Abstract
Ischemic stroke induces irreversible cerebral tissue damage, a condition exacerbated by the brain’s limited endogenous neuroplasticity and inability to regenerate neurons. While neural circuit reorganization holds therapeutic potential, its efficacy is hindered by pathological barriers such as glial scarring, chronic inflammation, and neurotrophic factor deficiency. Although pharmacological and interventional methods for stroke have been well developed, their functional recovery outcomes remain suboptimal. Emerging neural regeneration paradigms, particularly stem cell-based strategies (encompassing neural stem cell transplantation, neural progenitors grafts, and 3D brain organoid implantation), offer novel solutions to these challenges. However, critical limitations persist in conventional stem cell approaches: (1) compromised synaptic integration efficacy hinders functional neural circuit reconstruction; (2) the absence of functional vascular niches coupled with deficient astrocyte-mediated support and extracellular matrix signaling; (3) restricted regenerative capacity despite theoretical multipotent differentiation potential. Recent breakthroughs in cerebral organoid technology have revolutionized neurological disease modeling and neural repair research. Building upon this paradigm shift, our study mechanistically interrogates neuroplastic remodeling processes following ischemic stroke, while critically evaluating the therapeutic efficacy and inherent limitations of stem cell-based interventions. This affirms the critical role of 3D human cerebral organoid transplantation in the reconstruction of neural circuits. Additionally, we further summarize the utility of organoid-based disease models and address associated ethical and societal concerns. Future efforts should prioritize the clinical translation of organoid transplantation for ischemic stroke, aiming to mitigate neurological deficits and restore functional recovery.
Keywords: Ischaemic stroke, Neuroplasticity, Stem cell therapy, Organoid transplantation, Neurological recovery, Regenerative medicine
Introduction
Stroke, a cerebrovascular disease, is the second leading cause of death globally and the third leading cause when disability and mortality are included as compounding factors [1]. Accounting for 62.4% of all strokes, ischemic stroke is the most frequent type and is the leading cause of neurological dysfunction encountered in clinical practice. Stroke imposes a significant economic and social burden and severely impacts the healthy lives of individuals worldwide [2]. Despite the high incidence of cerebrovascular diseases such as stroke, only a limited number of effective therapies are available in clinical practice. This, along with the fact that most patients do not seek timely medical attention for treatment, delays the optimal time for treatment and leads to extensive necrosis in the infarcted area, which can cause irreversible damage to the neurons in the brain. After patients progress through the acute phase of a stroke, they are left with serious sequelae, such as motor dysfunction. Post-stroke motor impairments typically affect the face, arms, and legs on one side of the body, impacting approximately 80% of patients [3]. Recovery is often prolonged and can severely impact the patient’s daily work and life. Longitudinal follow-up data reveal that approximately 50% of patients continue to experience motor deficits four years after their stroke occurred [4]. Clinics currently use pharmacological thrombolysis as the primary treatment to restore cerebral perfusion [5]. Although many drugs show neuroprotective effects in both preclinical studies and animal models, they have not demonstrated satisfactory results in clinical practice. Differences in physiology between species pose a challenge to the clinical translation of disease research [6].
Neurorestorative therapies for stroke typically extended therapeutic timeframes from days to weeks after stroke onset and aim to improve neurovascular remodelling and synaptogenesis while decreasing inflammatory and immune responses [7]. Stem cell therapy in regenerative medicine offers novel therapeutic paradigms [9]. Cell therapy enhances the recovery of neurological function of the body through sophisticated neuroimmune modulation and trophic factor secretion [8], thereby avoiding the suboptimal functional outcomes associated with conventional stroke treatments and showing substantial developmental potential [10]. Neural stem cells (NSCs) exhibit multimodal therapeutic actions that can stimulate functional neural replacement or induce multimodal therapeutic actions across multiple central nervous system (CNS) regions [11]. Effectively combining the anti-neuroinflammatory, anti-apoptotic, and pro-angiogenic capacities properties of NSCs with other interventions (e.g., recombinant tissue plasminogen activator) may mitigate apoptosis and necrosis caused by the generation of excess reactive oxygen species (ROS). This excess ROS is generated when the therapeutic time window for restoring blood flow during rt-PA therapy is missed, resulting in reperfusion injury (RI). However, transplantation of cells after 2D monolayer culture is less effective in repairing the tissue structure of the infarcted core compared with culturing cortical organoid systems in a 3D structure. More importantly, cerebral organoids (COs) not only demonstrate efficacy in repairing the ischemic core but also exhibit lesion-targeted neurogenesis into lesion-specific neurons. These neurons extend axons to cortical-basal ganglia-thalamic circuits, establishing functional synaptic connections with the host neural network to reconstruct complete neural circuits. They also demonstrates measurable efficacy in ameliorating post-stroke sensorimotor dysfunction [12]. Currently, several technologies are available to improve the reproducibility of cerebral organoid culture, such as 3D printing to help manipulate the size, shape, and organisation of organoids [13] and Notch signalling inhibition to prevent cell proliferation, induce terminal differentiation, and promote cerebral organoid maturation [14]. Therefore, based on the feasibility and therapeutic potential of generating and optimizing human cerebral organoids, the clinical translation of organoids for enhancing the neuroregenerative capacity and functional remodelling of the brain is a translational research priority.
Mechanisms of neuroplasticity after stroke onset
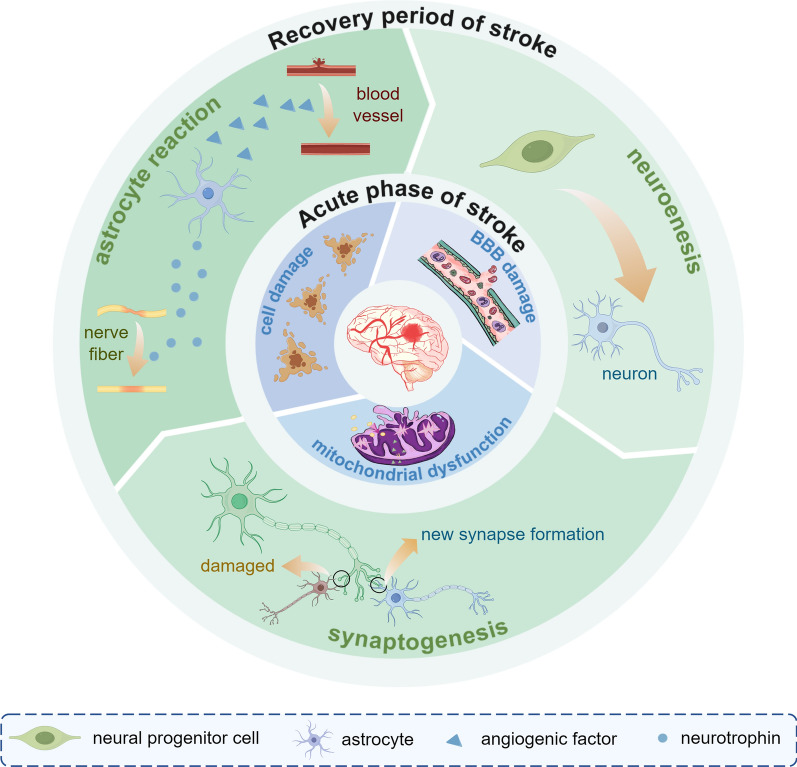
Brain recovery after stroke injury is dynamic and phase-dependent process. In early neurodevelopment (0–3 years), as the brain begins to develops, a large number of synapses are formed to sensory processing and multimodal integration and explore the comprehensive neural network [15]. During the initial development of the brain, the number of dendritic spines and synapses increases substantially, peaking at five years of age, after which the number decreases [15]. As brain volume expansion slows in the later stages, the energy needs of neuronal and synaptic activities cause the brain to adjust the density of these structures through selective synaptic elimination, transitioning from high plasticity to functional [16]. Stroke survivors show a sustained recovery of motor abilities over many years following brain injury. This recovery process is somewhat similar to how infants learn motor function [17]. The Stroke Recovery and Rehabilitation Roundtable has established a time frame for recovery of stroke patients, which is differentiated into the following four phases: the hyper-acute phase (0–24 h), the early subacute phase (7 days–3 months), the late subacute phase (3–6 months), and the chronic phase (>6 months) [18]. After a stroke occurs, the brain undergoes a series of ischemic cascade changes during the acute phase. Due to the disruption of blood flow to the brain and ATP depletion, neurons are unable to maintain normal transmembrane ion concentrations, resulting in a cascade of apoptosis and necrosis [19]. Hypoxia and depletion of metabolic substrates, as well as a substantial reduction in neuronal energy failure, subsequently causes structural damage to the brain [20].Therefore, ionic imbalance and cell death are the primary mechanisms at the onset of a stroke. In the subacute phase of stroke recovery, neuroinflammation diminishes while functional brain networks exhibit activity-dependent neuroplastic changes [21]. Microglia and monocyte-derived macrophages (MDMs) release factors that facilitate adaptation to resolving inflammation by removing neutrophils, cellular debris, and apoptotic cells [22]. These spontaneous neuroplastic changes include the release of brain-derived neurotrophic factor [23], structural remodelling at synaptic, axonal, and dendritic levels [24], and the activation, migration, and differentiation of endogenous neural stem cells [25]. During the chronic phase, experience-dependent reorganization of the neural network stabilizes, and long-term adaptation begins. At this point, “auto-reactive” lymphocytes sensitive to brain antigens return to the brain, triggering chronic inflammation and cytotoxicity, which likely contribute to the chronic sequelae of stroke [26]. Figure 1 Rehabilitation during the early subacute phase, when heightened neuroplasticity is at its peak, substantially increases both the speed and extent of patient recovery [18]. Although spontaneous remodeling occurs after ischemic stroke, these changes alone are insufficient to produce significant functional recovery [27]. In the future, functional recovery through cortical neuronal replacement may be the key to curing human neurodegenerative diseases [28, 29]. Compared with the use of dissociated neural progenitor cells, cerebral organoid transplantation offers several advantages, including a diverse cellular composition, maintenance of a native microenvironment, greater surgical feasibility, and higher vascularization potential, making it a promising therapeutic approach for restoring brain function in both the subacute and chronic phases of the disease [30].
Fig. 1.
The main pathophysiological mechanisms of IS in the acute and convalescent phases. In the acute phase of IS, the ischaemia and reperfusion injury lead to mitochondrial dysfunction, cellular damage, and impairment of the blood–brain barrier. As IS interrupts the normal synaptic plasticity, during the recovery period, homeostatic plasticity is up-regulated and neurogenesis and synaptic remodelling occur. In addition, new synapse formation builds new circuits and aids brain recovery. In the cellular response mode, astrocytes secrete neurotrophic and angiogenic factors to repair damaged blood vessels and nerves. IS ischaemic stroke
Homeostatic plasticity and Hebbian plasticity mechanisms
Reduced cerebral blood flow to tissues adjacent to those affected by the infarction disrupts the typical pattern of synaptic activity in functionally relevant structures [31, 32], as well as in those distant from the stroke [33]. The attenuation of synaptic activity causes upregulation of both presynaptic neurotransmitter release and postsynaptic receptor responses. This homeostatic plasticity is also observed in isolated, cultured hippocampal neurons [34]. This results in increased excitability of surviving neurons, creating a favourable environment for axon sprouting [35]. After triggering new synapse formation, the function is remapped from the damaged region to the surviving tissue surrounding the infarct [36], which compensates for the lost structural circuitry. Therefore, axonal sprouting [37] and increased dendritic spine generation [38] after stroke are homeostatic regulatory processes that contribute to the return of synaptic activity to normal levels. Hebbian theory refers to the idea that sustained repetitive stimulation of a postsynaptic neuron by a presynaptic neuron can lead to an increase in the efficacy of synaptic transmission and a strengthening of that synaptic connection. When homeostatic plasticity restores synaptic structure to the target level, the Hebbian mechanism comes into play and can cause simultaneous activation of both presynaptic and postsynaptic neurons; these simultaneously active connections form behaviourally relevant circuits [39]. Therefore, following a stroke, the reorganisation of synaptic connections is further optimised based on Hebbian plasticity. Moreover, circuits generated by active synaptic connections can be further enhanced during activity-dependent processes, enabling the brain to finely regulate synapses [40].This phenomenon was confirmed by observing histology brain sections of infarcted tissue 7–10 days after stroke surgery [41]. Additionally, homeostatic mechanisms regulate total synaptic strength, whereas Hebbian mechanisms can redistribute synaptic strength. When neuronal damage results in weak synaptic connections, the presence of Hebbian plasticity allows for compensatory reconnection of brain circuits. The interaction of these two plasticity mechanisms is crucial in helping the brain’s recovery.
Connection of cellular response modes to recovery modes
Astrocytes play a dual effects in ischaemic stroke (IS) and are a crucial target for treatment and prognostic improvement. During the acute phase of stroke, cytokines are released from the injured region, and a large amount of excitatory neurotransmitter glutamic acid (Glu) accumulates in the synaptic cleft; astrocytes take up GLu via surface receptors to attenuate excitotoxic damage [42].Cytokines stimulate the activation of astrocytes into reactive astrocytic cells (RACs), which proliferate in large numbers. The intermediate filament systems, such as glial fibrillary acidic protein (GFAP) and vimentin, accelerate the production of glial scarring and impede the infiltration of inflammatory cells in the ischaemic penumbra [43]. The reactive oxygen species (ROS) produced by RAC, such as nitric oxide (NO), induce apoptosis and necrosis. During the recovery period of stroke, after the synapses ensheathed by astrocytes are damaged, astrocytes not only secrete a large number of neurotrophic and angiogenic factors (e.g., cholesterol—raw material for synaptic growth; antioxidant glutathione -to enhance neuronal resistance) and sonic hedgehog, which upregulates the expression level of tight junction proteins in capillary endothelial cells to ensure the integrity of the blood brain barrier (BBB), but their long protrusions connect the subventricular zone of the lateral ventricle to the ischaemic striatum and also form a neural network involved in neurogenesis and revascularisation [44, 45]. However, the secretion of growth-inhibitory molecules and the formation of mature glial scar reduce neurologic recovery, resulting in poor connectivity of surviving neural pathways [46]. In summary, it can be concluded that astrocytes, and the glial scar they form, have a dual role in neuronal regeneration and functional restoration.
Changes in cellular activation status, regulated by homeostatic plasticity mechanisms, have implications for the brain microenvironment. The extracellular matrix (ECM), glial structures, neuronal growth, apoptosis, and angiogenesis after stroke cause the reappearance of relevant developmental proteins, such as the differentiation factor Neuro D, brain-derived neurotrophic factor (BDNF), cell cycle-related protein Cyclin D, and basic fibroblast growth factor (BFGF) [47, 48]. The presence of these factors builds a bridge between cell recovery and behavioural activity. The presence of cell cycle proteins demonstrates the transition of neuronal stem and progenitor cells from a quiescent to an activated state. Inflammatory responses after stroke cause the release of neuroinflammatory cytokines leading to upregulation of peri-infarct synaptic transmission; the introduction of BFGF in post-stroke rats increased the intensity of staining for a non-stroke hemispheric growth-associated protein (the axonal growth marker GAP43) and significantly improved motor function [49]. This improvement is most pronounced in the first few weeks after stroke, reaching a plateau after three months, after which improvements in motor function are less pronounced [50, 51]. This continuous production of neurons in the brain is known as endogenous neurogenesis. Current treatments for neurological damage in stroke focus on enhancing endogenous neurogenesis by identifying drugs that promote neuroprotection and repair after ischaemic stroke.
Current status of brain functional remodelling and regenerative medicine strategies
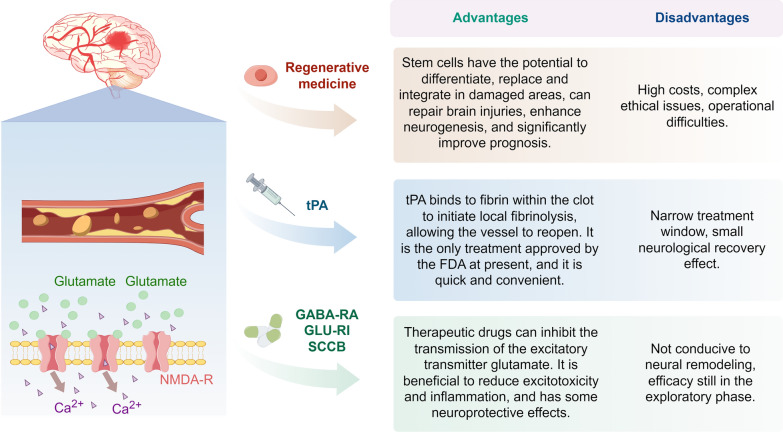
The core of motor function restoration therapy for post-stroke patients lies in promoting neuroplasticity-mediated [52]. Although new neurons are neurogenic capacity in some areas of the human brain, the circuit reorganization of neuronal circuits in patients with stroke is more difficult than in healthy brains based on vascular lesions, chronic inflammation and maladaptive plasticity. To rebuild neuronal differentiation and motor circuitry after stroke, contemporary therapeutic approaches for functional recovery in stroke patients converge on pharmacological treatments targeting critical signaling cascades, active rehabilitation, and regenerative cell-based interventions in regenerative medicine [53]. Pharmacologic therapy consists primarily of tissue plasminogen activator (tPA), gamma-aminobutyric acid (GABA) receptor agonists, glutamate receptor inhibitors, and sodium- and calcium-channel blockers. Currently the only FDA (Food and Drug Administration)-approved treatment option is tPA, but its use has minimal neurorestorative effects and is limited by a narrow therapeutic window [54]. GABA receptor agonists are neuroprotective [55], but their efficacy in reducing glutamate receptor-mediated toxicity is limited. Glutamate receptors contribute to neuronal damage via excitotoxicity and depolarization, owing to the untimely release of glutamate [56], drugs have been developed in recent years to target the pathway downstream of the receptor (N-methyl-d-aspartic acid receptor [NMDAR]-associated signalling pathway) to inhibit this pathway [57]. Sodium-and calcium-channel blockers are useful for reducing excitotoxicity and inflammation [58]. On one hand, their efficacy remains under investigation, on the other hand, inhibition of the neuroinflammatory response may diminish the body’s up-regulation of synaptic responses and impede neuroplasticity in the brain [59] as mentioned above. Activity-based rehabilitation involves designing hands-on tasks with robot-assisted upper limb training, which holds promise for improving difficulties with activities of daily living after stroke [60]. However, its therapeutic effect is subject to further in-depth study by researchers.
Stem cell therapy has great potential to promote neurological recovery from ischemic stroke [61]. In early subacute stroke, patients treated with mesenchymal stromal stem cells (MSCs) protect white matter in the corticospinal tract from post-stroke degeneration and enhance the reorganisation of brain networks. A study by Lee et al. assessed motor function after 90 days of treatment with MSCs. The strength, efficiency and density of connections in the motor network were evaluated using resting-state functional magnetic resonance imaging (rs-fMRI), revealing significantly increased interhemispheric and ipsilateral connectivity in the MSC group compared to controls [62]. Additionally, fMRI detected significant activation in the primary motor cortex and premotor cortex following stem cell intervention. This result was confirmed in a rat middle cerebral artery model [63, 64] as well as in stroke patients [65]. However, not all transplanted stem cells survived or functioned in the post-stroke brain [30], the number of successfully differentiated and surviving NSCs in the host brain remained suboptima [66]. The quantity of implanted cells, transplantation timing post-stroke [30] and the interplay between anaerobic/microaerobic metabolic adaptation of stem cells and their impaired self-renewal/differentiation [67] capacity critically influence human NSC neurogenesis. Furthermore, cells are modulated by the host microenvironment and may fail to acquire target neural phenotypes.Therefore, developing strategies to address the low post-transplant maturation rates and incomplete cellular subtype regeneration remains a priority [68].
The proposed 2D monolayer culture system for organoid-based stem cell growth provides enhanced scalability. The constrained oxygenation and nutrient availability in organoid cultures recapitulate the physiological microenvironment of native tissues [69]. The specific steps for cerebral organoid culture were as follows:
Generation of embryoid bodies
Human embryonic stem cells (hESCs) at approximately 80% confluence were dissociated into single cells using Accutase (Gibco, MA, USA). The cells were then added to each well of a super-attached 96-well plate (Corning, NY, USA) containing various supplements and inhibitors to generate embryoid bodies (EBs), which were maintained for 4–5 days.
Induction of neuroepithelial cell generation
When individual EBs reached a diameter of 500 μm, they were transferred to a super-attached 24-well tissue culture plate containing neural induction medium.
Rostral neuroepithelial structures were generated through 4–6 days of adherent culture under serum-free conditions.
Culturing of COs
The neuroepithelial tissues were embedded in a droplet of Matrigel supplemented with CO differentiation medium and maintained in a static conditions for 4 days to form neuroepithelial buds. These buds were then transferred to a rotationa culture system (Wheaton, Germany) set at 85 rpm for further culture. The COs were maintained in COS differentiation medium containing 1% B27 supplement (with vitamin A), with medium replenishment every 4–7 days) [70]. In 3D cell cultures within ECM microenvironments, cells such as hippocampal neurons and astrocytes develop distinct phenotypic profiles with significantly lower apoptotic rates and higher neuron-to-astrocyte ratios [71]. Additionally, due to complex cell–matrix interactions and intercellular interactions, 3D organoids have a more robust cell migration capability than 2D-cultured stem cells [72]. More surprisingly, transplantation of cerebral organoids into rats with middle cerebral artery occlusion (MCAO) not only promoted axonal regrowth and synaptic reorganization after stroke but also had no significant effect on apoptosis or neuroinflammation in the ipsilateral cortex, demonstrating the compatibility of the cerebral organs with their hosts [70].
With further advances in transplantation techniques, cerebral organoids are likely to play a significant role in post-stroke recovery as well as facilitating the reorganization of neuronal function (Fig. 2).
Fig. 2.
Comparison of different treatment stratagies for IS. Current treatments for IS include regenerative medicine, tPA, and pharmacological treatments (GABA receptor agonists, glutamate receptor inhibitors, and sodium-calcium channel blockers). NMDA-R N-methyl-d-aspartic acid receptors, GABA-RA gamma-aminobutyric acid receptor agonists, GLU-RI glutamate receptor inhibitors, SCCB sodium and calcium channel blockers, IS ischaemic stroke, tPA tissue plasminogen activator
Mechanisms of regenerative medicine for the treatment of stroke
Stem cell therapy
Stroke-induced neuronal loss constitutes the primary pathological basis for motor and cognitive dysfunction in patients. Stem cell-based regenerative strategies demonstrate the potential to to reverse neurological sequelae [73], with endogenous stem cells enhancing the brain’s normal response to injury and inducing neural differentiation through multiple pathways [74]. Neural stem cell (NSC) transplantation may not only promote the regeneration of lost tissue but also enhance functional outcomes through a range of bystander mechanisms [75]. Transplantation of exogenous stem cells into a mouse model of ischaemic stroke with BBB disruption rebuilt the integrity of the BBB and prevented neuronal apoptosis, effectively promoting neurological recovery in tMCAO mice [76]. Some degree of restoration of sensorimotor deficits was observed in post-stroke murine models [70], providing novel therapeutic prospects for stroke patients. Intravenous injections of bone marrow and umbilical cord blood-derived mesenchymal stem cells [77, 78], or intracerebral injections of pluripotent foetal progenitor cells [79, 80] and neural progenitor cells derived from hESCs are currently the primary modalities of ischaemic stroke treatment in clinical trials.
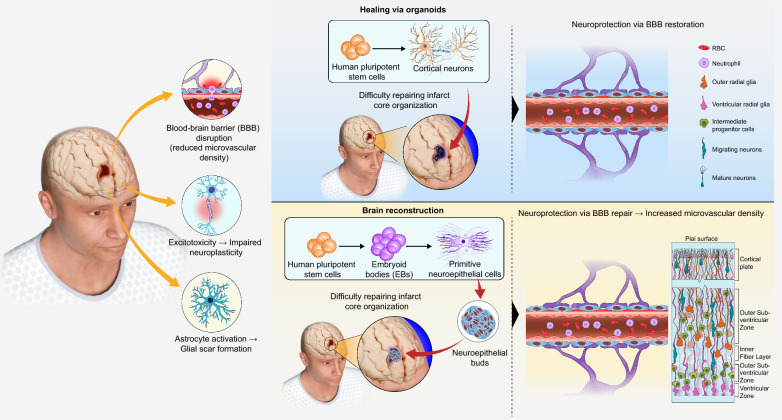
However, the therapeutic efficacy of cell therapy is limited; the transplantation of dissociated organoid cells alone is insufficient to reconstruct cytoarchitecture in the ischaemic core, whereas intact human cerebral organoids exhibit robust survival in the core of the infarct and subsequently differentiating into mature neurons and astrocytes in murine stroke models [12]. Additionally, cerebral organoids contain multiple cell types, recapitulate neuronal function mirror key developmental milestones of the development and maturation of complex neuronal tissues, and are more representative of physiological relevance than 2D cell cultures. Recent quantitative analyses demonstrated that transplanted Sox2-positive neural progenitor cells (NPCs) progressive depletion from 2 wpt (weeks post transplantation) to 4 wpt, whereas stable and sufficient numbers of NSCs were present in organoid transplants at both time points [81]. Transplanted stem cells were all exposed to hostile inflammatory milieu of the host brain, resulting in low survival [82]. Donor cell transplantation into organized cerebral organoids with a 3D cellular creates a neuroprotective niche against the host's hostile elements and achieve enhanced graft viability [81]. Similarly, isolated NPCs showed a rich vascular system at 2 wpt, but at 4 wpt, the number of CD31+ vascular network density was lower than that of transplanted COs. Furthermore, although the percentage of apoptotic cells did not change significantly between the two grafts, there was an increase in host Iba1+ microglia with hypertrophic morphology and a decrease in DCX+ neuroblasts at 4 wpt in NPC transplants, with a corresponding absence of NF-H+ mature neurons, thus suggesting a neuroprotective advantage of the human multicellular 3D microenvironment provided by Cos [83]. Methods for transplantation of human cerebral organoids into the adult mouse brain have been established, and organoids demonstrate neuronal differentiation, axonal pathfinding, and integration with host neuronal circuits in the brains of normal and neonatal thymus-less rats, which represent important research advances in the integration of cerebral organoids with neural circuitry in the host brain [12] (Fig. 3).
Fig. 3.
Comparison of Stem Cell Transplantation and Organoid Transplantation in Therapeutic Approaches for Ischemic Stroke. When a stroke occurs, a series of pathological changes take place in the ischemic core region. Transplantation of exogenous stem cells into the brain repairs the damaged BBB and replenishes the neuronal deficit in the brain; transplantation of mature human brain organoids cultured in vitro into the brain not only repairs the damaged BBB and vascularises the organoids, but also promotes the process of neurogenesis, in which neural stem cells and precursor cells continue to generate and differentiate into mature neurons, which subsequently migrate gradually to form the six-layered structure of the cerebral cortex. BBB blood brain barrier
Integration of organoid and host brain
Functional vascularisation
Cerebral organoids constructed in vitro lack the connectivity present in vivo, which limits their maturation and preventing integration with other circuits that control behaviour. This is evidenced by the differences in the internal microenvironment, neuronal circuits, vascular circulation and immune system compared to the human brain. Vascular structures are essential for the effective transport of oxygen and nutrients. Therefore, vascular formation is particularly necessary for establishing oxygen/nutrient gradients through perfusable networks, and metabolic support for proper differentiation of neural progenitor cells (NPCs). Lack of oxygen permeation leads to necrosis of organoid centres, which adversely affects normal development and potential neuronal transfer pathways [84]. Vascularisation can be achieved via the intracerebral transplantation of mouse cerebral organoids. When human cerebral organoids were transplanted into the brains of immunodeficient mice, initially showing only cortical lobules, the transplanted organoids reduced in size and contained typical progenitor cell areas layered with neural progenitor cells and neurons. At 7–10 days after transplantation, using in vivo two-photon imaging, it was observed that the host’s vascular network began to invade and nourish the organoid, and by 14 days, extensive vascularisation had occurred. Neural progenitor cells disappeared and were replaced by neurons and glial cells. Due to their non-neural origin, host microglia are typically absent in vitro neural organoid models [85]. This leads to improved neuronal survival compared to ex vivo preserved organoids, where more extensive neuronal processes form within the organoid and project to the host brain and gradually form neural networks [86]. Such grafts have been successfully applied to a wide variety of solid (brain [87, 88], small intestine [89] colon epithelium [90]), normal, and cancerous organ tissues. For example, kidney-like organs also become functionally vascularised after transplantation in vivo [91].
Axonal projections and host neuronal integration
When transplanted into the rodent cerebral cortex, human neurons can survive, extend axonal projections, and form synaptic connections with host neurons, demonstrating functional integration into local neural circuits [92–98]. Synaptic connections form the basis for signalling between neurons. Transplanted neuronal progenitor cells must differentiate into specific types of neurons that extend axons through the mature CNS milieu to establish synapses synaptic connections with their targets. Simultaneously, the transplanted neurons receive appropriate synaptic inputs from the host neurons to ensure that the activity of the transplanted cells is homeostatically controlled [99]. Spontaneous firing of action potentials by neurons provided evidence of synaptogenesis and elicited excitatory postsynaptic potentials in response to extracellular electrical stimulation, demonstrating functional synaptic integration [100]. Similarities between graft-reconstructed neural circuits and endogenous neural networks regarding the integration of presynaptic and postsynaptic cell types [101]. This demonstrates the ability of human pluripotent stem cells (hPSC)-derived neuronal subtypes to mediate specific circuit repair and promote functional recovery in the adult brain.
Furthermore, when constructing chimeras through xenotransplantation of human cortical pyramidal neurons as single cell units into the mouse cortex, the resultant neurons will exhibit coordinated morphological and physiological maturation at the single-cell level. This process would begin with robust dendritic spine remodeling, followed by stabilisation and synaptogenesis, enhanced functional synaptic plasticity, and sensory-evoked responses to sensory stimuli similar to those of endogenous cortical neurons [95]. For example when GABAergic interneuron precursors are transplanted into the brain, the precursor cells migrate long distances, survive, and differentiate into GABAergic interneurons that form neural circuits and enhance GABAergic tone [86]. When 3D-cultured human corticocortical organoids (hCO) derived from human induced pluripotent stem cells were transplanted into the primary somatosensory cortex of immunodeficient rats at early developmental stages, the transplanted hCO neurons demonstrated robust maturation, established thalamocortical and cortical connectivity that would evoke a sensory response, and forming axonal projections to the rat brain [102].
Organoid transplantation for ischaemic stroke: enhancing endogenous regeneration/reconstructing neural circuits
After transplantation of human brain organoids into the cortex of stroke mice, the organoids undergo growth and differentiation, and pathological changes occur in the vicinity of the cerebral infarct foci, as evidenced by neuronal cell formation, endogenous regeneration of neurons, and development of the cerebral cortex and reconstruction of circuits associated with the recovery of motor function. In particular, motor neuronal cell formation supports region-specific reconstruction of the motor cortex, contributing to brain injury repair and neuromotor recovery [103]. Additionally, cells generated by transplantation help reduce apoptosis, provide nutritional support through paracrine action, and enhance endogenous regeneration [104].The regeneration of endogenous neurons centres around GABAergic neurons, which serve as key inhibitory modulators of peri-infarct cortical plasticity [105] and are critical for post-stroke recovery [106]. These neurons originate from the medial ganglionic eminence (MGE), and migrate to the cerebral cortex via axonal projections, and integrate into local neural circuits during development. Human MGE-derived organoids(hMGEOs) transplanted into the infarct core and peri-infarct regions one week post-stroke demonstrated robust engraftment, as evidenced by human nuclear antigen expression and co-localization with GFAP or NeuN, confirming their differentiation into functional neural lineages [107].
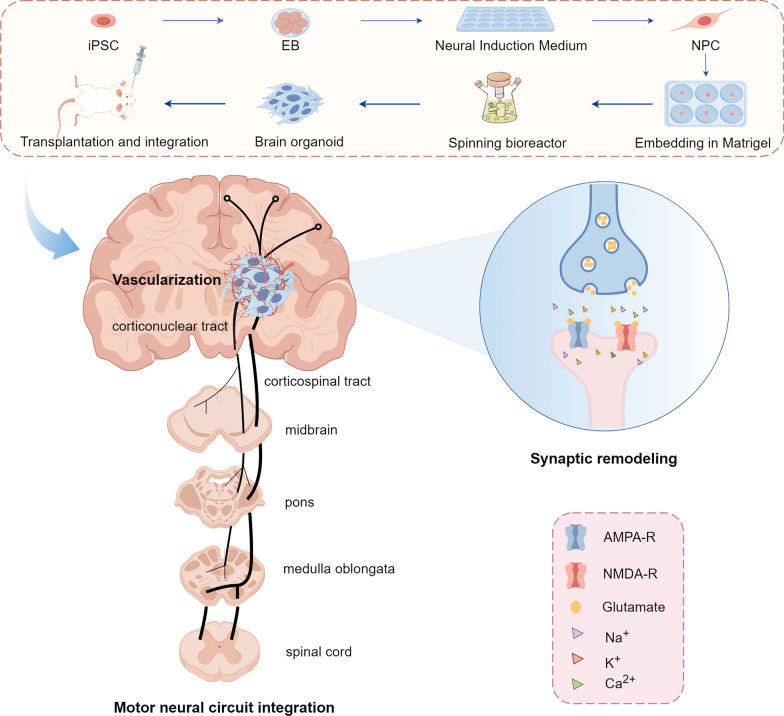
Transplanted cerebral organoids show increased expression of neurons and astrocytes and decreased expression of NSCs, similar to the in vivo differentiation and maturation of the developing cerebral cortex. The mammalian neocortex is a complex, highly organised six-layered structure containing hundreds of different types of neuronal cells and various types of glial cells [108]. It is a key region responsible for cognitive functions, sensory perception and consciousness, where projection neurons are essential for constructing and functioning in neural networks by transmitting information between different brain regions through their axons, which extend to distant targets. In the cerebral cortex, different subtypes of projection neurons have different projection pathways: layer II/III corpus callosum projection neurons project through the corpus callosum to the contralateral cortex; layer VI intrahemispheric projection neurons project to subcortical regions such as the thalamus; and layer V corticospinal projection neurons extend their axons to the brainstem and spinal cord [9]. Following transplantation, human cerebral organoid first survives and differentiates into target neurons in the host brain through neuronal integration and differentiation, thereby mimicking the developing human cerebral cortex. At this point, axons cross the corpus callosum (CC) (the bundle of nerve fibres connecting the two cerebral hemispheres) and eventually reach the contralateral and ipsilateral CC, striatum and hippocampus, the ipsilateral internal capsule, the sensory cortex, and the ventral posterior nucleus of the thalamus. This suggests that the corticospinal tract (CST) establishes a critical circuit for the recovery of motor function after stroke [12]. Ultimately, transplanted neurons establish long-distance projections, reconstructing functional CST pathways to promote motor functional rehabilitation (Fig. 4).
Fig. 4.
Transplantation and functional integration of iPSC-derived brain organoids into the brain. After transplantation of the brain organoids into the mouse brain: the host vascular network invades and nourishes the organoids to achieve vascularisation of the organoids and provide nutrients to the organoids; the human neurons search for the target host neurons through projections and establish functional synaptic connections with them, which generates spontaneous action potentials and triggers excitatory post-synaptic potentials (synaptic remoulding); The organoid grows and differentiates to mimic the motor nerve circuits of the human brain, travelling down through the midbrain, the pons, the medulla oblongata and the spinal cord to complete the corticospinal tracts-a key circuit for locomotion, and the motor nerve circuits are thus reconstructed and integrated. iPSC induced pluripotent stem cells, AMPA-R α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor
Synthetic biology: a new perspective on organoid transplantation
Organoids serve as in vitro models of human development and disease, preserving the genetic and epigenetic characteristics of the original tissue or tumour. These self-organizing three-dimensional structures enable the recapitulation of developmental processes, disease pathogenesis, and therapeutic responses in a controlled ex vivo environment [92]. The seminal work by Lancaster et al. embedded aggregates in a laminin-rich extracellular matrix (Matrigel™) secreted by the Engelbreth-Holm-Swarm tumour cell line. This approach yielded neuroepithelial buds that self-organized into regionally specified neural structures, effectively constituting a"mini-brain"with discrete anatomical domains [109]. Additionally, organoids mimic a wide variety of diseases. These include cystic fibrosis, infectious diseases (e.g., Zika virus) that reduce neurodevelopment of brain cells as neurospheres and brain organoids during development [110], and different types of cancers such as gastric [111], liver [112] and pancreatic cancers [113].
Fusion culture of organoids
Current research shows that brain-like organs produce different brain regions [110]. However, the growth and differentiation of many of these cells demonstrate greater vitality only when stimulated by in vivo conditions. For example, the ventral (NKX2-1) interneuron progenitor cell region exhibits variable but infrequent properties [114]. Therefore, Bagley et al. pioneered a one-species organ co-culture fusion paradigm using the smooth receptor inhibitor cycloheximide A to enhance dorsal forebrain identity in the 2D neuronal differentiation of hPSCs [115]. Separately treated dorsal and ventral (IWP2+SAG) forebrain organoid EBs were embedded together in a single Matrigel droplet. After a certain period of growth to facilitate fusion, and without affecting the structure of the organoid fusion tissue, a dorsal–ventral forebrain axis and long-distance cell migration were established [116]. This illustrates that this co-culture approach allows for the juxtaposition of two brain regions to occur in a process similar to brain development.
Constructing organoid-brain-computer interfaces
Classical brain-computer interfaces (BCIs) that enable direct communication between the brain and external computers [117] allow users to spell words, move cursors, and control wheelchairs or robotic arms [118]. In contrast, brain organoids can recapitulate key aspects of human brain development, including neurogenesis [92], neuronal migration, and the formation of neural circuits [119]. By integrating these two approaches, delivering electrical stimulation to grafted cells can promote in situ cellular regeneration and axon germination [120]. Flexible electrodes—with appropriate mechanical properties and excellent biocompatibility—are superior to rigid electrodes in maintaining the long-term quality of recorded neural signals and establishing stable electrode–neuron interfaces [121]. Consequently, organoid–brain–computer interfaces (OBCIs) have been innovatively constructed in vitro, and modulation through the OBCI is expected to facilitate graft development and establish effective functional connections with the host [122]. This integration of organoids with in vivo bioelectronics could contribute to a more intuitive and systematic mode of communication with the brain in the future.
Multidimensional organoids for in vitro drug screening and evaluation of drug efficacy
In the field of drug discovery, phenotype-based screening strategies has emerged as a superior alternative to traditional target-based drug screening methods and have become a new direction in drug discovery. Experiments are conducted using various animal disease models and applied to compound screening to achieve relatively high-throughput screening (HTS) [123]. This model has certain complexity and reproducibility issues. Many drugs are successful in preclinical experiments but fail in late-stage clinical studies. Cell-based phenotypic screening is more efficient for identifying lead compounds that are physiologically and disease-relevant for drug development. Organoids derived from hPSCs are potentially powerful tools for HTS. Organoids can exhibit near-physiological cellular compositions and grow extensively in culture while maintaining genomic stability [124, 125]. These attributes position organoid models as a crucial bridge between preclinical research and clinical trials [126]. Although organoid culture is challenging, liquid handling robots have been developed to perform all steps of cell culture, from plate laying to differentiation to analysis in a 21-day protocol [127]. Additionally, high-content microscopy is fundamental in providing a characterisation of cellular components, and the Single-cell RNA sequencing (scRNA-seq) platform allows the characterisation of cell types and states. The combination of high-content microscopy and scRNA-seq methods, along with advances in computational tools, will provide a more reliable and cost-effective analysis of heterogeneous cellular models, including neural organoids. This will help facilitate large-scale clinical translation of organoids for drug screening [128].
Ethical considerations
Due to the great potential of organoid tissues in human development and disease, as well as their role in advancing precision and regenerative medicine, these tissues may significantly impact the development of biomedicine and influence the balance between the use of animals, embryos and tissues. Therefore, it is essential to determine the moral and legal status of organoids and to engage in ethical discussion and inquiry concerning these issues.
Complementarity of organoid and animal experiments
In traditional animal experiments, one needs to choose between causing less harm to a larger number of animals or more harm to a smaller number [129]. For this controversial issue, organoids may represent a long-awaited alternative to animal experiments. The variable shapes of organoids result in their inability to fully reproduce the original structure of human organs [130]. This problem can be standardised by using bioengineered scaffolds to control the size of the organoid [131, 132]. Additionally, organoids lack immune cell interactions as well as peripheral nerve tissue innervating the organ, which can be co-cultured to overcome these technical challenges [133]. However, interactions between multiple organs can only be discovered in an animal context, therefore, the use of organoids can serve to complement the studies of animals. Based on the various limitations of animal experiments, scientists often justify their use based on their scientific value and biomedical importance [134]. However, this justification is insufficient; scientists should take additional steps, and scientific journals should fulfil their duty to enforce high ethical standards [135]. Additionally, organoid researchers must recognise the importance of animal experiments in gaining fundamental insights and informing clinical treatments.
Problems with the embryonic origin of organoids
The introduction of organoids has also had an impact on the study of human embryos and foetuses. Some researchers hold the view that the destruction of embryos is ethically unacceptable, a position that orthodox Christianity also supports [136]. Conversely, some argue that early pre-sentient embryos lack moral status as they are not yet capable of consciousness or perception [137]. A third position, independent of the first two, holds that embryos can be created for research under certain conditions and are not accorded an independent moral status. The key factor in determining these positions is the consent of the provider of the gamete, foetus, or developing embryo. The use of embryos remaining from in vitro fertilisation is now widely accepted, but it also faces enormous ethical challenges. The introduction and development of organoid research does not diminish the need for human embryonic material. Many developing organ tissues begin with human ES cells, and their establishment requires the use and destruction of embryos obtained through in vitro fertilisation or generated specifically for research purposes. The International Society for Stem Cell Research has revised its Guidelines for Stem Cell Research and Clinical Translation to require approval and oversight by a specialised human embryo research oversight committee [138]. It is indispensable for the concerned researchers to provide detailed information on the origin of the tissue or cells and on the social value derived from such research. At the same time, there is a urgent need to re-examine and reorganise ethical and legal policies on human embryo research.
Social acceptance
The introduction of organoid technology is significantly contingent upon its social impact and public acceptance, which are pivotal to the advancement of the field. Effectively addressing the public enthusiasm and expectations in this emerging field is an issue that warrants attention [139]. As organoid transplantation involves invasive procedures, it may entail unexpected risks and challenges, thereby drawing intense scrutiny from the community [140]. Moreover, organ transplantation is ethically categorized as a complex translational trial, encompassing multiple invasive interventions and research procedures [70]. Thus, a meticulous interdisciplinary approach to performing organoid transplantation in an ethical manner is essential.
Conclusions
Complete recovery from ischaemic stroke is influenced by multiple factors, and the mechanisms of recovery are closely related to neurogenesis, neuroplasticity, and angiogenesis [86]. The development of regenerative medicine technologies, such as organoids, and their clinical translation are indispensable for improving patient prognosis, and hold potential as an intervention in stroke treatment.Organoid transplant hosts will generate functional vascular networks that facilitate synaptic connections and integration between neurons. Reconstruction of neural networks after brain injury through axonal regeneration is expected to be highly effective in improving neuromotor function in post-stroke patients.
The time window for organoid transplantation, similar to that for neurologic remodelling, is an important parameter for preclinical studies. Transplantation at 6 h or even 24 h of the onset of obstruction remains highly effective [103]. Neuroglial scarring forms within seven days, and replacement of the organoid graft site within this period may still be effective. However, the efficacy of COs transplantation within seven days after stroke remains a question for further exploration in future studies. Meanwhile, given that organoid transplantation is an invasive procedure, surgeons question whether the graft might compress organs or tissues like a tumour. Although studies have shown that organoids do not proliferate as aggressively as tumour cells, concerns about transplantation remain. Therefore, in future approaches, promoting cerebral organoids to become more mature prior to transplantation and minimising the size will be necessary to improve the safety of transplantation.
Currently, pharmacological thrombolysis f is the mainstay of treatment for neurological recovery in ischaemic stroke. This review compiles the pathophysiological mechanisms associated with neuroplasticity in stroke and the mainstream therapeutic tools for motor recovery and details the changes that occur in the host with cerebral organoid transplantation from a neurobiological point of view. Innovative research tools and ethical issues in organoid research are objectively summarised. In the future, it is crucial to continue monitoring developments related to organoid technology, address potential opportunities and risks, and conduct more detailed ethical analyses to minimise public concerns about the organoid field. Therefore, the realisation of the clinical translation of cerebral organoids to ischaemic stroke treatment is a great expectation for the study of human brain recovery after injury in the context of the whole organism.
Acknowledgements
None.
Abbreviations
- BBB
Blood brain barrier
- BDNF
Brain-derived neurotrophic factor
- CC
Corpus callosum
- CNS
Central nervous system
- COs
Cerebral organoids
- CST
Corticospinal tract
- EBs
Embryoid bodies
- ECM
Extracellular matrix
- FDA
Food and Drug Administration
- GABA
Gamma-aminobutyric acid
- GFAP
Glial fibrillary acidic protein
- GLU
Glutamic acid
- HTS
High-throughput screening
- IS
Ischaemic stroke
- hCO
Human cortical organoids
- hESC
Human embryonic stem cells
- hMGEOs
Human medial ganglionic eminence organs
- hPSC
Human pluripotent stem cells
- IS
Ischaemic stroke
- MCAO
Middle cerebral artery occlusion
- MDMs
Monocyte-derived macrophages
- MGE
Medial ganglionic eminence
- MSCs
Mesenchymal stromal stem cells
- NMDAR
N-methyl-D-aspartic acid receptor
- NO
Nitric oxide
- NPCs
Neural progenitor cells
- NSCs
Neural stem cells
- RACS
Reactive astrocytic cells
- tPA
Tissue plasminogen activator
- wpt
Weeks post transplantation
Author contributions
Yixuan Hao and Naili Wei conceived the review article. Yixuan Hao wrote the manuscript with support from Naili Wei. Chenrui Li completed the article illustrations. Naili Wei is a guarantor of the article. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82001447), the Natural Science Foundation of Guangdong Province (2214050005144), special funds for science and technology of Guangdong Province (210728166901906; STKJ2021075), Guangdong Basic and Applied Basic Research Foundation (2023B1515230008).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-Xuan Hao, Chen-Rui Li, Zhi-Jie Lu contributed equally to this work.
Contributor Information
Hai-Liang Tang, Email: 081105229@fudan.edu.cn.
Jian Chen, Email: dr.sword@163.com.
Nai-Li Wei, Email: nlwei@stu.edu.cn.
References
- 1.Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke Off J Int Stroke Soc. 2022;17(1):18–29. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–492. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL. Stroke: practical management. JAMA. 2008;300(19):2311. [Google Scholar]
- 4.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–54. [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–110. [DOI] [PubMed] [Google Scholar]
- 6.Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–81. [DOI] [PubMed] [Google Scholar]
- 7.Venkat P, Shen Y, Chopp M, et al. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology. 2018;134(Pt B):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinden JD, Vishnubhatla I, Muir KW. Prospects for stem cell-derived therapy in stroke. Prog Brain Res. 2012;201:119–67. [DOI] [PubMed] [Google Scholar]
- 9.Molyneaux BJ, Arlotta P, Menezes JRL, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–37. [DOI] [PubMed] [Google Scholar]
- 10.Rw M, Si S. Intravenous cellular therapies for acute ischemic stroke. Stroke. 2018;49(5):1058–65. [DOI] [PubMed] [Google Scholar]
- 11.Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96(12):7029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao SY, Yang D, Huang ZQ, et al. Cerebral organoids transplantation repairs infarcted cortex and restores impaired function after stroke. NPJ Regen Med. 2023;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayavenkataraman S, Yan WC, Lu WF, et al. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296–332. [DOI] [PubMed] [Google Scholar]
- 14.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85. [DOI] [PubMed] [Google Scholar]
- 15.Pr H. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. [DOI] [PubMed] [Google Scholar]
- 16.Neniskyte U, Gross CT. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017;18(11):658–70. [DOI] [PubMed] [Google Scholar]
- 17.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23(6):265–71. [DOI] [PubMed] [Google Scholar]
- 18.Bernhardt J, Hayward KS, Kwakkel G, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair. 2017;31(9):793–9. [DOI] [PubMed] [Google Scholar]
- 19.Besancon E, Guo S, Lok J, et al. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29(5):268–75. [DOI] [PubMed] [Google Scholar]
- 20.Murphy TH, Li P, Betts K, et al. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28(7):1756–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing Y, Bai Y. A review of exercise-induced neuroplasticity in ischemic stroke: pathology and mechanisms. Mol Neurobiol. 2020;57(10):4218–31. [DOI] [PubMed] [Google Scholar]
- 22.Fan PL, Wang SS, Chu SF, et al. Time-dependent dual effect of microglia in ischemic stroke. Neurochem Int. 2023;169:105584. [DOI] [PubMed] [Google Scholar]
- 23.Luo HY, Rahman M, Bobrovskaya L, et al. The level of proBDNF in blood lymphocytes is correlated with that in the brain of rats with photothrombotic ischemic stroke. Neurotox Res. 2019;36(1):49–57. [DOI] [PubMed] [Google Scholar]
- 24.Brown CE, Li P, Boyd JD, et al. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27(15):4101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H, Wang J, Shen L, et al. Phosphatase and tensin homolog deletion enhances neurite outgrowth during neural stem cell differentiation. Neuropathology. 2020;40(3):224–31. [DOI] [PubMed] [Google Scholar]
- 26.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130(6):2777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tennant KA, Taylor SL, White ER, et al. Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nat Commun. 2017;8:15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tornero D, Tsupykov O, Granmo M, et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140(3):692–706. 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- 29.Tornero D, Wattananit S, Grønning Madsen M, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136(12):3561–77. [DOI] [PubMed] [Google Scholar]
- 30.Darsalia V, Allison SJ, Cusulin C, et al. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31(1):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94(15):8179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao TM, Pulsinelli WA, Xu ZC. Changes in membrane properties of CA1 pyramidal neurons after transient forebrain ischemia in vivo. Neuroscience. 1999;90(3):771–80. [DOI] [PubMed] [Google Scholar]
- 33.Bolay H, Gürsoy-Ozdemir Y, Sara Y, et al. Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke. 2002;33(5):1369–75. [DOI] [PubMed] [Google Scholar]
- 34.Bütefisch CM, Netz J, Wessling M, et al. Remote changes in cortical excitability after stroke. Brain J Neurol. 2003;126(Pt 2):470–81. [DOI] [PubMed] [Google Scholar]
- 35.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. [DOI] [PubMed] [Google Scholar]
- 36.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22(14):6062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmichael ST, Wei L, Rovainen CM, et al. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8(5):910–22. [DOI] [PubMed] [Google Scholar]
- 38.Brown CE, Aminoltejari K, Erb H, et al. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci Off J Soc Neurosci. 2009;29(6):1719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32(2):339–50. [DOI] [PubMed] [Google Scholar]
- 40.Ploughman M, Corbett D. Can forced-use therapy be clinically applied after stroke? An exploratory randomized controlled trial. Arch Phys Med Rehabil. 2004;85(9):1417–23. 10.1016/j.apmr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Hagemann G, Redecker C, Neumann-Haefelin T, et al. Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Ann Neurol. 1998;44(2):255–8. [DOI] [PubMed] [Google Scholar]
- 42.Roitbak T, Syková E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28(1):40–8. [DOI] [PubMed] [Google Scholar]
- 43.Huang L, Wu ZB, Zhuge Q, et al. Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci. 2014;11(4):344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goss JR, O’Malley ME, Zou L, et al. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149(2):301–9. [DOI] [PubMed] [Google Scholar]
- 45.Weidemann A, Krohne TU, Aguilar E, et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58(10):1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abeysinghe HCS, Phillips EL, Chin-Cheng H, et al. Modulating astrocyte transition after stroke to promote brain rescue and functional recovery: emerging targets include rho kinase. Int J Mol Sci. 2016;17(3):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31(1):223–30. [DOI] [PubMed] [Google Scholar]
- 48.Stork O, Welzl H. Memory formation and the regulation of gene expression. Cell Mol Life Sci CMLS. 1999;55(4):575–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. [DOI] [PubMed] [Google Scholar]
- 50.Kwakkel G, Kollen BJ, Van Der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–6. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura Y, Onoe H, Morichika Y, et al. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318(5853):1150–5. [DOI] [PubMed] [Google Scholar]
- 52.Richards LG, Cramer SC. Advances in stroke: therapies targeting stroke recovery. Stroke. 2021;52(1):348–50. [DOI] [PubMed] [Google Scholar]
- 53.Shehjar F, Maktabi B, Rahman ZA, et al. Stroke: Molecular mechanisms and therapies: update on recent developments. Neurochem Int. 2023;162: 105458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–8. [DOI] [PubMed] [Google Scholar]
- 55.Spithoven AW, Lodder GM, Goossens L, et al. Adolescents’ loneliness and depression associated with friendship experiences and well-being: a person-centered approach. J Youth Adolesc. 2017;46(2):429–41. 10.1007/s10964-016-0478-2. [DOI] [PubMed] [Google Scholar]
- 56.Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. 2020;21(20):7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong X-x, Wang Y, Qin Z-h. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank D, Zlotnik A, Boyko M, et al. The development of novel drug treatments for stroke patients: a review. Int J Mol Sci. 2022;23(10):5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Qian J, Yang B, et al. Challenges and improvements of novel therapies for ischemic stroke. Front Pharmacol. 2021;12: 721156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EEH, et al. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair. 2017;31(2):107–21. [DOI] [PubMed] [Google Scholar]
- 61.Boese AC, Le QSE, Pham D, et al. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res Ther. 2018;9(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Chang WH, Chung JW, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke. 2022;53(1):20–8. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Cabrer P, Justicia C, Wiedermann D, et al. Stem cell mediation of functional recovery after stroke in the rat. PLoS ONE. 2010;5(9): e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32(3):1080–9. [DOI] [PubMed] [Google Scholar]
- 65.Bhasin A, Srivastava M, Bhatia R. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012;8(3):181–9. 10.46582/jsrm.0803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oki K, Tatarishvili J, Wood J, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells Dayt Ohio. 2012;30(6):1120–33. [DOI] [PubMed] [Google Scholar]
- 67.Sandvig I, Gadjanski I, Vlaski-Lafarge M, et al. Strategies to enhance implantation and survival of stem cells after their injection in ischemic neural tissue. Stem Cells Dev. 2017;26(8):554–65. [DOI] [PubMed] [Google Scholar]
- 68.Wang P, Miao CY. NAMPT as a therapeutic target against stroke. Trends Pharmacol Sci. 2015;36(12):891–905. [DOI] [PubMed] [Google Scholar]
- 69.Edmondson R, Broglie JJ, Adcock AF, et al. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang SN, Wang Z, Xu TY, et al. Cerebral organoids repair ischemic stroke brain injury. Transl Stroke Res. 2020;11(5):983–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peretz H, Talpalar AE, Vago R, et al. Superior survival and durability of neurons and astrocytes on 3-dimensional aragonite biomatrices. Tissue Eng. 2007;13(3):461–72. [DOI] [PubMed] [Google Scholar]
- 72.Gjorevski N, Piotrowski AS, Varner VD, et al. Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep. 2015;5:11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao LR, Willing A. Enhancing endogenous capacity to repair a stroke-damaged brain: an evolving field for stroke research. Prog Neurobiol. 2018;163–164:5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J, Huang Y, Gong J, et al. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat Commun. 2020;11:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reekmans K, Praet J, Daans J, et al. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev Rep. 2012;8(1):262–78. [DOI] [PubMed] [Google Scholar]
- 76.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song M, Kim YJ, Kim YH, et al. Long-term effects of magnetically targeted ferumoxide-labeled human neural stem cells in focal cerebral ischemia. Cell Transpl. 2015;24(2):183–90. [DOI] [PubMed] [Google Scholar]
- 78.Laskowitz DT, Bennett ER, Durham RJ, et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl Med. 2018;7(7):521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet Lond Engl. 2016;388(10046):787–96. [DOI] [PubMed] [Google Scholar]
- 80.Muir KW, Bulters D, Willmot M, et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91(4):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daviaud N, Friedel RH, Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. eNeuro. 2018;5(6):ENEURO.0219-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bliss T, Guzman R, Daadi M, et al. Cell transplantation therapy for stroke. Stroke. 2007;38(2):817–26. [DOI] [PubMed] [Google Scholar]
- 83.Bellotti C, Samudyata S, Thams S, et al. Organoids and chimeras: the hopeful fusion transforming traumatic brain injury research. Acta Neuropathol Commun. 2024;12(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–40. 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 85.Brain organoids get vascularized - PubMed.
- 86.Southwell DG, Nicholas CR, Basbaum AI, et al. Interneurons from embryonic development to cell-based therapy. Science. 2014;344(6180):1240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giandomenico SL, Lancaster MA. Probing human brain evolution and development in organoids. Curr Opin Cell Biol. 2017;44:36–43. [DOI] [PubMed] [Google Scholar]
- 88.Gage FH, Björklund A. Intracerebral grafting of neuronal cell suspensions into the adult brain. Cent Nerv Syst Trauma J Am Paralys Assoc. 1984;1(1):47–56. [DOI] [PubMed] [Google Scholar]
- 89.Watson CL, Mahe MM, Múnera J, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20(11):1310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18(4):618–23. [DOI] [PubMed] [Google Scholar]
- 91.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4. 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Chiola S, Yang G, et al. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. Nat Commun. 2022;13:5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van den Berg CW, Ritsma L, Avramut MC, et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 2018;10(3):751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Espuny-Camacho I, Michelsen KA, Gall D, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77(3):440–56. 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Linaro D, Vermaercke B, Iwata R, et al. Xenotransplanted human cortical neurons reveal species-specific development and functional integration into mouse visual circuits. Neuron. 2019;104(5):972-986.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mansour AA, Gonçalves JT, Bloyd CW, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36(5):432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Real R, Peter M, Trabalza A, et al. In vivo modeling of human neuron dynamics and down syndrome. Science. 2018;362(6416):eaau1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitahara T, Sakaguchi H, Morizane A, et al. Axonal extensions along corticospinal tracts from transplanted human cerebral organoids. Stem Cell Rep. 2020;15(2):467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002;3(5):401–9. [DOI] [PubMed] [Google Scholar]
- 100.Lodato S, Arlotta P. Generating neuronal diversity in the mammalian cerebral cortex. Annu Rev Cell Dev Biol. 2015;31:699–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiong M, Tao Y, Gao Q, Feng B, Yan W, Zhou Y, Kotsonis TA, Yuan T, You Z, Wu Z, Xi J, Haberman A, Graham J, Block J, Zhou W, Chen Y, Zhang SC. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell. 2021;28(1):112-126.e6. 10.1016/j.stem.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kichula EA, Huntley GW. Developmental and comparative aspects of posterior medial thalamocortical innervation of the barrel cortex in mice and rats. J Comp Neurol. 2008;509(3):239–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vonderwalde I, Azimi A, Rolvink G, et al. Transplantation of directly reprogrammed human neural precursor cells following stroke promotes synaptogenesis and functional recovery. Transl Stroke Res. 2020;11(1):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weston NM, Sun D. The potential of stem cells in treatment of traumatic brain injury. Curr Neurol Neurosci Rep. 2018;18(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hakon J, Quattromani MJ, Sjölund C, et al. Multisensory stimulation improves functional recovery and resting-state functional connectivity in the mouse brain after stroke. NeuroImage Clin. 2018;17:717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tyson JA, Anderson SA. GABAergic interneuron transplants to study development and treat disease. Trends Neurosci. 2014;37(3):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao SY, Tao MD, Lou SN, et al. Functional reconstruction of the impaired cortex and motor function by hMGEOs transplantation in stroke. Biochem Biophys Res Commun. 2023;671:87–95. [DOI] [PubMed] [Google Scholar]
- 108.Histology of the nervous system: of man and vertebrates.
- 109.Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. [DOI] [PubMed] [Google Scholar]
- 111.Huo C, Zhang X, Gu Y, et al. Organoids: construction and application in gastric cancer. Biomolecules. 2023;13(5):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vazin T, Ball KA, Lu H, et al. Efficient derivation of cortical glutamatergic neurons from human pluripotent stem cells: a model system to study neurotoxicity in Alzheimer’s disease. Neurobiol Dis. 2014;62:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bagley JA, Reumann D, Bian S, et al. Fused dorsal-ventral cerebral organoids model complex interactions between diverse brain regions. Nat Methods. 2017;14(7):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen KG, Mallon BS, Park K, et al. Pluripotent stem cell platforms for drug discovery. Trends Mol Med. 2018;24(9):805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McFarland DJ, Wolpaw JR. Brain-computer interfaces for communication and control. Commun ACM. 2011;54(5):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain-computer interfaces for communication and rehabilitation. Nat Rev Neurol. 2016;12(9):513–25. [DOI] [PubMed] [Google Scholar]
- 119.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18(10):573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patil N, Korenfeld O, Scalf RN, et al. Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury. Stem Cell Res Ther. 2023;14:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Purushothaman G, Scott BB, Bradley DC. An acute method for multielectrode recording from the interior of sulci and other deep brain areas. J Neurosci Methods. 2006;153(1):86–94. [DOI] [PubMed] [Google Scholar]
- 122.Hu N, Shi JX, Chen C, et al. Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury. Nat Commun. 2024;15:9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duarte AA, Gogola E, Sachs N, et al. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat Methods. 2018;15(2):134–40. [DOI] [PubMed] [Google Scholar]
- 124.Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaushik G, Ponnusamy MP, Batra SK. Concise review: current status of three-dimensional organoids as preclinical models. Stem Cells Dayt Ohio. 2018;36(9):1329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Czerniecki SM, Cruz NM, Harder JL, et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell. 2018;22(6):929-940.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Costamagna G, Comi GP, Corti S. Advancing drug discovery for neurological disorders using iPSC-derived neural organoids. Int J Mol Sci. 2021;22(5):2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Festing S, Wilkinson R. The ethics of animal research Talking Point on the use of animals in scientific research. EMBO Rep. 2007;8(6):526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma L, Ja K. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. [DOI] [PubMed] [Google Scholar]
- 130.Gjorevski N, Ranga A, Lutolf MP. Bioengineering approaches to guide stem cell-based organogenesis. Dev Camb Engl. 2014;141(9):1794–804. [DOI] [PubMed] [Google Scholar]
- 131.Lancaster MA, Corsini NS, Wolfinger S, et al. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35(7):659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Magré L, Verstegen MMA, Buschow S, et al. Emerging organoid-immune co-culture models for cancer research: from oncoimmunology to personalized immunotherapies. J Immunother Cancer. 2023;11(5): e006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Paul J. Why animal experimentation matters: the use of animals in medical research. 1st ed. New Brunswick, NJ: Routledge; 2000. p. 258. [Google Scholar]
- 134.Olsson IAS, Hansen AK, Sandøe P. Ethics and refinement in animal research. Science. 2007;317(5845):1680. [DOI] [PubMed] [Google Scholar]
- 135.Congregation for the Doctrine of the Faith. The Congregation for the Doctrine of the Faith, Donum Vitae. Instruction on respect for human life in its origin and the dignity of procreation. 1987.
- 136.Bredenoord AL, Braude P. Ethics of mitochondrial gene replacement: from bench to bedside. BMJ. 2010;341: c6021. [DOI] [PubMed] [Google Scholar]
- 137.Bredenoord AL, Pennings G, Smeets HJ, et al. Dealing with uncertainties: ethics of prenatal diagnosis and preimplantation genetic diagnosis to prevent mitochondrial disorders. Hum Reprod Update. 2008;14(1):83–94. [DOI] [PubMed] [Google Scholar]
- 138.King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. 2014;5(4):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niemansburg SL, van Delden JJ, Dhert WJ, et al. Regenerative medicine interventions for orthopedic disorders: ethical issues in the translation into patients. Regen Med. 2013;8(1):65–73. [DOI] [PubMed] [Google Scholar]
- 140.Kimmelman J. Ethics, ambiguity aversion, and the review of complex translational clinical trials. Bioethics. 2012;26(5):242–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.