Abstract
Previous investigations in our laboratory have found that the stimulus effects of the hallucinogenic serotonergic agonists DOM and LSD are potentiated by phencyclidine [PCP], a non-competitive NMDA antagonist. Also suggestive of behaviorally significant serotonergic/glutamatergic interactions is our finding that stimulus control by both PCP and LSD is partially antagonized by the mGlu2/3 agonist, LY 379268. These observations coupled with the fact that the stimulus effects of LSD and DOM are potentiated by selective serotonin reuptake inhibitors [SSRI’s] led us in the present investigation to test the hypothesis that stimulus control by PCP is potentiated by the SSRI, citalopram. Stimulus control was established with PCP [3.0 mg/kg; 30 min pretreatment time] in a group of 12 rats. A two-lever, fixed ratio10, positively reinforced task with saline controls was employed. Potentiation by citalopram of an intermediate dose of PCP was observed. In an attempt to establish the mechanism by which citalopram might interact with PCP, subsequent experiments examined the effects on that interaction of antagonists at serotonergic receptors. It was found that the selective 5-HT2C-selective antagonists, SDZ SER 082 and SB 242084, significantly, albeit only partially, blocked the effects of citalopram on PCP. In agreement with our previous conclusions regarding the interaction of citalopram with DOM, the present data suggest that potentiation of the stimulus effects of PCP by citalopram are mediated in part by agonist activity at 5-HT2C receptors.
Keywords: Drug discrimination, Phencyclidine, Citalopram, SB 242084, SDZ SER 082, Rat
1. Introduction
Previous studies in our laboratory have found that the stimulus effects in rats of both indoleamine and phenethylamine hallucinogens are augmented by the co-administration of the selective serotonin reuptake inhibitors fluoxetine, fluvoxamine, and venlafaxine [SSRIs; Fiorella et al., 1996; Winter et al., 1999a; 2002]. These findings are in general agreement with an anecdotal report of an increase in the effects of lysergic acid diethylamide [LSD] in an individual who co-administered fluoxetine in an attempt to augment the LSD experience [Bonson and Murphy, 1996] and of LSD flashbacks in persons with a history of LSD abuse subsequently treated with SSRI’s [Markel et al., 1994]. Interpretation of the animal data is confounded by the fact that SSRIs may partially mimic the stimulus effects of the phenethylamine hallucinogen, [-]-2,5-dimethoxy-4-methylamphetamine [DOM; Winter et al., 1999b] or may inhibit the metabolism of DOM [Eckler et al., 2002]. However, these interpretational problems are largely overcome by the use of citalopram, an SSRI which appears to be truly selective for the serotonin transporter [Bymaster et al., 2002; Hyttel, 1994; Milne and Goa, 1991] and which neither mimics the stimulus effects of DOM nor alters its metabolism [Eckler et al., 2002].
On the basis of reports that phencyclidine [PCP] and dizocilpine, non-competitive antagonists of the NMDA subtype of ionotropic glutamate receptors, increase serotonin levels in rat brain [Yan et al., 1997; Martin et al., 1998] we reasoned that NMDA antagonists might potentiate the stimulus effects of hallucinogens in a fashion similar to that of the SSRI’s. Indeed, it subsequently was found that stimulus control by both DOM [Winter et al., 2000a] and LSD [Winter et al., 2004] is potentiated by PCP. Thus having observed potentiation of serotonergic hallucinogens by serotonergic agents, the SSRIs, and by an NMDA antagonist, PCP, the present investigation tested the symmetry of these serotonergic/glutamatergic interactions by examining the effects of citalopram in rats trained with PCP as a discriminative stimulus. Furthermore, on the basis of a previous investigation which concluded that the effects of citalopram on stimulus control by DOM are partially mediated by 5-HT2C receptors [Eckler et al., 2004], we tested the effects of selective serotonergic antagonists.
2. Materials and Methods
2.1. Subjects
A group of 12 male Fischer 344 rats was obtained at an age of approximately 6 weeks from Harlan Sprague-Dawley Inc. [Indianapolis, IN, U.S.A.], housed in pairs under a 12-hr light-dark cycle beginning at 6:00 a.m., and allowed free access to water in their home cages. All training and testing took place during the light cycle. Caloric intake was controlled to maintain a mean body weight of approximately 275 g. Subjects were fed standard rat chow following experimental sessions. Caloric control has been shown to lengthen the life span and decrease the incidence of a variety of pathologies in Fischer 344 rats [Keenan et al. 1994]. Based on a recent sample of 25 rats, the average life span under these conditions is 34.3 months [SEM 1.1]. Animals used in these studies were maintained in accordance with U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals as amended August 2002. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
2.2. Discrimination training
Six small animal test chambers [MED Associates ENV-008] were used for all experiments. These were housed in larger light-proof, sound-insulated boxes which contained a house light and an exhaust fan. Chambers contained two levers mounted at opposite ends of one wall. Centered between the levers was a dipper which delivered 0.1 ml of sweetened condensed milk diluted 2:1 with tap water. Sessions were managed by a micro-computer using operant control software [MED-PC State Notation, Version IV].
After learning to drink from the dipper, rats were trained to press first one and then the other of the two levers. The number of responses for each reinforcement was gradually increased from 1 to 10. During this time, the reinforced lever was alternated on a random basis. All subsequent training and testing sessions used a fixed-ratio 10 (FR10) schedule of reinforcement. Discrimination training was then begun. Subjects were trained to discriminate PCP [3.0 mg/kg, 30 min pretreatment time, IP; N = 12] from saline as described previously (Hirshhorn and Winter, 1971; Fiorella et al., 1995a). Following the administration of PCP, every tenth response on the PCP-appropriate lever was reinforced. Similarly, responses on the saline-appropriate lever were reinforced on a FR10 schedule following the injection of saline. For half of the subjects, the left lever was designated as the PCP-appropriate lever. During discrimination training, PCP and saline were alternated on a daily basis. PCP-induced stimulus control was assumed to be present when, in five consecutive sessions, 83 % or more of all responses prior to the delivery of the first reinforcer were on the appropriate lever, i.e., no more than 2 incorrect responses prior to completion of the FR10 on the correct lever.
2.3. Test procedures
After stimulus control with PCP was well established, tests of generalization were conducted once per week in each animal. Tests were balanced between subjects trained on the previous day with saline and PCP, respectively. During test sessions, no responses were reinforced and the session was terminated after the emission of 10 responses on either lever. The distribution of responses between the two levers was expressed as the percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated for each session by dividing total number of responses emitted prior to lever selection, that is, prior to the emission of 10 responses on either lever, by elapsed time. Data for any subjects failing to emit 10 responses within the constraints of the 10 min test session were not considered in the calculation of the percent drug-appropriate responding but were included in the analysis of response rates. For purposes of discussion of these data, an intermediate degree of generalization or antagonism is defined as being present when the mean response distribution after a test drug or combination of drugs is less than 80% drug-appropriate and is statistically significantly different from the results following both training conditions.
The effects of citalopram on PCP-induced stimulus control were assessed by co-administration of citalopram [3.0 mg/kg, 90 min pretreatment] and PCP [30 minutes before testing]. The interactions of serotonergic antagonists with the effects of citalopram on PCP-induced stimulus control were assessed in experiments in which the antagonists were administered in combination with citalopram and PCP.
2.4. Drugs
The following drugs were generously provided by the organizations indicated: PCP HCl [National Institute on Drug Abuse, Rockville, MD, USA], racemic citalopram hydrobromide [H. Lundbeck A/S, Copenhagen, Denmark], SB 242084 [GlaxoSmithKline, Great Britain]. The following were purchased from the commercial sources indicated: m-chlorophenylpiperazine [mCPP] and pirenperone [Sigma-Aldrich USA], SDZ SER 082 and WAY 100635 [Tocris, USA]. A stock solution of pirenperone [1 mg/ml] was made in a minimal volume of a 45 percent w/v aqueous solution of 2-hydroxy-propyl-β-cyclodextrin and solutions for injection were made by diluting the stock with sterile 0.9% NaCl. M100907 was synthesized at the Laboratory of Medicinal Chemistry, National Institute of Diabetes, Digestive and Kidney Disorders at the National Institutes of Health [Bethesda, MD]. A stock solution of M100907 [0.5 mg/ml] was made by dissolving M100907 in a minimal volume of 0.2% w/v tartaric acid and diluting with water. All other drugs were dissolved in 0.9% saline. Doses are expressed as mg/kg of the salts. The IP route was employed for all drugs with the exception of WAY-100635 which was administered SC. An injection volume of 1 ml/kg body weight was employed for all drugs.
2.5. Statistical analysis
The statistical significance of the interaction between citalopram and the stimulus effects of PCP was determined using 2-way repeated measures ANOVA with dose of PCP and treatment with citalopram as factors. For assessment of the statistical significance of antagonism by various drugs of the potentiation of PCP by citalopram, 1-way repeated measures ANOVA compared the results of PCP alone, PCP + citalopram, and PCP + citalopram + antagonist. Pairwise comparisons following ANOVA were made using the Holm-Sidak method. Differences were considered to be statistically significant if the probability of their having arisen by chance was < 0.05. All analyses were conducted using SigmaStat 3.0 for Windows™ [Jandel Scientific Software, San Rafael, CA]. Control data were repeated for each comparison and statistical analyses were applied using the appropriate control sessions. However, for purposes of clarity, mean values for control data are shown in all figures.
3. Results
3.1 Potentiation of PCP by citalopram
Preliminary experiments examined the time course of interaction of citalopram [3 mg/kg] with an intermediate dose of PCP [1.0 mg/kg]. Although the stimulus effects of PCP were enhanced using pretreatment times as brief as 15 min, maximum enhancement occurred using a pretreatment time of 90 minutes and all subsequent experiments used that pretreatment time.
Figure 1 shows an orderly dose-related increase in PCP-appropriate responding in rats trained and tested with PCP. When the same doses were tested in rats pre-treated with a fixed dose of citalopram, PCP-appropriate responding increased for all doses of PCP less than the training dose. For PCP doses of 0.6 and 1.0 mg/kg, two-way repeated measures ANOVA revealed a significant increase in PCP-appropriate responding following the combination of citalopram and PCP compared with PCP alone [F[1,9] = 59.31; p = 0.001]. Response rates were not altered by pretreatment with citalopram.
Figure 1.
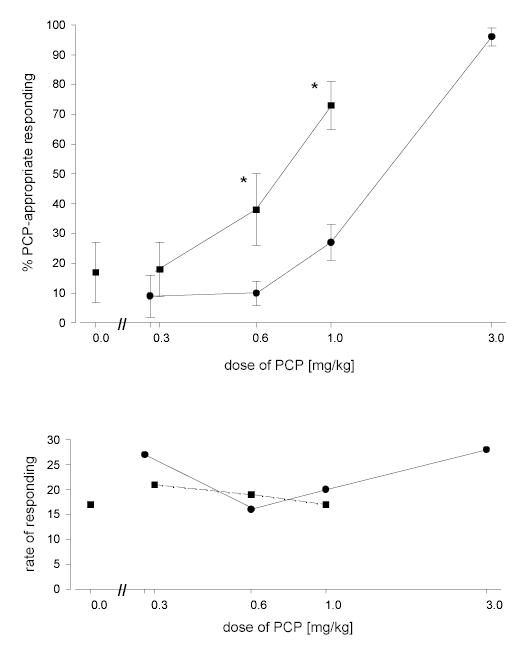
Dose-response relationship for PCP alone and in combination with citalopram. Circles represent the effects of PCP alone in rats trained with PCP as a discriminative stimulus [3.0 mg/kg; 30 minute pretreatment time]. Squares represent the effects of PCP in combination with citalopram [3.0 mg/kg; 90 minute pretreatment time]. Each point represents the mean of one determination in each of 10 rats with the exception of the training dose where the mean of 4 determinations in each of the subjects is shown. Standard errors of the means are indicated. An asterisk indicates a statistically significant difference between PCP alone and in combination with citalopram. The point at a dose of 0.0 is for citalopram alone. Ordinate: upper panel: percent PCP-appropriate responding. Lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
3.2 Antagonism of the potentiation of PCP by citalopram
In figure 2 are shown the results of tests of interactions between a series of serotonergic antagonists in combination with an intermediate dose of PCP [1.0 mg/kg] following pretreatment with citalopram. It is seen that the selective 5-HT2A antagonist, M-100907, the non-selective 5-HT2 receptor antagonist, pirenperone, and the selective HT1A receptor antagonist, WAY-100635, do not block the potentiation of the stimulus effects of PCP by citalopram. In contrast, the selective 5-HT2C receptor antagonist, SDZ SER 082, at doses of 0.3 and 1.0 mg/kg antagonized the interaction of citalopram with PCP [F (2,9) = 22.040, P < 0.001; F (2,9) = 20.689, P < 0.001, respectively]. Likewise, the selective 5-HT2C receptor antagonist, SB 242,084, at a dose of 2.0 mg/kg significantly decreased the interaction between PCP and citalopram [F (2,9) = 30.899, P < 0.001]. Subsequent pair-wise comparisons revealed significant differences between PCP [1.0 mg/kg] alone, PCP+citalopram, and PCP+citalopram+antagonist thus meeting our criteria for intermediate antagonism. In separate experiments, no statistically significant antagonism of the training dose of PCP was observed in the presence of M-100907, pirenperone, WAY-100635, SDZ SER 082, or SB 242084 [data not shown].
Figure 2.
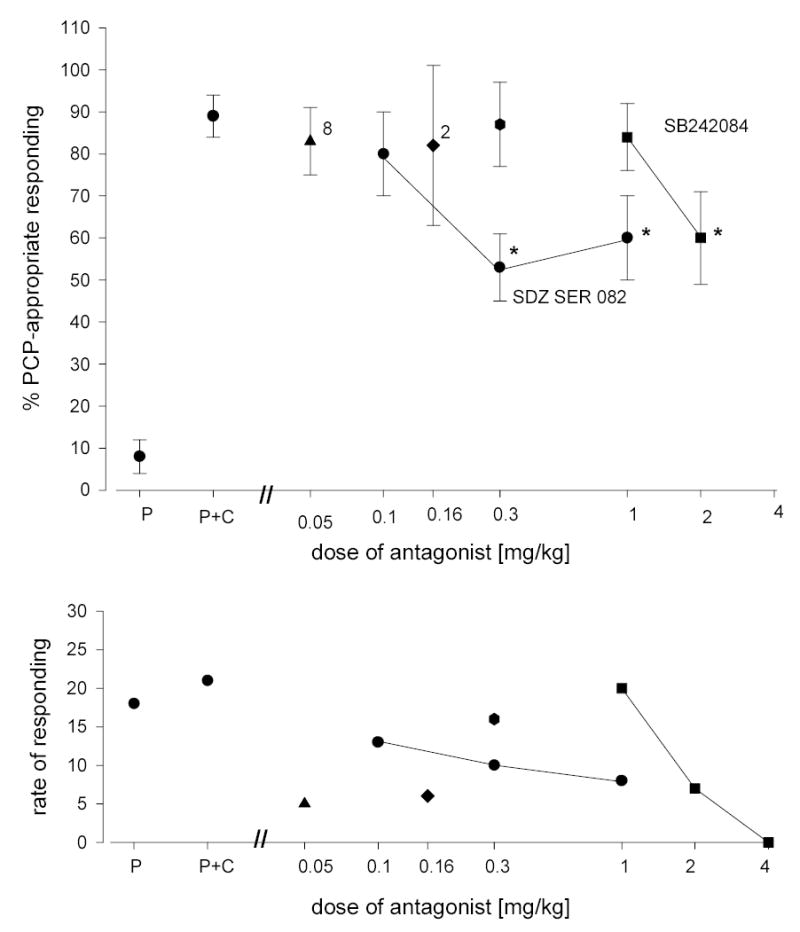
The effects of selected serotonergic antagonists on the potentiation of the stimulus effects of PCP [1.0 mg/kg; 30 minute pretreatment time] following the administration of citalopram [3.0 mg/kg; 90 minutes pretreatment time]. The point indicated by P on the abscissa is for PCP [1.0 mg/kg] alone. The point indicated by P+C on the abscissa is for the combination of PCP and citalopram. Other points shows the effects of P+C in combination with the 5-HT2A antagonist, M-100907 [triangle], the 5-HT2 antagonist, pirenperone [diamond], the 5-HT1A antagonist, WAY-100635 [hexagon], and the 5-HT2C antagonists, SDZ SER 082 [circles] and SB 242084 [squares], respectively. All points represent the mean of one determination in each of 10 rats. An asterisk indicates a statistically significant difference between P+C alone and in combination with an antagonist. A numeral adjacent to a point indicates the number of subjects completing the test if other than 10. Ordinate: upper panel: percent PCP-appropriate responding. Lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
3.3 Interaction of the non-selective 5-HT2C receptor agonist, mCPP, with PCP.
Based upon the results seen in figure 2 with selective antagonists at 5-HT2C receptors, we examined the effect of a non-selective 5-HT2C receptor agonist, meta-chlorophenylpiperazine [mCPP; Callahan and Cunningham, 1994; Fiorella et al., 1995b; Pauwels, 2003], on the stimulus effects of an intermediate dose of PCP. For doses of PCP and mCPP of 1.0 and 0.3 mg/kg, respectively, repeated measures ANOVA revealed a significant increase in PCP-appropriate responding following the combination of mCPP and PCP compared with PCP alone [N = 6; F(2,5) = 11.077; P = 0.003].
4. Discussion
We previously provided evidence that stimulus control by LSD and by DOM is enhanced both by SSRIs [Fiorella et al., 1996; Winter et al., 1999a; 2002] and by non-competitive NMDA antagonists including PCP [Winter et al., 2000a]. The latter observation of behaviorally significant serotonergic/glutamatergic interactions is extended by the present data [figure 1] to include potentiation of PCP-induced stimulus control by the SSRI, citalopram. Because citalopram is selective for the serotonin transporter [Millan et al., 2000], we may rule out a direct role for increased levels of either dopamine or norepinephrine. However, in as much as citalopram would be expected to increase levels of serotonin at all serotonin receptors, the data provided in figure 1 do not define the specific receptor or receptors involved. Of the 14 serotonin receptors now recognized [Hoyer et al., 2002], we chose to examine 5-HT1A, 5-HT2A, and 5-HT2C because each may play a role in glutamate release, one of the proposed mechanisms by which not only PCP but also LSD and the phenethylamine hallucinogens exert their behavioral effects [Aghajanian and Marek, 1999; 2000; Winter et al., 2004].
A functionally significant role for activation or antagonism of 5-HT1A receptors in the effects of citalopram as well as non-competitive NMDA antagonists is suggested by several studies. The selective 5-HT1A-selective antagonists WAY-100135 and WAY-100635 antagonize citalopram-induced hypothermia [Oerther and Ahlenius, 2001] and certain of the behavioral effects of the non-competitive NMDA antagonist, dizocilpine [MK-801] [Loscher and Honack, 1993; Wedzony et al., 2000]. In addition, dizocilpine increases the number of 5-HT1A receptors in rat brain [Wedzony et al., 1997] and Czyrak and her colleagues [2003] have identified 5-HT1A receptors in the rat cingulate cortex which they believe may regulate glutamate release. However, it is seen in figure 2 that the selective 5-HT1A antagonist, WAY-100635, does not alter the potentiating effects of citalopram. The dose chosen, 0.3 mg/kg, was previously shown in our laboratory to fully block the effects of the 5-HT1A agonist, 8-hydroxy-2-dipropylaminotetralin [8-OH-DPAT; Winter et al., 2000b]. The absence of an effect of WAY-100635 on the potentiation of PCP by citalopram is in keeping with our previous observation that WAY-100635 does not alter the effects of citalopram on stimulus control by DOM [Eckler et al., 2004].
It has long been recognized that the stimulus effects of both indoleamine and phenethylamine hallucinogens are mediated by serotonergic receptors [Browne and Ho, 1975; Winter, 1975; 1978] specifically those of the 5-HT2 subtype [Glennon et al., 1983; 1984; Fiorella et al., 1995a] and that PCP acts via blockade of NMDA receptors [Anis et al., 1983; Zukin and Zukin, 1979; Koek, 1999]. In a most provocative hypothesis, Aghajanian and Marek [1999; 2000] proposed on the basis of electrophysiological evidence that release of glutamate represents a final common pathway for hallucinogens whose direct effects are on either NMDA or serotonergic receptors. We have recently provided direct support for this hypothesis by demonstrating that LSD-induced stimulus control is potentiated by a glutamate releaser, LY341495, and partially antagonized by LY379268, an mGlu2/3 agonist which inhibits glutamate release [Winter et al., 2004]. In addition, it has recently been shown using in vivo microdialysis that LSD as well as the phenethylamine hallucinogens, 2,5-dimethoxy-4-methylamphetamine [DOM] and 2,5-dimethoxy-4-iodoamphetamine [DOI], increase extracellular glutamate in rat brain [Scruggs et al., 2003; Muschamp et al., 2004]. Moghaddam and Adams [1998] observed similar increases in serotonin levels in rat brain following systemic treatment with PCP. Because LSD-induced release of glutamate is antagonized by the selective 5-HT2A antagonist, M100907 [Muschamp et al., 2004], we tested the hypothesis that citalopram potentiates the stimulus effects of PCP via agonism at 5-HT2A receptors. However, neither M100907 nor pirenperone diminished the effect of citalopram on stimulus control by PCP [figure 2]. It should be noted that the doses of pirenperone [0.16 mg/kg] and M-100907 [0.05 mg/kg] used in the present study have previously been found to antagonize completely the stimulus effects of LSD in F 344 rats [Winter and Rabin, 1988; Winter et al., 2004]. A puzzling aspect of the interaction between the combination of PCP and citalopram and the 5-HT2 receptor antagonists, M100907 and pirenperone, is the rate decreasing effect seen in figure 2; indeed, following pirenperone, only 2 of 10 subjects completed the interaction tests. Previously we observed similar rate-suppressing effects of pirenperone in combination with agents presumed to act as agonists at 5-HT1A receptors [Winter and Rabin, 1988]. Although we are unaware of definitive behavioral data with respect to the selectivity of pirenperone for 5-HT2 receptor subtypes, in vitro second messenger studies suggest a 250-fold higher affinity for the 5-HT2A subtype as compared with the 5-HT2C subtype [Hoyer et al., 1994].
Abundant evidence suggests that serotonin plays a significant role in glutamate release or glutamatergic functions and most studies implicate the 5-HT2A receptor [Arvonov et al., 1999; Meller et al., 2002; Regina et al., 2004]. However, the fact that there is a highly significant correlation between agonist activity at 5-HT2A and 5-HT2C receptors [Nichols, 2004] has made difficult an assessment of the part played by the latter receptor. Nonetheless, glutamate release by serotonergic agonists such as LSD or DOI is fully blocked by M100907 [Scruggs et al., 2003; Muschamp et al., 2004] and, in those studies in which selective 5-HT2C antagonists have been employed, negative results have been obtained [Marcoli et al., 2001; Martin-Ruiz, 2001; Dawson et al., 2002; Pei et al., 2004]. Despite these caveats, a prominent role for 5-HT2C receptors in the actions of citalopram is suggested by the studies of Millan and his colleagues [Millan et al., 1999; Dekeyne et al., 2001] in which citalopram was trained as a discriminative stimulus in the rat. Citalopram generalized to the selective 5-HT2C agonist, Ro 60-0175, and was blocked by the selective 5-HT2C antagonist, SB-242084 [Kennett et al., 1997]. Furthermore, previous work in our laboratory provided evidence that sensitization to the stimulus effects of LSD following serotonin depletion in the rat is accompanied by upregulation of the 5-HT2C receptor [Fiorella et al., 1995c]. In addition, we previously observed that SB-242084 significantly but incompletely blocks augmentation of the stimulus effects of DOM by citalopram [Eckler et al., 2004]. For these reasons, we examined SB-242084 [Kennett et al., 1997] and SDZ SER 082 [Nozulak et al., 1995] in combination with citalopram and PCP [figure 2]. The results obtained strongly suggest that, indeed, the 5-HT2C receptor is a significant factor in the effects of citalopram on DOM. However, the fact that antagonism by both drugs was intermediate in nature, i.e., statistically significant but less than complete, leaves open the possibility that other factors are involved. A further test of the importance of actions at the 5-HT2C receptor would be a demonstration that agonists at 5-HT2C receptors potentiate the stimulus effects of PCP. To that end, we examined the interaction with PCP of the 5-HT2C/2B agonist, mCPP and significant potentiation of an intermediate dose of PCP was observed. Unfortunately, the more selective 5-HT2C receptor agonist, Ro 60-0175, used by Millan et al. [1999] to characterize the stimulus effects of citalopram was not available to us due to institutional constraints. Nonetheless, the observed effects of mCPP on PCP-induced stimulus control together with the observation that potentiation of PCP by citalopram is significantly antagonized by SB-242084 and by SDZ SER 082 support the hypothesis that citalopram acts to potentiate stimulus control by DOM via a 5-HT2C-mediated mechanism.
It should be noted that a racemic mixture of citalopram was employed in the present studies. In future investigations it would be well to examine the respective contributions of the [R]- and [S]- isomers of citalopram. Recent evidence suggests that the [S]-isomer [escitalopram] is the more active of the two [Hyttel et al., 1992] and that, indeed, [R]-citalopram may antagonize certain of the effects of escitalopram [Mork et al., 2003; Sanchez, 2003; Fish et al., 2004]. In our previous studies of the potentiation of the phenethylamine hallucinogen, DOM, by citalopram [Eckler et al., 2002], pharmacokinetic factors were ruled out by the measurement of DOM levels in the brain. Although citalopram is believed to interact minimally with cytochrome P450 [CYP] enzymes [for reviews, see Brosen and Naranjo, 2001; Spina et al., 2003], the fact that PCP is metabolized by CYP enzymes [Laurenzana and Owens, 1997] makes plausible a pharmacokinetic contribution to the present behavioral interaction between PCP and citalopram. This hypothesis was not tested in the present investigation.
The present data add to the body of evidence which indicates that functionally significant interactions occur between glutamatergic and serotonergic systems [Aghajanian and Marek, 2000; Carlsson et al., 2001; Winter et al., 2000a; 2004]. These interactions may provide important clues as to the mechanisms of action of multiple classes of hallucinogenic drugs as well to reconcile and to integrate current hypotheses as to the etiology of psychotic disorders. We suggest that drug-induced stimulus control, a behavioral technique which over the past three decades has contributed to our understanding of a variety of psychoactive drugs may provide fresh insight into serotonergic/glutamatergic interactions.
Acknowledgments
This study was supported in part by U.S. Public Health Service grant DA 03385 [J. C. W.; R. A. R.] and by National Research Service Award F30 DA14238 [J. R. E.]. We thank Ms. B.A. Winter for technical contributions.
References
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurons by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Russo A, Wang RY. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci. 1999;11:3064–3072. doi: 10.1046/j.1460-9568.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- Berendsen HHG, Broekkamp CLE. Comparison of stimulus properties of fluoxetine and 5-HT receptor agonists in a conditioned taste aversion procedure. Eur J Pharmacol. 1994;253:83–89. doi: 10.1016/0014-2999(94)90760-9. 06 07 05 Deleted from final proofs. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Murphy DL. Alterations in responses to LSD in humans associated with chronic administration of tricyclic antidepressants, monoamine oxidase inhibitors or lithium. Behav Brain Res. 1996;73:229–233. doi: 10.1016/0166-4328(96)00102-7. [DOI] [PubMed] [Google Scholar]
- Brosen K, Naranjo CA. Review of pharmacokinetic and pharmacodynamic interaction studies with citalopram. Eur Neuropsychopharmacol. 2001;11:275–283. doi: 10.1016/s0924-977x(01)00101-8. [DOI] [PubMed] [Google Scholar]
- Browne RG, Ho BT. Role of serotonin in the discriminative stimulus properties of mescaline. Pharmacol Biochem Behav. 1975;3:429–435. doi: 10.1016/0091-3057(75)90052-0. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine but not other selective serotonin uptake inhibitors increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology. 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine. Eur J Pharmacol. 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Czepiel K, Mackowiak M, Chocyk A, Wedzony K. Serotonin 5-HT1A receptors might control the output of cortical glutamatergic neurons in rat cingulate cortex. Brain Res. 2003;989:42–51. doi: 10.1016/s0006-8993(03)03352-3. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Galandak J, Djali S. Attenuation of ischemic efflux of endogenous amino acids by the novel 5-HT1A/5-HT2 receptor ligand adatanserin. Neurochem Int. 2002;40:203–209. doi: 10.1016/s0197-0186(01)00082-1. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Loretta I, Millan MJ. Following long-term training with citalopram, both mirtazapine and mianserin block its discriminative stimulus properties. Psychopharmacology. 2001;153:389–393. doi: 10.1007/s002130000632. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Doat M, Rabin RA, Winter JC. Potentiation of DOM-induced stimulus control by fluoxetine and citalopram: the role of pharmacokinetics. Life Sci. 2002;71:1341–1347. doi: 10.1016/s0024-3205(02)01861-1. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Chang-Fong J, Rabin RA, Smith C, Teitler M, Glennon RA, Winter JC. Behavioral characterization of 2’ and 5’ O-demethyl metabolites of the phenethylamine hallucinogen DOM. Pharmacol Biochem Behav. 2003;75:845–852. doi: 10.1016/s0091-3057(03)00159-x. 06 07 05 Deleted from final proofs. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT2C receptor-mediated interaction between 2,5-dimethoxy-4-methylamphetamine [DOM] and citalopram in the rat. Pharmacol Biochem Behav. 2004;79:25–30. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995a;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects m-chlorophenylpiperazine. Psychopharmacology. 1995b;119:222–230. doi: 10.1007/BF02246164. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley SE, Lorrain DS, Palumbo PA, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology. 1995c;121:364–372. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley S, Winter JC, Rabin RA. Potentiation of LSD-induced stimulus control by fluoxetine in the rat. Life Sci. 1996;59:PL283–PL287. doi: 10.1016/0024-3205(96)00490-0. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidoma S, Gupta S, Miczek KA. Anxiolytic-like effects of escitalopram, citalopram and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther. 2004;308:474–480. doi: 10.1124/jpet.103.058206. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported antagonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983;91:189–196. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Hirschhorn ID, Winter JC. Mescaline and lysergic acid diethylamide [LSD] as discriminative stimuli. Psychopharmacology. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clark DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine [serotonin] Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological, and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors [SSRIs] Int Clin Psychopharmacol. 1994;9(suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Hyttel J, Bogeso KP, Perregaard J, Sanchez C. The pharmacological effect of citalopram resides in the (S)-(+)-enantiomer. J Neural Transm Gen Sect. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Smith PF, Hertzog P, Soper K, Ballam GC, Clark RL. The effects of overfeeding and dietary restriction on Sprague-Dawley rat survival and early pathology biomarkers of aging. Toxicologic Pathology. 1994;22:300–315. doi: 10.1177/019262339402200308. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacol. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Koek W. N-Methyl-D-aspartate antagonists and drug discrimination. Pharmacol Biochem Behav. 1999;64:275–281. doi: 10.1016/s0091-3057(99)00055-6. [DOI] [PubMed] [Google Scholar]
- Laurenzana EM, Owens SM. Metabolism of phencyclidine by human liver microsomes. Drug Metab Dispos. 1997;25:557–563. [PubMed] [Google Scholar]
- Loscher W, Honack D. Effects of the novel 5-HT1A receptor antagonist, (+)-WAY 100135, on stereotyped behavior induced by the NMDA receptor antagonist dizocilpine in rats. Eur J Pharmacol. 1993;242:99–104. doi: 10.1016/0014-2999(93)90015-a. [DOI] [PubMed] [Google Scholar]
- Marcoli M, Rosu C, Bonfanti A, Raiteri M, Maura G. Inhibitory presynaptic 5-HT2A receptors regulate evoked glutamate release from rat cerebellar mossy fibers. J Pharmacol Exp Ther. 2001;299:1106–1111. [PubMed] [Google Scholar]
- Markel H, Lec A, Holmes RD, Domino EF. LSD flashback syndrome exacerbated by selective serotonin reuptake inhibitor antidepressants in adolescents. J Pediatrics. 1994;125:817–819. doi: 10.1016/s0022-3476(94)70083-4. [DOI] [PubMed] [Google Scholar]
- Martin P, Carlsson ML, Hjorth S. Systemic PCP treatment elevates brain extracellular 5-HT: a microdialysis study in awake rats. Neuroreport. 1998;9:2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by 5-HT2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Harrison P, Elliott J, Sharp T. In vitro evidence that 5-HT increases efflux of glial glutamate via 5-HT2A receptor activation. J Neurosci Res. 2002;67:399–405. doi: 10.1002/jnr.10126. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Girardon S, Dekeyne A. 5-HT2C receptors are involved in the discriminative stimulus effects of citalopram in rats. Psychopharmacology. 1999;142:432–434. doi: 10.1007/s002130050910. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic, and noradrenergic transmission in the frontal cortex: relevance to the actions of antidepressant agents. J Psychopharmacol. 2000;14:114–138. doi: 10.1177/026988110001400202. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Goa KL. Citalopram. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in depressive illnesses. Drugs. 1991;41:450–477. doi: 10.2165/00003495-199141030-00008. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mork A, Kreilgaard M, Sanchez C. The R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharmacology. 2003;48:167–173. doi: 10.1016/s0028-3908(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. LSD and DOM increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nozulak J, Kallman HO, Floersheim P, Hoyer D, Schoeffter P, Buerki HR. (+0-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolol[1,7-bc][2,6]-naphthyridine: a selective 5-HT2B/2C receptor antagonist with low 5-HT2A receptor affinity. J Med Chem. 1995;38:28–33. doi: 10.1021/jm00001a007. [DOI] [PubMed] [Google Scholar]
- Oerther S, Ahlenius S. Involvement of 5-HT1A and 5-HT1B receptors for citalopram-induced hypothermia in the rat. Psychopharmacology. 2001;154:429–434. doi: 10.1007/s002130000659. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ. 5-HT receptors and their ligands. Tocris Reviews. 2003;No. 25:1–10. [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacol. 2004;46:331–339. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Regina MJ, Bucelli RC, Winter JC, Rabin RA. Cellular mechanisms of serotonin 5-HT2-receptor-mediated cGMP formation: the essential role of glutamate. Brain Research. 2004;1003:168–175. doi: 10.1016/j.brainres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Sanchez C. R-citalopram attenuates anxiolytic effects of escitalopram in a rat ultrasonic vocalization model. Eur J Pharmacol. 2003;464:155–158. doi: 10.1016/s0014-2999(03)01376-1. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane [DOI] increases cortical extracellular glutamate levels in rats. Neurosci Ltr. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Spina E, Scordo MG, D’Arrigo C. Metabolic drug interactions with new psychotropic agents. Fundamental Clin Pharmacol. 2003;17:517–538. doi: 10.1046/j.1472-8206.2003.00193.x. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Mackowiak M, Czyrak A, Fijat K, Michalska B. Single doses of MK-801, a non-competitive antagonist of NMDA receptors, increase the number of 5-HT1A serotonin receptors in the rat brain. Brain Res. 1997;756:84–99. doi: 10.1016/s0006-8993(97)00159-5. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Mackowiak M, Zajaczkowski W, Fijat K, Chocyk A, Czyrak A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology. 2000;23:547–559. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- Winter JC. Blockade of the stimulus properties of mescaline by a serotonergic antagonist. Arch int Pharmacodyn. 1975;214:250–253. [PubMed] [Google Scholar]
- Winter JC. Stimulus properties of phenethylamine hallucinogens and lysergic acid diethylamide: The role of 5-hydroxytryptamine. J Pharmacol Exp Ther. 1978;204:416–423. [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol Biochem Behav. 1988;30:617–624. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Helsley SE, Fiorella D, Rabin RA. The acute effects of monoamine reuptake inhibitors on the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 1999a;63:607–613. doi: 10.1016/s0091-3057(99)00039-8. [DOI] [PubMed] [Google Scholar]
- Winter JC, Fiorella DJ, Helsley SE, Rabin RA. Partial Generalization of [-]DOM to Fluvoxamine in the Rat: Implications for SSRI-induced Mania and Psychosis. Int J Neuropsychopharmacol. 1999b;2:165–172. doi: 10.1017/S1461145799001510. [DOI] [PubMed] [Google Scholar]
- Winter JC, Doat M, Rabin RA. Potentiation of DOM-induced stimulus control by non-competitive NMDA antagonists: A link between the glutamatergic and serotonergic hypotheses of schizophrenia. Life Sci. 2000a;68:337–344. doi: 10.1016/s0024-3205(00)00934-6. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RF, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: A hallucinogen which induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000b;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckle JR, Doat MM, Rabin RA. The Effects of Acute and Chronic Treatment with Fluoxetine and Citalopram on Stimulus Control by DOM. Pharmacol Biochem Behav. 2002;74:95–101. doi: 10.1016/s0091-3057(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: The effects of mGlu2/3 receptor ligands in rats trained with phencyclidine and LSD as discriminative stimuli. Psychopharmacology. 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Yan Q, Reith MEA, Jobe PC, Dailey JW. Dizocilpine [MK-801] increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997;765:149–158. doi: 10.1016/s0006-8993(97)00568-4. [DOI] [PubMed] [Google Scholar]
- Zukin SR, Zukin RS. Specific [3H]phencyclidine binding in rat central nervous system. Proc Nat Acad Sci USA. 1979;76:5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]


