunohistochemisty: Her2/neu expression
Anti-Her2/neu monoclonal antibody (MAb 7.16.4) [31] was used to detect the presence of the receptor in 5 micron frozen sections of rat mammary carcinoma tissues. The MAb was biotinylated and diluted 1:50 according to manufactures instructions (DAKO Animal Research Immunohistochemistry Kit). Non-specific IgG2a antibodies (Sigma) was used as a negative control. Tissues were fixed in acetone, treated with biotin blocking buffer and peroxidase according to manufactures instructions (DAKO). Sections were then incubated with biotinylated antibodies for 15 minutes, followed by another 15-minute incubation with streptavidin-peroxidase. DAB with substrate chromogen (DAKO) was added to the rinsed slides in order to detect bound MAb 7.16.4. Sections were subsequently counterstained for 10 seconds with Mayer s Hematoxylin solution (Sigma). Staining intensity was independently scored on a scale of 1–10 by two of the authors and background staining (IgG2a) scores were subtracted from MAb 7.16.4 stained sections to derive the total score to the agreement of both evaluators (IHC Net–Table II).
Table II.
Histologic Grade of Rat Mammary Tumors
|
No Tamoxifen |
Tamoxifen |
||||||
|---|---|---|---|---|---|---|---|
|
All |
Sham |
Irradiated |
All |
Sham |
Irradiated |
||
| Mean Histologic Pattern Grade | 1.7 | 2.4 | 1.6 | 2.3 | 2.4 | 2.3 | |
| Histologic subtype: | Solid | 23% (13/57) | 60% (3/5) | 19% (10/52) | 57% (21/37) | 60% (3/5) | 56% (18/32) |
| Papillary | 77% (44/57) | 40% (2/5) | 81% (42/52) | 43% (16/37) | 40% (2/5) | 44% (14/32) | |
| Mean Latency: | Solid | *17.5 mo. | *22.4 mo. | ||||
| Papillary | **13.2 mo. | **22.7 mo. | |||||
p value=.0986
** p value=.0001
Focus Formation Assay
Cells were released from culture flasks with Trypsin-EDTA (Sigma) and resuspended at a concentration of 15 cells/ml in 10mls of growth media and placed in 10mm culture dishes. The cells were allowed to grow for 9 days at which point they were washed with phosphate buffered saline (PBS) (pH 7.4) and stained with 1mg/ml p-Iodonitrotetrazolium violet in DMEM overnight.
Flow Cytometric Analyses
Cells were released from tissue culture flasks and 50,000 cells were incubated in the presence of 20ug/ml anti-Her2/neu (MAb 7.16.4). Binding was detected by anti-mouse immunoglobulin conjugated to a fluorescene label (Sigma Immunochemicals) and 8,000 cells were analyzed per sample by flow cytometry. Replicates of three samples were analyzed.
Her2/neu Transmembrane Point Mutation Analyses
Procedures were performed according to Buzard, et al, [32] and described briefly here. RNA was isolated from rat mammary tumor cells using the RNeasy Mini-kit (Qiagen) and reverse-transcribe and amplified by reverse-transcription polymerase chain reaction (RT-PCR) using primers homologous to the rat neu transmembrane domain (Forward–CAGCCCGGTGACATTCATCA; Reverse–TACTTCCGGATCTTCTGTCT). 40 cycles of amplification were performed (Denature - 95° C, Anneal - 55° C, Elongate - 72° C). The cDNA thus produce was digested with Mnl I (New England Biologics) and analyzed by 10% polyacrylamide gel electrophoresis. The gel was stained with ethidium bromide and visualized under ultraviolet light. NIH 3T3 cells that were transfected with transforming rat Her2/neu cDNA were used as a positive control.
Cell Viability Assay: MTT
Cells were released from culture flasks and resuspended at a concentrations of 2,000 cells/ml (RMT 50 & 56), 5,000 cells/ml (RMT 91) and 1,000 (NIH3T3-t-Her2/neu) in growth media and 100ul were distributed to each well of a 96-well plate. Tamoxifen (Sigma), MAb 7.16.4, or combinations of each were added to respective wells at concentrations as indicated in the text. Five identically treated wells were seeded for each treatment group and replicate 96-well plates were analyzed for each day. On indicated days the media from each well was replaced with 100ul of 3-(4,5-dimethylthiazol-2-yl-)-2,5-diphenyltetrazolium bromide (MTT)(Sigma). Cells were incubated in the MTT for 3 hours at which point they were lysed in acidified isopropanol and colorimetric change in the media, which is associated with viable cell numbers, was quantitated by spectrophometric analysis at 570nm using a fluorescent plate reader (BioRad, Model 3550) [33].
Western Blots
The cells were lysed in 0.5 mls of lysis buffer (1% NP40, 0.5%Deoxycholate, 0.1%SDS, 0.15M NaCl, 0.1 M Sodium Phosphate, pH 7.4, 200U/ml, 2mM EDTA, Aprotinin, 1 mM phenylmethylsulfonyl fluoride). The protein concentration of each sample was determined by the Bradford Method (BioRad Protein Assay, Hercules, CA), and equal amounts of protein were loaded on a 10% SDS-Polyacrylamide Gel. After electrophoresis the proteins were transferred to PVDF membrane (Immobilon-P-Millipore, Bedford, MA). Blotting was performed using rabbit anti-estrogen receptor-α (MC-20) and β (H-150) (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:500 in blocking buffer : TTBS (100 mM Tris·Cl, pH 7.5, 0.9% (w/v) NaCl, 0.05% Tween-20) with 5% powdered milk. Horse-radish peroxidase conjugated anti-rabbit immunoglobulin (Amersham Pharmacia, Piscataway, NJ) diluted 1: 1,000 in blocking buffer was added to the blots after the primary antibody was washed with TTBS. Enhanced chemiluminescence (ECL) reagent and CL-X Posure film (Pierce, Rockford, IL) was used to visualize protein bands. The blots were stripped and the procedure was repeated with anti-βactin antibodies (AC-74) Sigma-Aldrich, St. Louis MO). The blots were electronically scanned and band intensities were quantified using the Scion analysis program (Fredrick, MD).
Results
Tamoxifen Chemoprevention of Carcinomas Induced by Ionizing Radiation
The effect of continuous tamoxifen chemoprevention in a rat mammary carcinoma model analyzing time to first carcinoma over the complete lifespan is shown in Figure 1. In the absence of tamoxifen chemoprevention, ionizing radiation resulted in a marked increase in the cumulative incidence of first mammary carcinomas in female rats at all ages for 300 cGy, 500 cGy, and 900 cGy whole body exposure compared to sham-exposed individuals. Tamoxifen chemoprevention, initiated 30 days after exposure, decreased the cumulative incidence of mammary carcinomas at all three doses of ionizing radiation that were studied. The effect of tamoxifen on the mean carcinoma latency of each group is shown in Figure 2. Carcinomas in the sham irradiated, TAM group (n = 5 for TAM, n = 5 for No TAM), had a slightly increased latency compared to the sham irradiated No TAM group, but the trend failed to reach significance. However, tamoxifen clearly prolonged the latency of carcinomas that arose in irradiated rats. If all irradiated rats were analyzed as a group, the mean carcinoma latency for all receiving tamoxifen was 21.5 months compared to a mean carcinoma latency of 13.1 months for all irradiated rats that did not receive tamoxifen (p<−0.0001). If divided according to dose, tamoxifen significantly prolonged the latency of mammary carcinomas at 300cGy and 500 cGy exposure and showed a trend toward prolonged latency at the 900 cGy dose. We conclude that tamoxifen is effective as a chemopreventive against radiation-induced mammary carcinomas even when initiated 30 days following radiation exposure.
Fig. 1: Time to First Carcinoma and Cumulative Incidence.
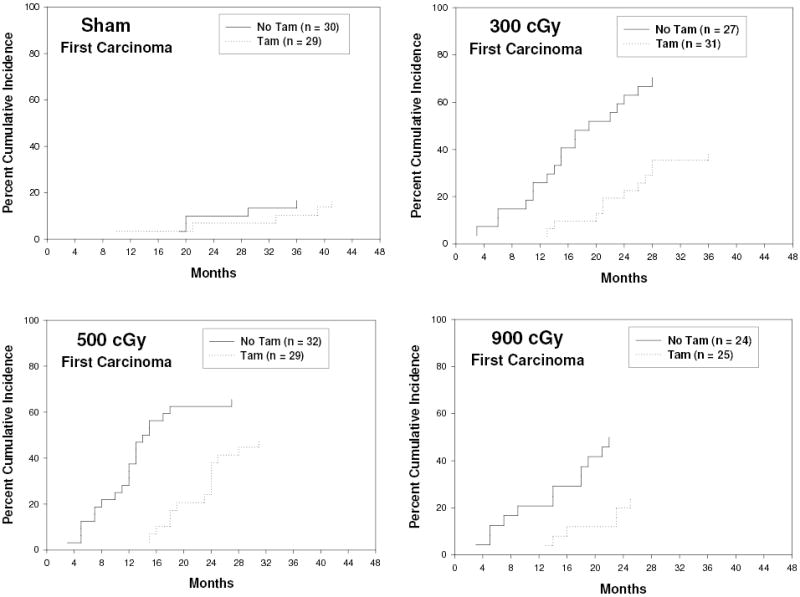
Fig. 2: Mean Mammary Carcinoma Latency.

Effectiveness of Tamoxifen Chemoprevention during Aging
In this lifetime study, we had the opportunity to examine the effectiveness of tamoxifen chemoprevention both early and late following radiation exposure. The results of longitudinal analysis of new carcinoma incidence are summarized in Table 1. Overall, tamoxifen had a positive impact on mean survival in both irradiated and nonirradiated females. In addition, tamoxifen was remarkably effective in preventing early carcinomas as no carcinomas occurred in any irradiated females during the first 12 months following irradiation compared to a 28.9% incidence of new mammary carcinomas in irradiated females. At 13–24 months after irradiation, tamoxifen chemoprevention was less effective, but still reduced the incidence of new mammary carcinomas by almost 50% in irradiated females (25/56 with No TAM compared to 19/80 with TAM). However, tamoxifen chemoprevention failed to reduce the incidence of new mammary carcinomas in the oldest females at more than 24 months post irradiation (25% in No TAM compared to 32.4% in TAM).
Table I.
Longitudinal Analysis of New Mammary Carcinomas
| Incidence of New Carcinomas | ||||
|---|---|---|---|---|
| Latency: |
0–12 months |
13–24 months |
>24 months |
Mean Survival (months) (entire cohort) |
| SHAM No TAM | 0 (n=30) | 10.7% (3/28) | 14% (2/14) | 23.5 (n=30) |
| Sham + TAM | 3.4% (1/29) | 3.4% (1/28) | 12% (3/25) | 32.8 (n=29) p=.0001 |
| 300 cGy No TAM | 25.9% (7/27) | 50% (10/20) | 33% (2/6) | 21 (n=27) |
| 300 cGy + TAM | 0 (n=31) | 23.3% (7/30) | 33% (5/15) | 24.3 (n=31) p=.0451 |
| 500 cGy No TAM | 37.5% (12/32) | 44% (8/18) | 20% (1/5) | 18.8 (n=32) |
| 500 cGy + TAM | 0 (n=29) | 24.1% (7/29) | 33% (5/15) | 23.3 (n=29 p=.0066 |
| 900 cGy No TAM | 20.8% (5/24) | 38.9% (7/18) | 0 (0/1) | 18.9 (n=24) |
| 900 cGy + TAM | 0 (n=25) | 23.8% (5/21) | 25% (1/4) | 20.2 (n=25) p=.3826 |
| All Irrad No TAM | 28.9% (24/83) | 44.6% (25/56) | 25% (3/12) | 19.5 (n=83) |
| All Irrad TAM | 0 (n=85) | 23.5% (19/80) | 32.4% (11/34) | 22.8 (n=85) p=.0007 |
Histological patterns associated with Tamoxifen Failure Later in Life
In order to gain a better understanding of underlying causes of tamoxifen failures that occurred later in life following radiation exposure, we compared the histolopathogical features of the mammary carcinomas in detail and summarized the results in Table 2. Previous work has suggested that for rat mammary carcinomas, a predominantly papillary pattern may be consistent with a lower grade malignancy while more extensive solid areas of growth within a mammary carcinoma indicates higher grade malignancies[34]. Recent studies correlating rat mammary carcinoma gene expression profiles with histological pattern have lent further support to this notion [35]. The mammary carcinomas were classified as either predominantly papillary (low grade) or as solid if they had a significantly solid growth pattern (high grade). A histological pattern grade (1–3) was assigned to each carcinoma based on its histological pattern of growth. The mean histological pattern grade was higher in the carcinomas that arose in the presence of tamoxifen compared to carcinomas that arose in the absence of tamoxifen. In addition, while papillary and solid carcinomas arose in both TAM and No TAM groups at a variety of ages, the majority of carcinomas that arose in the presence of tamoxifen were classified as having a solid pattern (57%) compared to a papillary pattern (43%). This was different from the majority of the carcinomas which arose in the absence of tamoxifen which had predominantly a papillary pattern (77%).
We examined the effectiveness of tamoxifen chemoprevention against these two types of carcinomas by examining the mean latency of each tumor type in the presence or absence of tamoxifen. The results in Table 2 revealed that mean latency of papillary carcinomas was significantly increased by tamoxifen chemoprevention (13.2 months vs 22.7 months; p < 0.0001). However, the latency of solid tumors was not significantly altered by tamoxifen chemoprevention (p = 0.0986). Interestingly, in the No TAM group, the carcinomas that had a solid pattern of growth exhibited a longer latency than carcinomas of the papillary subtype. We conclude that the incidence of carcinomas was increased in irradiated rats, and those exposed to long-term tamoxifen chemoprevention developed tumors later in life.
Her-2/neu expression in rat mammary carcinomas
In order to further examine the relationship between tamoxifen chemoprevention and expression of Her-2/neu in mammary tumors, a subset of mammary carcinomas excised from tamoxifen treated and untreated irradiated rats was compared for Her-2/neu expression. Consecutive tissue sections were stained with MAb 7.16.4 and an isotype control (IgG2a) and staining intensity of each slide was independently evaluated by two of the authors (NP & DH) who were blinded to the treatment groups (Table 3 & Figure 3). Scores never varied by more than 2 points between the two evaluators and a consensus score was determined for each slide with minor discrepancies. Table 3 demonstrates that tumor tissues with the highest expression level of Her2/neu expression were taken from tamoxifen treated rats. Low or intermediate Her2/neu expression in the mammary tumors occurred in both the presence and absence of tamoxifen chemoprevention in the tissues analyzed.
Table III.
Her-2/neu staining of Mammary Carcinomas
| No TAM | ||
|---|---|---|
| Score* | Latency** | Histology*** |
| 6 | 10 | Papillary |
| 6 | 17 | Solid |
| 5 | 12 | Solid |
| 4 | 11 | Papillary |
| 4 | 18 | Papillary |
| 2 | 12 | Papillary |
| 2 | 7 | Papillary |
| 2 | 19 | Papillary |
| TAM | ||
| 9 | 16 | Solid |
| 8 | 23 | Solid |
| 5 | 19 | Solid |
| 5 | 13 | Solid |
| 3 | 15 | Solid, |
| 3 | 18 | Papillary |
| 2 | 13 | Papillary |
| 1 | 15 | Solid |
Her-2/neu expression based on immunohistochemistry
** Latency period for the carcinoma in months measured from the time of radiation exposure
*** Predominant histological pattern
Fig. 3: Example - Immunohistochemical Analysis of Her-2/neu Expression in a Rat Mammary Tumor.
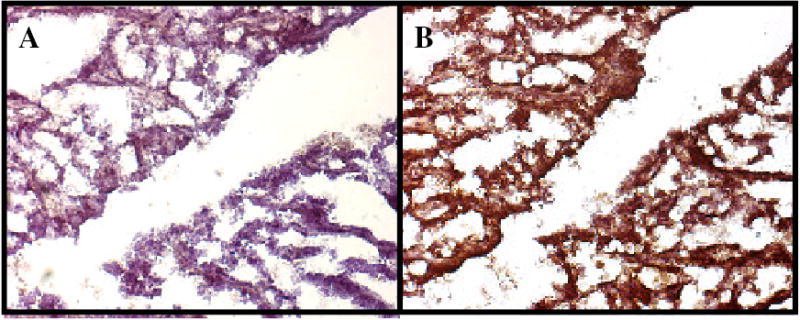
Paraffin embedded mammary tumor tissue taken from rat #538 was analyzed as described in Table 2 legend and the Methodology Section and presented here as an example. Brownish pigment is indicative of the presence of erbB-2/neu expression. A: IgG2a control antibody, B: Anti-rat Her-2/neu monoclonal antibody, 7.16.4.
In order to investigate the effects of tamoxifen and anti-Her2/neu targeted chemoprevention at the cellular level, cell lines were developed from mammary tumors of post-irradiated rats and one sham-irradiated rat (Table 4). Cultures were established from primary tumors and passaged for a minimum of 20 times before assays were performed. Cell growth and morphology were characteristic of mammary carcinoma cells. Flow cytometric analysis of six of the rat mammary tumor (RMT) cell lines using anti-Her2/neu monoclonal antibody (MAb) 7.16.4 revealed that the tyrosine kinase receptor was expressed in varying amounts in all of the cell lines (Figure 4). The cell line with the highest level of Her2/neu expression (RMT 50) was derived from an irradiated tamoxifen treated rat, while that with the lowest expression level (RMT 91) was obtained from non-tamoxifen treated irradiated rat. Similar to results observed in the immunohistochemistry analysis of a different subset of tumors, RMTs with intermediate levels of Her2/neu expression were derived from both treatment groups. cDNAs from each of the cell lines were also analyzed by the RT-PCR - MnlI digestion assay to determine if a transforming point mutation in the transmembrane codon 664 (T -> A) of Her2/neu may have been associated with the development of mammary tumors in the irradiated rats [32]. Unlike neurologic tumors in mutagen treated rats and hamsters, there was no evidence of this mutation in the transmembrane codons of cDNAs obtained from RMTs (data not shown).
Table IV.
Rat Mammary Tumor (RMT) Cell Lines
| ID | Radiation Type (cGy)* | Tamoxifen** |
|---|---|---|
| 18 | Cesium (500) | No |
| 50 | Proton (500) | Yes |
| 56 | Iron (200) | No |
| 58 | Iron (90) | Yes |
| 87 | Iron (160) | Yes |
| 91 | Cesium (50) | No |
| 99 | Spontaneous (00) | No |
Type and dose (Centigrey) of radiation exposure in rats from which the cell lines were derived
** Tamoxifen exposure history (see Materials & Methods) of the rats
Fig. 4: Relative Her-2/neu Cell-Surface Expression.
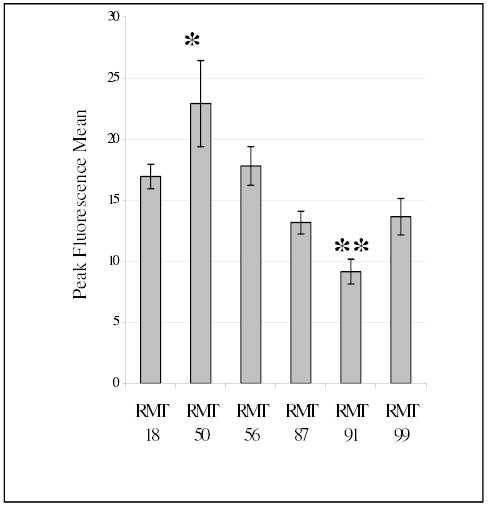
Flow cytometric analysis was performed on RMT cell lines reacted with MAb 7.16.4 to determine their relative levels of erbB-2/neu expression. The mean peak fluorescence of erbB-2 transfected NIH 3T3 cells was 162 +/−33 (not shown). Three groups from each cell line were independently assayed and the mean of the peak fluorescence mean is graphed with error bars showing the standard deviation. * mean peak fluorescence was significantly greater than all the other cell lines presented ** mean peak fluorescence was significantly less than RMT 18, 50, and 56. ANOVA (p<0.05), Tukey 95% Simultaneous Confidence Interval.
Tamoxifen exposure history in the rat affected the in vitro growth characteristics of the RMT cell lines. The ability of cells to form foci when seeded at low density is characteristic of a transformed phenotype [36]. When this assay was used to compare the RMTs, cell lines derived from two of the tamoxifen treated rats (RMT 58 and 87) formed the largest and most foci, whereas the RMTs from rats that did not receive the chemopreventative formed fewer foci (RMT 99 and 18) or none at all (RMT 91) (Figure 5). Although RMT 50 (tamoxifen treated rat) had the highest level of Her2/neu expression level, the number of foci and staining intensity was less than expected. However, RMT 50 grew at a much faster rate, reached a higher density, and had a higher level of Her2/neu expression, when compared to RMTs derived from untreated rats (RMT 56 and 91) (Figure 6, compare 0 uM Tamoxifen doses - A,B, & C). Although the growth rates of RMT 56 and RMT 91 were similar during the first 5 days after seeding, RMT 91 cells began to die over the next 2 days, whereas the viability of RMT 56 remained stable at its peak during this time period (data not shown). We conclude that at least a subset of mammary carcinomas that develop after radiation exposure and long-term tamoxifen chemoprevention, express high levels of Her-2/neu.
Fig. 5: Focus Formation Assay of Rat Mammary Tumor Cell Lines.
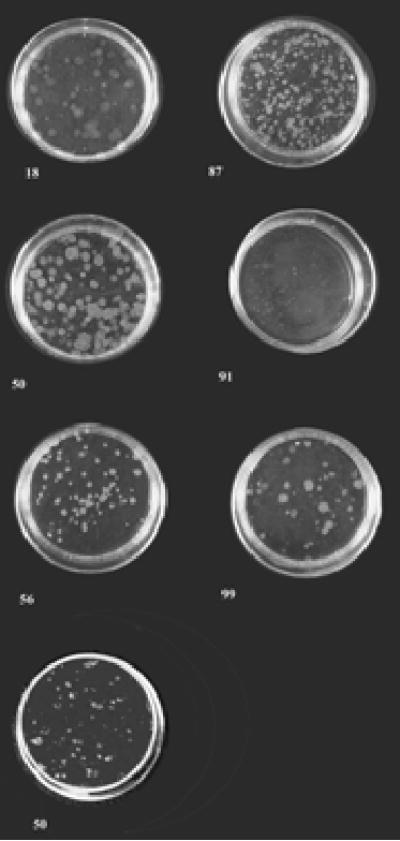
Cells were seeded at a density of 150 cells/plate in 10mm cell culture dishes and grown for 9 days in 10% Fetal Bovine Serum/DMEM. At the end of this growth period the media was removed from the cells and they were stained with 1mg/ml p-Iodonitrotetrazolium violet overnight. Numbers correspond to rat mammary tumor (RMT) cell line
Fig. 6: In Vitro RMT Cell Growth Response to Tamoxifen.
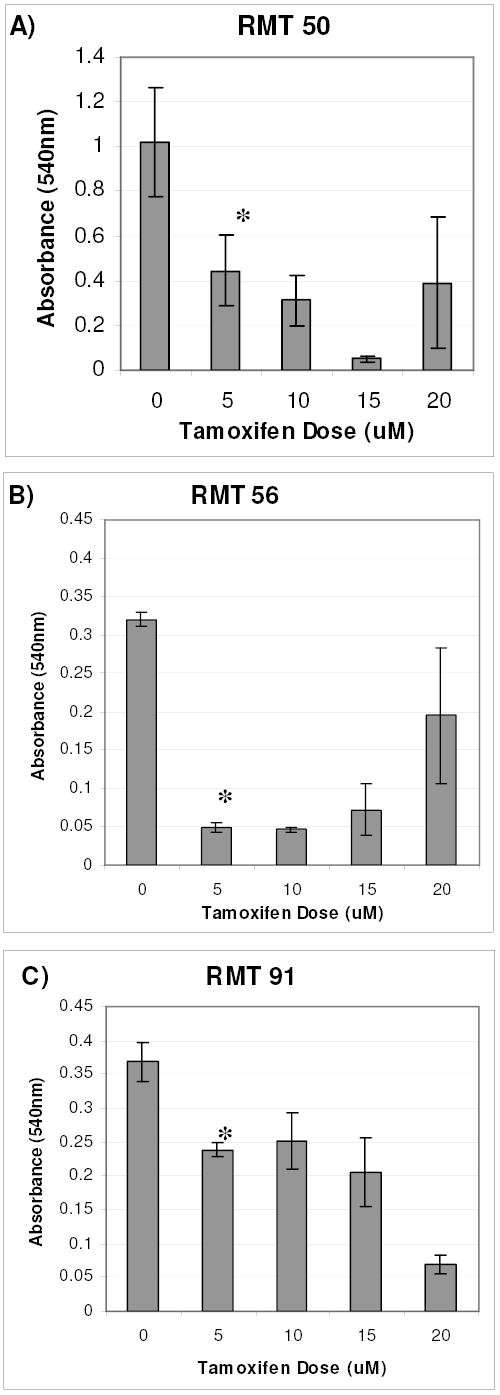
RMTs 50, 56, and 91 were exposed to the indicated concentrations of tamoxifen for 5 days. The MTT assay was performed to determine relative numbers of viable cells in each group. Replicates of 5 were analyzed for each treatment group and the means +/− standard deviations are represented. The assay was repeated and similar results were obtained (data not shown). * mean absorbance (540nm) of 5 uM tamoxifen treated cells was significantly less than untreated cells. ANOVA (p<0.05), Fisher 95% Independent Confidence Interval.
Estrogen Receptor Expression in Rat Mammary Tumor Cell Lines
Western blot analysis was performed to determine if in vivo tamoxifen exposure influenced estrogen receptor expression in six rat mammary tumor cell lines. Estrogen receptor-α (ERα) was expressed in all of the cell lines with the highest relative level in the cell line derived from the non-irradiated, untreated rat (Figure 7, RMT 99). Similar, to that of some human cell lines [37], but not MCF-7s, a lower molecular weight form of ERα was observed in most of the RMTs. Estrogen receptor β (ERβ), which has been speculated to play a role in mammary tumorogenesis [38], was also detected in the RMTs (Figure 7). Variation in the size of ERβ has been described in human breast cancer cell lines [39], and also occurs in RMTs, as two predominant isoforms of ERβ were detected in RMT 99 (Figure 7). In summary, estrogen receptor expression or subtype (α or β) in RMTs was not consistently affected by in vivo tamoxifen selection pressure, but heterogenity occurs, as in human mammary cancer tissues and cell lines.
Fig. 7: Estrogen Receptor Protein Expression.
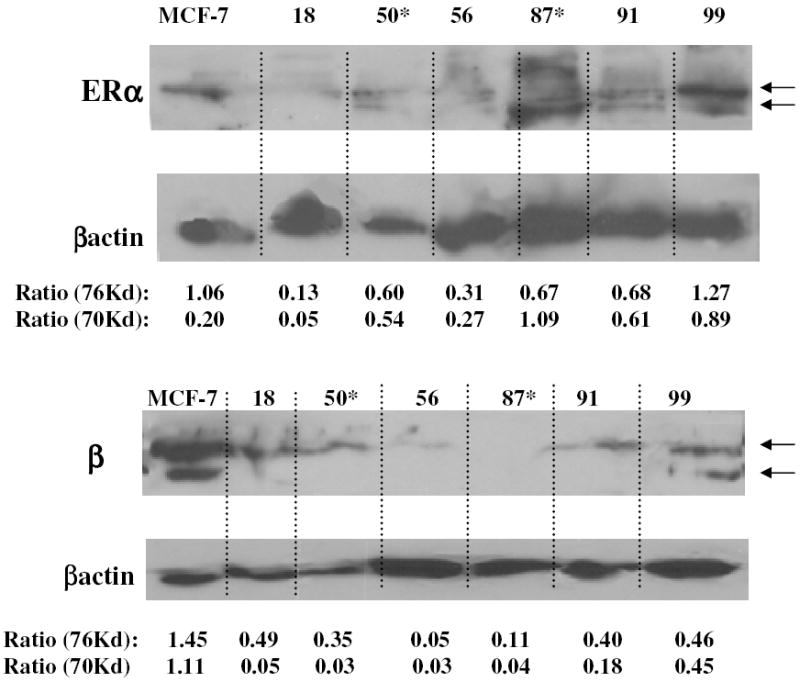
Western Blot analysis was performed on cell lysates as described in the Materials & Methods section of the text. The expression of the estrogen receptor forms was standardized to β-actin expression by dividing the Scion digitized band intensities of each ER form by their respective re-blotted β- actin intensities (Ratio 76 and 70 Kd). Arrows depict 76 and 70Kd ER forms.
RMT cell response to tamoxifen and Her-2/neu targeted treatment
Because the RMTs expressed both Her-2/neu and estrogen receptors, selected cell lines were investigated to develop an in vitro assay to study cell sensitivity to receptor targeted therapies. Each of the three cell lines that were analyzed had different growth characteristics in the presence of increasing doses of tamoxifen (Figure 6). All were significantly inhibited by 5uM of tamoxifen. Interestingly, high concentrations of tamoxifen (20 uM) had an agonistic effect on RMT 50 and 56, but not upon RMT 91. In short, there were no obvious associations between estrogen receptor and Her-2/neu expression levels and tamoxifen sensitivity. However, the heterogenity of these cell lines may be useful for studies to elucidate the mechanisms of receptor interactions that govern cellular responses to treatment.
Having demonstrated that the response of RMTs to tamoxifen treatment varies, and that p185Her2/neu is expressed on their cell-surface, the potential of this tyrosine kinase receptor to serve as an anti-cancer target in this model was further investigated in vitro. RMTs and rat Her2/neu transformed NIH 3T3 fibrocytes were exposed to 50 and 200ug/ml MAb 7.16.4 (Figure 8). In order to allow sufficient time for growth differences to be detected, cells were seeded at a lower density than the previous MTT assays. RMT 91 did not grow well at this lower density and was therefore not included in our analysis. In each of the cell lines analyzed, the higher dose of MAb 7.16.4 was growth inhibitory as evidenced by the decreased slope of the growth curve. By 6 days post-seeding, the growth rates of NIH3T3–Her2/neu, RMT 50, and RMT 56 were significantly lower than their respective controls and 50ug/ml treatment groups (p<0.05). However, antibody treatment alone was insufficient to completely abolish cell growth and by 7 days post-seeding, the number of viable cells in high dose treatment groups of each of these lines was not significantly different than some of the controls (p<0.05). These results are concurrent with observations in MAb 7.16.4 treated athymic mice, in that growth of transplanted NIH3T3–Her2/neu cells was inhibited, but not killed [40].
Fig. 8. In Vitro RMT Cell Growth Response to Anti-Her-2/neu MAb 7.16.4 Treatment.
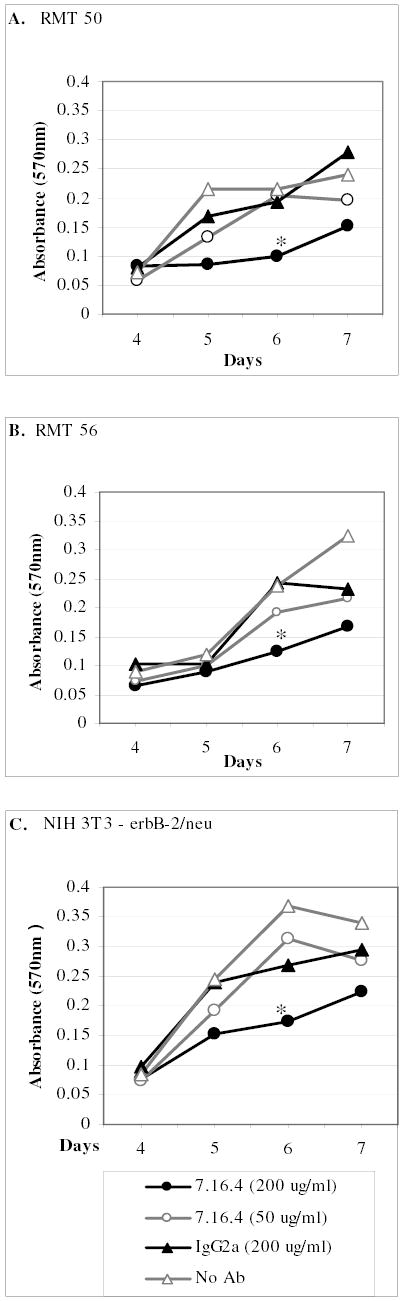
RMTs 50, 56, and 91 were seeded in 4 replicate plates and incubated with the indicated concentrations of MAb 7.16.4. The MTT assay was performed on days 4, 5, 6 and 7 to determine relative numbers of viable cells in each group. Replicates of 5 were analyzed for each treatment group on each day and the means +/− standard deviations are represented. * mean absorbance (540nm) was significantly less than other treatment groups. ANOVA (p<0.05), Tukey 95% Simultaneous Confidence Interval.
As described above, tamoxifen treatment significantly delayed the onset and decreased the incidence of mammary tumors in irradiated rats, but did not completely inhibit their occurrence. In order to test the hypothesis that combined therapies may be a more effective means of cancer treatment and further evaluate the potential of this model, the effects of combined tamoxifen-anti-Her2 treatment upon RMT growth in vitro was analyzed (Figure 9). The low doses of tamoxifen (0.25uM) and MAb 7.16.4 (50ug/ml) applied independently to the RMTs for this study were insufficient to cause significant decreases in cell growth. However, when treatments were combined, growth of RMT 50 cells was markedly inhibited throughout the duration of the study and the number of viable cells after 10 days of combined treatment was significantly lower than the other experimental groups (Figure 9A). Because the RMT 56 cells do not reach the same high density as the RMT 50 cells in this assay, differences between experimental groups could not be discerned at the lower treatment doses (Figure 9B). If, however, the concentration of tamoxifen and MAb 7.16.4 were increased (0.5uM and 200ug/ml, respectively), the synergistic effect of the combined treatment upon RMT 56 could also be appreciated (Figure 9C). Similar to our previous results, MAb 7.16.4 alone obviously delayed RMT 56 cell growth during the first 8 days of treatment, but cell numbers had markedly increased during the next two days. This is in contrast to the tamoxifen-MAb7.16.4 treated cells in which no cell growth was noted throughout the study. The sharp decline in viable cell numbers in the tamoxifen and tamoxifen-IgG2a treated groups from day 8 to 10 was due to cell death after peak cell concentrations had been reached on day 8. In each of these studies, the combined tamoxifen-Mab 7.16.4 treatment appeared to be toxic to the RMTs upon microscopic observation and the cells were unable to recover and grow.
Fig. 9: In Vitro RMT Cell Growth Response to Combined Tamoxifen and Anti-Her-2/neu MAb 7.16.4 Treatment.
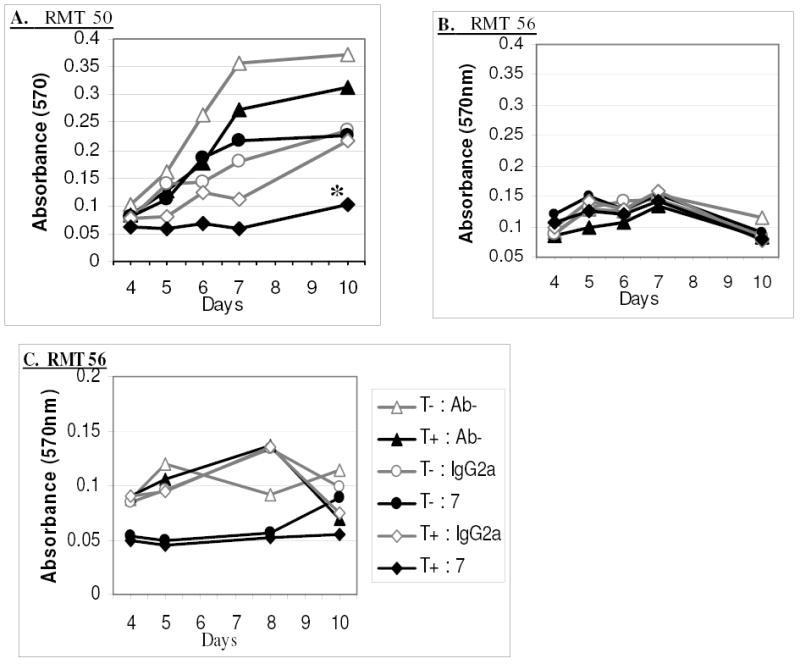
RMT 50 (A) and RMT 56 (B & C) were seeded in 5 replicate plates and incubated with 0.25 uM tamoxifen (T), 50 ug/ml MAb 7.16.4 (7), 50 ug/ml IgG2a, without antibody (Ab-), or any combination of these as indicated. C) 0.5 uM tamoxifen, 200 ug/ml MAb 7.16.4, and 200 ug/ml IgG2a were used. The MTT assay was performed on one of the plates at each of the specified days to determine the relative numbers of viable cells in each group. Replicates of 5 were analyzed for each treatment group on each day and the means are represented. * mean absorbance (540nm) was significantly less than other treatment groups. ANOVA (p<0.05), Tukey 95% Simultaneous Confidence Interval.
Discussion
The rat model provides an excellent setting for the development of combinatorial breast cancer treatments and chemopreventives that target estrogen and/or Her2/neu receptor signaling pathways. The results reported here, and those of others, demonstrate that the onset and incidence of mammary carcinomas in irradiated or mutagen treated rats is markedly reduced in the presence of tamoxifen chemoprevention [41] [29]. We also found that the highest expression levels of Her2/neu tend to be associated with cell lines and tumors obtained from tamoxifen treated rats (Table 3). Our results parallel those of human breast cancer studies that suggest increased Her2/neu expression is associated with tamoxifen treatment resistance and a more aggressive tumor cell phenotype [42] [43] [9] [44]. However, similar to breast cancer in humans, the positive association between tamoxifen selection, Her2/neu expression, and cell growth characteristics is not steadfast, and other genetic variables are also likely to influence cellular phenotype.
ER expression was detected in the RMTs that were obtained from the tamoxifen treated rats (Figure 7; RMT50 and 87). These results are similar to those of human clinical studies in which ER expression is retained in more than half of acquired tamoxifen resistant breast cancer cases[45]. Mutations that cause tamoxifen bound estrogen receptor to adopt an activating conformation have also been proposed as a method by which selection for tamoxifen resistant cells can occur without affecting gene expression levels[46] [47]. Yao et al, found that MCF-7 cells grown in the presence of tamoxifen for less than one year would switch from a growth inhibitory to a growth stimulatory response to the anti-estrogen treatment [48]. Tamoxifen s agonistic effect was also observed in our RMT cell lines when exposed to high doses of anti-estrogen in vitro (Figure 6 compare C & D).
Multiple molecular weight forms of both the ERα and ERβ were observed on Western blots. Heterogeneous expression of 16 splice variants of ERα was also identified in human breast cancer tissues [49] and one of these variants was shown to have constitutive transactivating properties [50]. Additionally, expression of ERα receptor variants was also shown to be associated with human breast tumors when compared to normal mammary tissue [51]. The significance of splice variants and receptor isoform heterogeneity in breast cancer prognosis and response to tamoxifen therapy is currently poorly understood and further studies using the resources developed here are likely to provide additional insights.
Transgenic mouse models that over express Her2/neu are commonly used for studies involving Her2/neu targeted approaches to cancer treatment and prevention. However, the forced expression of Her2/neu in the transgenic mouse models limits their application in studies aimed at elucidating mechanisms of immunotherapeutic or chemopreventative resistance, whereas tumor development in the irradiated rat is more likely to mimic that of the cancer patient. Furthermore, MAb 7.16.4 treated rats have recently been used as a model for Trastuzumab (anti-Her2/neu therapeutic antibody) induced cardiotoxicity in people (Gabrielson, Peterson, et al, in preparation), further supporting the role of the irradiated rat as an in vivo model for Her2/neu targeted approaches to cancer treatment, prevention, and toxicity.
The mechanism by which MAb 7.16.4 inhibits tumor cell growth and causes cardiomyopathy in the rat is likely to be similar to that which occurs in humans, as it s structure and epitopes are similar to that of the therapeutic antibody Herceptin [52]. Because mammary tumor and heart tissue in the rat and humans respond to anti-Her2/neu approaches similarly, this model would be most appropriate for the therapeutic and toxicologic evaluation of many newly developed anti-cancer drugs which target the Her2/neu signaling pathways, such as antibody-based small molecules[13] and tyrosine kinase inhibitors[53]. While not ideal due to long latency periods, the radiation-induced mammary carcinoma model in rats described here offers perhaps a more accurate, predictive means to model complex events involving carcinogenesis, tamoxifen resistance, and aging in human breast cancer.
Combined approaches to cancer treatment and prevention are often more effective than mono-therapeutic strategies. Our results indicate that there is a synergistic tumoricidal effect when RMTs are cultured in the presence of tamoxifen and anti-Her2/neu MAbs. These results are similar to studies in which tamoxifen sensitivity of human MCF-7 breast cancer cells was increased with anti-Her2/neu antibodies or blockade of the tyrosine kinase receptor pathways [53] [54]. Further studies in the irradiated rat could be used to address if the synergistic effects of the combined treatment would be sufficient to lower the anti-Her2/neu MAb concentration enough to avoid cardiotoxic effects and yet maintain a high level of tumor cell killing. Clinical trials in people are also currently underway to investigate the effectiveness of Trastuzmab (Herceptin) in combination with paclitaxel, platinum and taxanes, and anthracyclines with less cardiotoxicity, such as liposomal doxorubicin (reviewed in [55]). Additionally, Her2/neu vaccines are currently being investigated in mouse models for potential therapeutic and immunopreventative use [56] [57]. As shown here, the irradiated rat model can serve as an invaluable model as a means to evaluate the effectiveness of these anti-cancer approaches while providing useful information on their cardiotoxic effects.
Acknowledgments
Thanks to Khalid Khan, Guosheng Liu, Ashti Hawaizi, Paul Eyabi, and Rodolfo Ricart for their excellent technical assistance. This work was supported by NASA and the National Space Biomedical Research Institute RE 00203, RE00205, and by NIH RR00171, CA62924.
References
- 1.Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745–51. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, DeLaat C, Fossati-Bellani F, Morgan E, Oberlin O, Reaman G, Ruymann FB, Tersak J, Meadows AT. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–94. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 3.van Leeuwen FE, Klokman WJ, Stovall M, Dahler EC, van’t Veer MB, Noordijk EM, Crommelin MA, Aleman BM, Broeks A, Gospodarowicz M, Travis LB, Russell NS. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–80. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 4.Peto, R. (2000) Adjuvant hormone therapy: tamoxifen. NIH Consensus Development Conference: Adjuvant Therapy for Breast Cancer, Bethesda, Maryland.
- 5. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 6.Borg A, Baldetorp B, Ferno M, Killander D, Olsson H, Ryden S, Sigurdsson H. ERBB2 amplification is associated with tamoxifen resistance in steroid- receptor positive breast cancer. Cancer Lett. 1994;81:137–44. doi: 10.1016/0304-3835(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 7.Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995;13:1129–35. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 8.Houston SJ, Plunkett TA, Barnes DM, Smith P, Rubens RD, Miles DW. Overexpression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. Br J Cancer. 1999;79:1220–6. doi: 10.1038/sj.bjc.6690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 10.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–46. [PubMed] [Google Scholar]
- 11.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–61. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J. Clinical trials of Herceptin(trastuzumab) Eur J Cancer. 2001;37(Suppl 1):S18–24. [PubMed] [Google Scholar]
- 13.Park BW, Zhang HT, Wu C, Berezov A, Zhang X, Dua R, Wang Q, Kao G, O’Rourke DM, Greene MI, Murali R. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18:194–8. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 14.Berezov A, Chen J, Liu Q, Zhang HT, Greene MI, Murali R. Disabling receptor ensembles with rationally designed interface peptidomimetics. J Biol Chem. 2002;277:28330–9. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 15.Xiong HQ, Abbruzzese JL. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29:31–7. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, Averbuch SD, Feyereislova A, Swaisland H, Rojo F, Albanell J. Phase I Safety, Pharmacokinetic, and Pharmacodynamic Trial of ZD1839, a Selective Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, in Patients With Five Selected Solid Tumor Types. J Clin Oncol. 2002;20:4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 17.Jolicoeur P, Bouchard L, Guimond A, Ste-Marie M, Hanna Z, Dievart A. Use of mouse mammary tumour virus (MMTV)/neu transgenic mice to identify genes collaborating with the c-erbB-2 oncogene in mammary tumour development. Biochem Soc Symp. 1998;63:159–65. [PubMed] [Google Scholar]
- 18.Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989;57:931–6. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Frequent induction of mammary carcinomas following neu oncogene transfer into in situ mammary epithelial cells of susceptible and resistant rat strains. Cancer Res. 1991;51:5649–54. [PubMed] [Google Scholar]
- 20.Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Difference in the response of neu and ras oncogene-induced rat mammary carcinomas to early and late ovariectomy. Cancer Res. 1992;52:4102–5. [PubMed] [Google Scholar]
- 21.Gould MN. The introduction of activated oncogenes to mammary cells in vivo using retroviral vectors: a new model for the chemoprevention of premalignant and malignant lesions of the breast. J Cell Biochem Suppl. 1993;17G:66–72. doi: 10.1002/jcb.240531113. [DOI] [PubMed] [Google Scholar]
- 22.Davies BR, Warren JR, Schmidt G, Rudland PS. Induction of a variety of preneoplasias and tumours in the mammary glands of transgenic rats. Biochem Soc Symp. 1998;63:167–84. [PubMed] [Google Scholar]
- 23.Watson PA, Kim K, Chen KS, Gould MN. Androgen-dependent mammary carcinogenesis in rats transgenic for the Neu proto-oncogene. Cancer Cell. 2002;2:67–79. doi: 10.1016/s1535-6108(02)00083-1. [DOI] [PubMed] [Google Scholar]
- 24.Gould MN. Rodent models for the study of etiology, prevention and treatment of breast cancer. Semin Cancer Biol. 1995;6:147–52. doi: 10.1006/scbi.1995.0023. [DOI] [PubMed] [Google Scholar]
- 25.Gillette CA, Zhu Z, Westerlind KC, Melby CL, Wolfe P, Thompson HJ. Energy availability and mammary carcinogenesis: effects of calorie restriction and exercise. Carcinogenesis. 1997;18:1183–8. doi: 10.1093/carcin/18.6.1183. [DOI] [PubMed] [Google Scholar]
- 26.Zan Y, Haag JD, Chen KS, Shepel LA, Wigington D, Wang YR, Hu R, Lopez-Guajardo CC, Brose HL, Porter KI, Leonard RA, Hitt AA, Schommer SL, Elegbede AF, Gould MN. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–51. doi: 10.1038/nbt830. [DOI] [PubMed] [Google Scholar]
- 27.Welsch CW, Goodrich-Smith M, Brown CK, Miglorie N, Clifton KH. Effect of an estrogen antagonist (tamoxifen) on the initiation and progression of gamma-irradiation-induced mammary tumors in female Sprague-Dawley rats. Eur J Cancer Clin Oncol. 1981;17:1255–8. doi: 10.1016/0014-2964(81)90004-9. [DOI] [PubMed] [Google Scholar]
- 28.Gotoh T, Yamada K, to A, Yin H, Kataoka T, Dohi K. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary cancer by miso and tamoxifen, alone and in combination. Jpn J Cancer Res. 1998;89:487–95. doi: 10.1111/j.1349-7006.1998.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thordarson G, Lee AV, McCarty M, Van Horn K, Chu O, Chou YC, Yang J, Guzman RC, Nandi S, Talamantes F. Growth and characterization of N-methyl-N-nitrosourea-induced mammary tumors in intact and ovariectomized rats. Carcinogenesis. 2001;22:2039–47. doi: 10.1093/carcin/22.12.2039. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Burford C, Lubet RA, Steele VE, Eto I, Bandy M, Juliana MM, Weiss HL, Grizzle WE, Kelloff GJ, Grubbs CJ. Effects of acute and chronic body weight gain reductions in the evaluation of agents for efficacy in mammary cancer prevention. Oncol Rep. 2001;8:373–9. doi: 10.3892/or.8.2.373. [DOI] [PubMed] [Google Scholar]
- 31.Drebin JA, Link VC, Weinberg RA, Greene MI. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc Natl Acad Sci U S A. 1986;83:9129–33. doi: 10.1073/pnas.83.23.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buzard GS, Enomoto T, Anderson LM, Perantoni AO, Devor DE, Rice JM. Activation of neu by missense point mutation in the transmembrane domain in schwannomas induced in C3H/HeNCr mice by transplacental exposure to N-nitrosoethylurea. J Cancer Res Clin Oncol. 1999;125:653–9. doi: 10.1007/s004320050330. [DOI] [PubMed] [Google Scholar]
- 33.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 34.Costa I, Solanas M, Escrich E. Histopathologic characterization of mammary neoplastic lesions induced with 7,12 dimethylbenz(alpha)anthracene in the rat: a comparative analysis with human breast tumors. Arch Pathol Lab Med. 2002;126:915–27. doi: 10.5858/2002-126-0915-HCOMNL. [DOI] [PubMed] [Google Scholar]
- 35.Shan L, Yu M, Snyderwine EG. Gene expression profiling of chemically induced rat mammary gland cancer. Carcinogenesis. 2005;26:503–9. doi: 10.1093/carcin/bgh330. [DOI] [PubMed] [Google Scholar]
- 36.Wada T, Myers JN, Kokai Y, Brown VI, Hamuro J, LeVea CM, Greene MI. Anti-receptor antibodies reverse the phenotype of cells transformed by two interacting proto-oncogene encoded receptor proteins. Oncogene. 1990;5:489–95. [PubMed] [Google Scholar]
- 37.Fasco MJ, Keyomarsi K, Arcaro KF, Gierthy JF. Expression of an estrogen receptor alpha variant protein in cell lines and tumors. Mol Cell Endocrinol. 2000;162:167–80. doi: 10.1016/s0303-7207(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 38.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–201. [PubMed] [Google Scholar]
- 39.Fuqua SA, Schiff R, Parra I, Friedrichs WE, Su JL, McKee DD, Slentz-Kesler K, Moore LB, Willson TM, Moore JT. Expression of wild-type estrogen receptor beta and variant isoforms in human breast cancer. Cancer Res. 1999;59:5425–8. [PubMed] [Google Scholar]
- 40.Drebin JA, Link VC, Greene MI. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene. 1988;2:387–94. [PubMed] [Google Scholar]
- 41.Lemon HM, Kumar PF, Peterson C, Rodriguez-Sierra JF, Abbo KM. Inhibition of radiogenic mammary carcinoma in rats by estriol of tamoxifen. Cancer. 1989;63:1685–1692. doi: 10.1002/1097-0142(19900501)63:9<1685::aid-cncr2820630907>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 42.Dowsett M, Harper-Wynne C, Boeddinghaus I, Salter J, Hills M, Dixon M, Ebbs S, Gui G, Sacks N, Smith I. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res. 2001;61:8452–8. [PubMed] [Google Scholar]
- 43.Kumar R, Mandal M, Lipton A, Harvey H, Thompson CB. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin Cancer Res. 1996;2:1215–9. [PubMed] [Google Scholar]
- 44.Bartsch C, Szadowska A, Karasek M, Bartsch H, Geppert M, Mecke D. Serial transplants of DMBA-induced mammary tumors in fischer rats as model system for human breast cancer: V. Myoepithelial-mesenchymal conversion during passaging as possible cause for modulation of pineal- tumor interaction. Exp Toxicol Pathol. 2000;52:93–101. doi: 10.1016/S0940-2993(00)80091-3. [DOI] [PubMed] [Google Scholar]
- 45.Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26:237–46. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- 46.Tonetti DA, Jordan VC. The role of estrogen receptor mutations in tamoxifen-stimulated breast cancer. J Steroid Biochem Mol Biol. 1997;62:119–28. doi: 10.1016/s0960-0760(97)00034-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhang QX, Borg A, Wolf DM, Oesterreich S, Fuqua SA. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57:1244–9. [PubMed] [Google Scholar]
- 48.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 49.Poola I, Speirs V. Expression of alternatively spliced estrogen receptor alpha mRNAs is increased in breast cancer tissues. J Steroid Biochem Mol Biol. 2001;78:459–69. doi: 10.1016/s0960-0760(01)00118-2. [DOI] [PubMed] [Google Scholar]
- 50.Chaidarun SS, Alexander JM. A tumor-specific truncated estrogen receptor splice variant enhances estrogen-stimulated gene expression. Mol Endocrinol. 1998;12:1355–66. doi: 10.1210/mend.12.9.0170. [DOI] [PubMed] [Google Scholar]
- 51.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered expression of estrogen receptor-alpha variant messenger RNAs between adjacent normal breast and breast tumor tissues. Breast Cancer Res. 2000;2:64–72. doi: 10.1186/bcr30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, Murali R. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Exp Mol Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 53.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen- resistant breast cancer cells. Cancer Res. 2000;60:5887–94. [PubMed] [Google Scholar]
- 54.Witters L, Engle L, Lipton A. Restoration of estrogen responsiveness by blocking the HER-2/neu pathway. Oncol Rep. 2002;9:1163–6. [PubMed] [Google Scholar]
- 55.Baselga J. Current and planned clinical trials with trastuzumab (Herceptin) Semin Oncol. 2000;27:27–32. [PubMed] [Google Scholar]
- 56.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu- expressing tumors. Cancer Res. 2001;61:880–3. [PubMed] [Google Scholar]
- 57.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER- 2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]


