Abstract
Contingency management (CM) rapidly reduces cocaine use, but its effects subside after treatment. Cognitive–behavioral therapy (CBT) produces reductions months after treatment. Combined, the 2 might be complementary. One hundred ninety-three cocaine-using methadone-maintained outpatients were randomly assigned to 12 weeks of group therapy (CBT or a control condition) and voucher availability (CM contingent on cocaine-negative urine or noncontingent). Follow-ups occurred 3, 6, and 12 months posttreatment. Primary outcome was cocaine-negative urine (urinalysis 3 times/week during treatment and once at each follow-up). During treatment, initial effects of CM were dampened by CBT. Posttreatment, there were signs of additive benefits, significant in 3- versus 12-month contrasts. Former CBT participants were also more likely to acknowledge cocaine use and its effects and to report employment.
Among the most reliable treatments for cocaine abuse is the set of behavioral interventions called contingency management (CM), in which a desired behavior such as cocaine abstinence is monitored (typically with repeated urine testing) and reinforced (Higgins & Wong, 1998). The range of reinforcers in CM depends partly on the setting and the targeted drug of abuse; for example, in methadone clinics, heroin abstinence can be reinforced with take-home doses of methadone. Because there is no homologous reinforcer for cocaine abstinence, CM has typically relied on vouchers that are exchangeable for goods and services. One advantage of CM for cocaine abuse is that the onset of its effects is rapid, even instantaneous; for example, the availability of a $100 voucher induced 2 days of cocaine abstinence in 40 of 50 cocaine abusers (Robles et al., 2000). Across periods of several weeks or months, robust results can be obtained with an escalating-reinforcement procedure developed by Higgins and colleagues (1991) and used in our clinic and others (Bigelow, Brooner, & Silverman, 1998; Higgins, 1997; Higgins et al., 1993; Preston, Umbricht, & Epstein, 2000; Preston, Umbricht, Wong, & Epstein, 2001; Silverman et al., 1996; Silverman et al., 1998). In this procedure, the first negative urine specimen is reinforced with a voucher of low value (typically $2.50), but each subsequent negative specimen is reinforced with a voucher of increasingly high value. The procedure has two salient drawbacks. First, its escalating monetary cost is obviously not sustainable indefinitely for any individual patient. (However, some success has been demonstrated with nonescalating-maintenance CM [Preston et al., 2000, 2001] and with low-cost lottery-based CM [Petry, 2000].) Second, when CM is discontinued, its beneficial effects—although not entirely transient (Higgins, Wong, Badger, Haug Ogden, & Dantona, 2000)—do subside (Silverman et al., 1996).
The advantages and drawbacks of escalating-schedule CM are neatly complemented by those of cognitive–behavioral therapy (CBT), a time-limited, present-oriented psychotherapy in which the cocaine-abusing patient is helped to identify the thoughts, feelings, and events that precede and follow each episode of cocaine use and to develop and rehearse coping skills, both drug specific (e.g., avoiding or resisting drug-associated cues) and general (e.g., managing negative affect and finding nondrug sources of reinforcement; Carroll, 2000). Unlike CM, CBT does not reduce cocaine use rapidly; in fact, during the course of active treatment, it appeared to be no better than a control condition (Carroll, Rounsaville, Gordon, et al., 1994). However, in follow-up evaluations 6 or 12 months after treatment cessation, patients who received CBT showed a greater improvement on the Cocaine scale of the Addiction Severity Index (Carroll, Rounsaville, Nich, et al., 1994). This finding suggests that the skills learned in CBT take time to be incorporated into daily behavior. In a more recent study from the same group (Carroll et al., 2000), the difference between a clinical-management control condition and either of two active psychotherapies increased across a 12-month follow-up; the authors suggested that each of the active psychotherapies became effective over time as former patients began to use a therapy-specific set of skills. The effect was small, failing to reach statistical significance. However, a study conducted with alcoholics (O’Malley et al., 1996) had similar and statistically significant findings: At posttreatment follow-up, former CBT participants continued to reduce their drinking, whereas former controls did not.
We hypothesized that CBT and CM, with their almost opposite shortcomings and strengths, might be more effective in combination than alone, producing reductions in cocaine use that would be both rapid and enduring. We tested this by randomly assigning participants to receive either, neither, or both treatments for 12 weeks.
Method
Participants
Participants were selected from 286 patients consecutively admitted for methadone maintenance at Archway Clinic, the treatment-research program of the National Institute on Drug Abuse Intramural Research Program in Baltimore, MD. Eligibility criteria for initial enrollment were: age 18–65, qualification for methadone maintenance according to Food and Drug Administration guidelines, and self-reported history of intravenous opiate use. Eligibility for randomization to a group was based on subsequent cocaine use (see Study Timeline and Groups section). Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; DSM–III–R; American Psychiatric Association, 1987) diagnoses of heroin or cocaine dependence were not required, although nearly all participants met criteria for heroin dependence. Exclusion criteria were current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives; unstable serious medical illness; current pregnancy or breast-feeding of a child; estimated IQ below 80 (Shipley Institute of Living Scale; Zachary, 1986); and urologic conditions that would preclude urine collection. Applicants were screened by telephone and in two on-site visits that included medical, psychiatric, and drug use histories; a physical examination; urine and blood screens; and a battery of assessment instruments, including the Addiction Severity Index (ASI; McLellan et al., 1985) and the Diagnostic Interview Schedule (Helzer, Croughan, Robins, & Ratcliff, 1981). This study was approved by the local institutional review board for human research; all participants gave informed written consent prior to participation.
Standard Treatment
All participants received, without charge, daily methadone and weekly individual counseling for 29 weeks. Methadone HCl (Mallinckrodt, Inc., St. Louis, MO) was administered orally in 35 ml of a cherry-flavored solution. Methadone dose was stabilized at 70 mg/day within the first week and held constant thereafter. Participants could request increases up to 80 mg/day; of the 193 participants whose data we discuss here, 81 requested and received 80 mg/day, 111 remained on 70 mg/day, and 1 could tolerate only 50 mg/day.
For individual-counseling sessions, counselors completed a semistructured psychosocial assessment and treatment plan for each participant. Reduction of substance use was the primary goal. Unlike the group sessions (described below), individual-counseling sessions were devoted to discussion of cessation of all drugs.
Study Timeline and Groups
The study comprised three consecutive phases: (a) baseline treatment (5 weeks), (b) intervention (12 weeks), and (c) maintenance (12 weeks). Every Monday, Wednesday, and Friday, urine specimens were collected under the observation of laboratory technicians.
Baseline began on study enrollment and continued until the participant had provided 15 urine specimens. At that point, the 255 (out of 286) participants who had not dropped out of treatment were eligible for randomization if their urine had tested positive for cocaine at least three times, provided that 3 of the last 12 and 2 of the last 6 specimens were positive. These criteria were met by 193 participants, and their data are reported here. The remaining 62 participants were treated identically to the randomized participants (although some received CM targeted to opiates rather than cocaine), but their data are not reported here. Participants were not told about the randomization criteria.
For the 12-week intervention, participants were randomized to one of four experimental groups (described below). For the 12-week maintenance, standard treatment was resumed. Thereafter, participants were encouraged to transfer to other treatment programs in the community; those who did not were offered a 10-week methadone taper.
Main Outcome Measures: Urine and Breath Toxicology
All urine specimens underwent qualitative testing with an enzyme multiplied immunoassay technique (Syva Corp., Palo Alto, California) system for cocaine (benzoylecgonine equivalents [BZE]), opiates (morphine), marijuana, and benzodiazepines (oxazepam). Cutoffs for positive specimens were 300 ng/ml for cocaine, opiates, and benzodiazepines, and 50 ng/ml for marijuana. Breath alcohol levels were determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO).
For a more precise measure of cocaine use, BZE concentrations were also assayed quantitatively by fluorescence polarization immunoassay using TDx Cocaine Metabolite Assay reagents (Abbott Laboratories, Abbott Park, IL). Cross-reactivity was 100% for BZE and less than 1% for cocaine, ecgonine methyl ester, and ecgonine. The linear range for BZE was 30–5,000 ng/ml; specimens with higher concentrations were diluted to concentrations within this range and reanalyzed. This quantitative assay was intended to be an objective measure of reductions in cocaine use because, in the specimens that were “positive” by qualitative assay, actual concentrations of BZE ranged from 300 ng/ml to greater than 1,000,000 ng/ml.
Other Outcome Measures
Data on self-reported drug use were obtained immediately after each urine collection; participants were asked how many times they had used cocaine or other drugs on each day since the previous urine collection. At the end of each group therapy session (described below), each patient was asked to indicate how much the session had helped him or her, on a Likert scale that ranged from 1 (not at all) to 5 (extremely well).
An exit assessment was administered to each participant when he or she left our program or began the dose taper (whichever came first); assessments included questionnaires about why participants had or had not reduced their cocaine use. Participants returned for follow-up assessments 3, 6, and 12 months after the exit assessment; each follow-up included urine collection and an abbreviated ASI.
CM Conditions
During intervention, participants could receive vouchers after submitting urine specimens every Monday, Wednesday, and Friday. Vouchers were exchangeable for goods and services (e.g., bus passes, clothes) consistent with a drug-free lifestyle. When the participant accumulated enough vouchers to make a purchase, a staff member reviewed the request, then purchased the item and gave it to the participant in the clinic; no money was given directly to participants.
The contingent groups (n = 96) earned vouchers for each specimen that tested negative for cocaine. Specimens were tested on-site with Abuscreen OnTrak immunoassay kits (Roche Diagnostic Systems, Somerville, NJ), which gave qualitative results for BZE (cutoff 300 ng/ml) within 5 min of specimen provision. Voucher values began at $2.50 and increased by $1.50 for each consecutive voucher earned. For every three consecutive vouchers earned, participants received an additional voucher worth $10.00. A participant who tested negative for 12 weeks could earn a total of $1,155 in vouchers. If the participant tested positive or did not provide a specimen, no voucher was given, and the value of the next earned voucher was reset to $2.50.
Each participant in the noncontingent groups (n = 97) was yoked to a randomly prior chosen participant in the contingent groups. (To accomplish the yoking, we assigned the first 6 patients to the contingent groups, but group assignment was random thereafter.) These participants were told that they would receive vouchers on a “totally unpredictable schedule,” contingent only on their providing a urine specimen. Like participants in the contingent groups, they were immediately informed of their immunoassay kit results.
Group Therapy Conditions
During intervention, concurrently with CM, participants were randomized to one of two group therapy conditions: (a) CBT (n = 97) or (b) a social-support control condition (n = 96). Groups met once per week for 90 min. The different types of group were led by different master’slevel counselors, who worked in two separate wings of the clinic building and were urged never to discuss study-related information. Sessions were audiotaped, and the tapes were monitored by David H. Epstein for drift from the protocol. During sessions, participants were reminded, when necessary, that the focus of groups was cocaine rather than heroin. Patients who missed (or arrived more than 15 min late for) more than two sessions without a legitimate reason were discharged from the study with a 21-day methadone dose taper. Thirteen participants were discharged specifically for this reason: 9 from the CBT groups and 4 from the social-support groups; this difference was not statistically significant, χ2(1, N = 193) = 2.0, p = .20.
The CBT procedure was based on a manual developed by two of the authors (Wesley E. Hawkins and Lino Covi), one of whom (Wesley E. Hawkins) trained the counselors. The manual combined elements of relapse-prevention methods (Marlatt & Gordon, 1985), coping methods (Lazarus & Folkman, 1984), behavioral-reinforcement methods (Lewinsohn, Steinmetz, Antonuccio, & Teri, 1984), and methods of generalizing skills to the environment (developed in our clinic; Covi, Hess, Schroeder, & Preston, 2002). Sessions included intensive use of worksheets and role plays, through which participants were taught skills in four main areas: (a) increasing nondrug sources of reinforcement, (b) developing adaptive emotional responses to drug-specific and general life stressors, (c) developing adaptive behavioral responses to such stressors, and (d) decreasing HIV-risk behaviors.
The social-support control condition was intended to provide the non-specific elements of group therapy without the active ingredients of structured exercises and coping skills training. Thus, no specific coping skills were taught by the counselors. In a nondirective setting, participants were encouraged to share ideas and feelings and to support each other.
The counselors who led the social-support groups were also responsible for the individual-counseling sessions. Therefore, many (41%) of the participants in the social-support groups had the same counselor for both individual and group sessions. This had no effect on participants’ ratings of group helpfulness, or on study retention or cocaine use (data not shown).
Overview of Analytic Approach
Intake measures.
To ensure comparability among groups, intake measures were analyzed by analysis of variance (ANOVA; for continuous variables), Pearson chi-square (for categorical variables), or Fisher’s exact test (for categorical variables with expected cell sizes under 5).
Study retention was compared among groups with a log-rank test of time until provision of the final urine sample; participants who left before the final week of the experimental phase (intervention or maintenance) were coded as dropouts from that phase. Participants with three or more sporadically missing urine tests (i.e., not because of dropout) during the experimental phase were coded as poor attenders; this was compared among groups with Fisher’s exact test.
Intervention and maintenance: Qualitative urinalyses for cocaine and opiates.
The time course of these results during intervention was analyzed with generalized linear mixed models fit with the SAS macro GLIMMIX (Littel, Milliken, Stroup, & Wolfinger, 1996). GLIMMIX analyzes dichotomous repeated measures with missing data points by invoking the SAS procedure MIXED (SAS Institute, 1997) with a logit link, and its output includes covariate-adjusted percentages. A first-order autoregressive error structure was used. The nonrandom nature of the missing data was controlled for by including dummy coded terms for dropout and poor attendance (Hedeker & Gibbons, 1997). Most models contained one within-subject factor (Time, with 36 levels) and five between-subjects factors: CM (1 vs. 0), CBT (1 vs. 0), baseline percentage negative (arcsine-transformed), cocaine dependence (1 vs. 0), poor attendance (1 vs. 0), and dropout (1 vs. 0). (Inclusion of the last four covariates did not appreciably change p values for any of the main effects or interactions.) All two- and three-way interactions for CM, CBT, and time were included, but additional interaction terms and covariates were forgone in order to avoid overspecifying the models. Data from intervention and maintenance were analyzed separately.
Quantitative urinalyses for cocaine were analyzed in models identical to those described above, using mixed regressions (SAS MIXED procedure). Values below the laboratory reporting cutoff (100) were coded as 0. Because the BZE data were heavily skewed to the right, all nonzero values were log-transformed (Delucchi, Jones, & Batki, 1997) to reduce the influence of extremely high values. Data from intervention and maintenance were analyzed separately. Self-reported cocaine use and weekly group-helpfulness ratings were similarly analyzed in mixed regressions, with no log transformation.
Longest duration of cocaine abstinence was calculated for each participant as the longest run of consecutive cocaine-negative urine specimens during each phase; groups were compared by 2 × 2 factorial ANOVA. Voucher earnings and group attendance during the intervention phase were also compared by 2 × 2 factorial ANOVA.
Use of alcohol and other drugs, assessed through urine/breath specimens and self-report, was analyzed in terms of whole-phase means; mixed models could not be fit to these data because of underdispersion (lack of variability). Intervention and maintenance means were compared across groups with 2 × 2 factorial analyses of covariance (ANCOVAs, with each participant’s baseline used as a covariate). Percentage data were arcsine-transformed to maintain homogeneity of variance.
Exit and follow-up assessments.
Participants’ open-ended responses to exit questionnaires were divided into nonmutually exclusive categories and compared across groups with Pearson chi-square.
Current methadone treatment.
Our outcome measures did not assess this directly; therefore, we defined the dichotomous variable “on methadone” as “reporting oral methadone use for at least 25 of the past 30 days on the ASI”—a reasonable proxy measure. “On methadone” status was analyzed in GLIMMIX models with two between-subjects factors (CM and CBT) and one within-subjects factor (month, with three levels: 3, 6, and 12).
Qualitative urinalyses were analyzed in GLIMMIX models with four between-subjects factors (CM, CBT, cocaine dependence at study intake, and the time-varying covariate “on methadone”) and one within-subjects factor (Time). ASI data were analyzed similarly in mixed regressions.
All analyses were two-tailed; alpha was set at .05, with trends noted at .10.
Results
For convenience, the groups are referred to as control, CBT only, CM only, and combination, although each group, as described above, received more treatment than these names imply.
Participant Characteristics
Participants were mostly male (57%) and African American (70%); their mean age was 39 years (SD = 6.8), and they had 11.5 (SD = 2.0) years of education. Fifty percent were unemployed, 25% were employed part time, and 25% were employed full time. Fifty-four percent had never been married, 32% were separated or divorced, and 14% were married. They reported a mean cocaine-use history of 11 years (SD = 7.5) and reported having used cocaine on 18.3 (SD = 10.1) of the past 30 days. Nine percent met DSM–III–R criteria for antisocial personality disorder. These characteristics did not differ significantly across the four treatment groups.
Participants with DSM–III–R diagnoses of cocaine dependence (41% of the total sample) were underrepresented in the combination group (24%, vs. 39%–53% in the other three groups). To determine whether cocaine dependence was associated with treatment response, we fit a GLIMMIX model using cocaine dependence and day as the sole predictors of percentage of cocaine-negative urine specimens during the intervention phase. The effect of cocaine dependence was significant, F(1, 191) = 25.99, p < .0001, although, counterintuitively, participants who met criteria for cocaine dependence had a higher adjusted percentage of cocaine-negative urines (40%, 95% confidence limits [CL95] 31%–49%) than those who did not meet criteria (16%, CL95 12%–21%). (We obtained a similar result after controlling for baseline cocaine use.) Cocaine dependence was therefore included as a covariate in subsequent outcome analysis; in general, the effect of its inclusion was to make the combination group appear slightly less successful, but not to a degree that changed the conclusions.
Comparability of Conditions
Groups did not differ on retention, methadone doses, or voucher earnings (see Table 1). Group therapy attendance and ratings of helpfulness did differ by condition. CBT groups were subject to more frequent absences (M = 4.2, SEM = 0.3) than were social-support groups (M = 3.3, SEM = 0.3), F(1, 189) = 4.50, p < .04. Nonetheless, ratings of helpfulness were higher for CBT (Least-squares mean [LSmean] = 3.9 points out of 5, SEM = 0.1) than for social support (LSmean = 3.4 points out of 5, SEM = 0.1, F(1, 189) = 16.3, p < .0001. Ratings did not differ among counselors within each type of therapy.
Table 1.
Retention, Missing Urine Specimens, and Voucher Earnings
| Variable | Control (n = 49) | CM only (n = 47) | CBT only (n = 48) | Combination (n = 49) | Analysis |
|---|---|---|---|---|---|
| Completed 12-week intervention | 37 (76%) | 38 (81%) | 38 (79%) | 34 (69%) | log-rank χ2(3, N = 193) = 2.63, p = 45. |
| Sporadically missing urine specimens during intervention | |||||
| M (SD) | 1.8 (2.2) | 1.0 (1.4) | 1.6 (1.8) | 1.6 (1.8) | F(3, 189) = 1.96, p = .12 |
| No. poor attenders (%) | 5 (10%) | 3 (6%) | 5 (10%) | 3 (6%) | Fisher exact p = .82 |
| Mean voucher earnings (SD) | $283 (413) | $292 (386) | $182 (316) | $231 (374) | F(3, 189) = 0.89, p = .45 |
| Median voucher earnings | $29 | $64 | $10 | $10 | |
| Range of voucher earnings | $0–$1,153 | $0–$1,155 | $0–$1,155 | $0–$1,155 | |
| Completed 12-week maintenance | 31 (63%) | 29 (62%) | 28 (58%) | 28 (57%) | log-rank χ2(3, N = 193) = 3.46, p = .33 |
| Sporadically missing urine specimens during maintenance | |||||
| M (SD) | 1.8 (2.2) | 1.0 (1.4) | 1.1 (1.8) | 1.9 (2.2) | F(3, 134) = 1.36, p = .26 |
| No. poor attenders (%) | 6 (12%) | 6 (13%) | 3 (6%) | 5 (10%) | Fisher exact p = .72 |
Note. Poor attenders are participants missing 3 or more urine specimens not because of dropout. CM = contingency management; CBT = cognitive–behavioral therapy.
Condition Differences During the Intervention Phase
As expected, CM had robust main effects on all cocaine-related outcome measures. In qualitative urinalyses (see Figure 1), cocaine-negative urine specimens were more frequent in the CM-only and combination groups than in the CBT-only and control groups. The same pattern was apparent in quantitative urinalyses: throughout intervention, BZE levels were lower in the CM-only and combination groups (M = 53,380 ng/mL, SEM = 8,870) than in the other two groups (M = 62,070, SEM = 8,600), F(1, 185) = 15.94, p < .001 (data not shown). The longest duration of cocaine abstinence (inferred from qualitative urinalyses) was also greater for the two CM groups (CM only: M = 11.3 successive specimens, SEM = 1.8; combination: M = 8.3, SEM = 1.7) than for the other two groups (CBT only: M = 3.7, SEM = 0.9; control: M = 2.3, SEM = 0.4), F(1, 189) = 29.29, p < .0001. This finding was essentially unchanged in an ANCOVA controlling for each participant’s baseline percentage of cocaine-negative urines and in an ANOVA controlling for current cocaine dependence. Consistent with the urine data, self-reported frequency of cocaine use (see Table 2) was lower in the two CM groups (LSmean from mixed regression = 0.35 uses per day, SEM = 0.04) than in the other two groups (LSmean = 0.45, SEM = 0.04), F(1, 185) = 5.76, p = .017.
Figure 1.
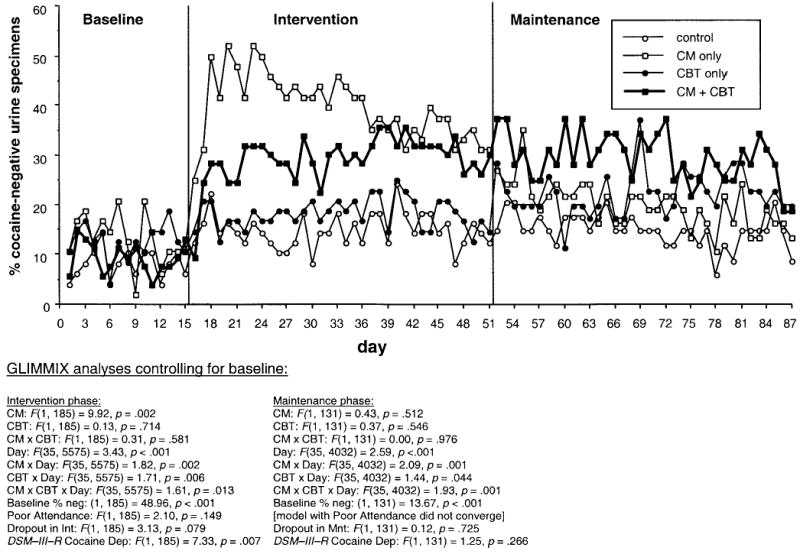
Percentages of participants who were cocaine abstinent on 87 successive urine test days in the baseline phase (15 specimens), intervention (Int) phase (36 specimens), and maintenance (Mnt) phase (36 specimens) in the four treatment groups: CM only (contingency management plus social support groups; n = 47 in baseline/intervention and 37 in maintenance), CBT only (cognitive–behavioral therapy plus noncontingent vouchers; n = 48 in baseline/intervention and 35 in maintenance), combination (CM plus CBT; n = 49 in baseline/intervention and 32 in maintenance), and control (social support groups plus noncontingent vouchers; n = 49 in baseline/intervention and 34 in maintenance). Statistical results are from a generalized linear mixed model (SAS GLIMMIX macro) that does not require imputation of missing data. However, the data shown are actual percentages, with missing specimens (including those due to dropout) treated as positive. neg = negative; DSM–III–R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987); Dep = dependence.
Table 2.
Self-Reported Frequency of Drug Use (Uses/Day)
| Control (n = 49)
|
CM only (n = 47)
|
CBT only (n = 48)
|
Combination (n = 49)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | ANCOVA controlling for baseline |
| Cocaine | |||||||||
| Baseline | 0.48 | 0.33 | 0.41 | 0.43 | 0.40 | 0.30 | 0.52 | 0.49 | |
| Intervention | 0.34 | 0.28 | 0.22 | 0.24 | 0.38 | 0.28 | 0.34 | 0.44 | Less frequent in CM, F(1, 188) = 5.87, p = .016; more frequent in CBT, F(1, 188) = 4.03, p = .046. |
| Maintenance | 0.24 | 0.21 | 0.29 | 0.33 | 0.25 | 0.30 | 0.24 | 0.45 | No effect of CM or CBT. |
| Heroin | |||||||||
| Baseline | 0.35 | 0.33 | 0.34 | 0.40 | 0.50 | 0.64 | 0.46 | 0.47 | |
| Intervention | 0.15 | 0.21 | 0.10 | 0.18 | 0.16 | 0.29 | 0.16 | 0.25 | No effect of CM or CBT. |
| Maintenance | 0.13 | 0.20 | 0.12 | 0.18 | 0.12 | 0.25 | 0.13 | 0.26 | No effect of CM or CBT. |
| Alcohol | |||||||||
| Baseline | 0.04 | 0.06 | 0.05 | 0.13 | 0.06 | 0.20 | 0.08 | 0.25 | |
| Intervention | 0.04 | 0.09 | 0.04 | 0.09 | 0.05 | 0.16 | 0.04 | 0.11 | No effect of CM or CBT. |
| Maintenance | 0.06 | 0.16 | 0.07 | 0.15 | 0.07 | 0.21 | 0.15 | 0.43 | No effect of CM or CBT. |
| Cannabis | |||||||||
| Baseline | 0.02 | 0.05 | 0.01 | 0.03 | 0.01 | 0.02 | 0.01 | 0.04 | |
| Intervention | 0.01 | 0.06 | 0.01 | 0.03 | 0.00 | 0.02 | 0.01 | 0.02 | No effect of CM or CBT. |
| Maintenance | 0.01 | 0.07 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | No effect of CM or CBT. |
| Benzodiazepines | |||||||||
| Baseline | 0.02 | 0.06 | 0.01 | 0.04 | 0.01 | 0.04 | 0.03 | 0.17 | |
| Intervention | 0.00 | 0.01 | 0.00 | 0.02 | 0.01 | 0.04 | 0.02 | 0.09 | No effect of CM or CBT. |
| Maintenance | 0.01 | 0.04 | 0.02 | 0.10 | 0.02 | 0.06 | 0.01 | 0.05 | No effect of CM or CBT. |
Note. CM = contingency management; CBT = cognitive–behavioral therapy; ANCOVA = analysis of covariance.
CBT, as expected, showed no main effects on any of the cocaine-related outcome measures. In fact, qualitative urinalyses showed that CBT appeared to reduce the effect of CM in the combination group during the first 7 weeks of the Intervention phase (CM × CBT × Day interaction; see Figure 1). Similarly, self-reported frequency of use appeared highest in the CBT-only group (LSmean = .48, SEM = .05), although this was not statistically significant: CM × CBT interaction, F(1, 185) = 0.42, p = .517.
There were few clear group differences in use of other drugs (see Tables 2 and 3). For opiates, a GLIMMIX analysis on the time course of qualitative urinalyses (data not shown) showed a trend toward a three-way interaction of CM × CBT × Day, F(35, 5575) = 1.37, p = .073, reflecting reduced use in the combination group during the middle of intervention. This analysis controlled for cocaine dependence, which was associated with fewer opiate-positive urines (adjusted percentages: 22% vs. 36%), F(1, 185) = 3.91, p = .049. For other drugs, there were no treatment effects or interactions, and rates of use were generally low, whether assessed by urinalysis or self-report.
Table 3.
Urine and Breath Analyses for Drugs Other Than Cocaine
| Control (n = 49)
|
CM only (n = 47)
|
CBT only (n = 48)
|
Combination (n = 49)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | ANCOVA controlling for baseline |
| Heroin, % negative | |||||||||
| Baseline | 34 | 33 | 40 | 34 | 30 | 31 | 27 | 30 | |
| Intervention | 40 | 30 | 55 | 35 | 44 | 39 | 47 | 38 | More negatives in CM, F(1, 188) = 3.49, p = .0631. |
| Maintenance | 40 | 32 | 44 | 37 | 52 | 40 | 54 | 39 | More negatives in CBT, F(1, 133) = 103.89, p < .0001. |
| Cannabis, % negative | |||||||||
| Baseline | 85 | 27 | 87 | 27 | 94 | 16 | 91 | 19 | |
| Intervention | 81 | 33 | 83 | 31 | 90 | 24 | 90 | 23 | No effect of CM or CBT. |
| Maintenance | 87 | 23 | 83 | 33 | 88 | 29 | 90 | 22 | No effect of CM or CBT. |
| Benzodiazepines, % negative | |||||||||
| Baseline | 97 | 79 | 96 | 11 | 97 | 11 | 94 | 14 | |
| Intervention | 97 | 8 | 94 | 14 | 97 | 10 | 89 | 22 | Fewer negatives in CM, F(1, 188) = 3.13, p = .0781. |
| Maintenance | 95 | 13 | 89 | 23 | 94 | 16 | 90 | 24 | No effect of CM or CBT. |
| Alcohol, % negative (BAC) | |||||||||
| Baseline | 98 | 5 | 98 | 5 | 97 | 11 | 99 | 2 | |
| Intervention | 98 | 5 | 95 | 10 | 96 | 13 | 98 | 8 | No effect of CM or CBT. |
| Maintenance | 97 | 5 | 93 | 13 | 94 | 18 | 96 | 9 | No effect of CM or CBT. |
Note. CM = contingency management; CBT = cognitive–behavioral therapy; ANCOVA = analysis of covariance; BAC = blood alcohol concentration.
Condition Differences During the Maintenance Phase
On visual inspection of the qualitative-urinalysis data for cocaine (see Figure 1), it appeared that the two CBT groups (especially the combination group) might be evincing lasting benefits from the intervention phase. However, this was not reflected in a main effect of CBT or an interaction of CM × CBT. All interactions with day were significant but, unlike in the intervention phase, these interactions seemed to reflect temporal fluctuations with unclear clinical significance. Similarly, in quantitative urinalyses for cocaine, the combination group tended to have the lowest BZE values throughout maintenance (data not shown), but none of the treatment effects or interactions approached significance. Longest duration of cocaine abstinence also showed no treatment effects or interactions (CM only: M = 4.1 successive specimens, SEM = 1.3; combination: M = 6.6, SEM = 1.7; CBT only: M = 4.2, SEM = 1.2; control: M = 2.9, SEM = 1.0). This finding was unchanged in an ANCOVA controlling for each participant’s baseline percentage of cocaine-negative urines. Self-reported frequency of cocaine use also did not differ among groups (see Table 2). Thus, any apparent delayed benefit of CBT during the maintenance phase could not be distinguished from random variability.
Use of most other drugs, except opiates, remained low (see Table 3). For opiates, a GLIMMIX analysis on the time course of qualitative-urinalysis data showed that the percentage of positive urine specimens was lower in the two CBT groups (56%, CL95 38%–73%) than in the other two groups (32%, CL95 19%–49%), F(1, 132) = 5.21, p = .024. There was no effect of CM and no CM × CBT interaction.
At study exit, participants’ short-answer responses to open-ended questionnaires confirmed that most had not enrolled to reduce cocaine use: Sixteen percent specifically cited a desire to reduce or stop their heroin use, 21% cited being tired of the lifestyle associated with avoiding withdrawal, and another 7% cited a desire to stop using all illicit drugs, but none specifically cited cocaine. These findings did not differ across groups (data not shown). Furthermore, most participants—nearly 70%—continued to cite heroin, not cocaine or polydrugs, as their major problem drug (a finding that continued across all follow-up assessments, again not differing across groups).
Condition Differences at Follow-Up
Of the 193 participants, 163 returned for at least one follow-up assessment (for a total of 402 visits); 134 returned for the 12-month assessment. Because of the need to control for current methadone treatment, we restricted data analyses to the 390 visits for which ASI data were available; this reduced the follow-up sample to 161 participants, of whom 133 returned for the 12-month assessment. The 161 participants in the follow-up sample were more likely than the other 32 to be African American (75% vs. 47%; Fisher exact p = .0034) but did not differ from the other 32 on other demographic or intake measures, or in their group assignment, or on their percentages of cocaine- or opiate-negative urines in any phase of the study (data not shown). The same was found when the 133 participants in the 12-month follow-up sample were compared to the other 60 participants (77% African American vs. 55% African American; Fisher exact p = .0018).
Current methadone use increased over time in all groups, main effect of month: F(2, 196) = 4.03, p = .019; adjusted percentages from the GLIMMIX analysis were 22% (CL95: 15%–32%) at 3 months, 35% (CL95: 26%–25%) at 6 months, and 36% (CL95: 27%–46%) at 12 months. Former control group participants appeared less likely to report current methadone use (adjusted percentage: 22%, CL95: 12%–36%, vs. 31%–37% in the other three groups), but this did not approach significance, CM × CBT interaction: F(1, 157) = 0.60, p = .439. Nonetheless, we included current methadone use as a time-varying dichotomous covariate in subsequent analyses.
The most suggestive finding can be seen in Figure 2, which shows qualitative urinalyses for cocaine: At the 12-month follow-up, the rank order of the four groups was as predicted, suggesting additive effects of CM and CBT. However, none of the effects of CM or CBT, nor their interactions with month, were statistically significant. Post hoc power analyses on covariate-adjusted percentages showed that a combination-versus-control contrast at 12 months would have reached significance with 49 participants per group, and a combination-versus-CM contrast would have reached significance with 128 participants per group. The respective effect sizes, expressed in terms of Cohen’s h (Cohen, 1988), were .575 (medium; equivalent to an r of about .28) and .336 (between small and medium; equivalent to an r of about .16). In exploratory contrasts of the 3-month time point versus the 12-month time point, the increase in cocaine negatives did reach significance for the two CBT groups, t(220) = 3.51, Tukey–Kramer adjusted p = .007, and did not for the two social-support groups, t(220) = 1.00, Tukey–Kramer adjusted p = .916.
Figure 2.
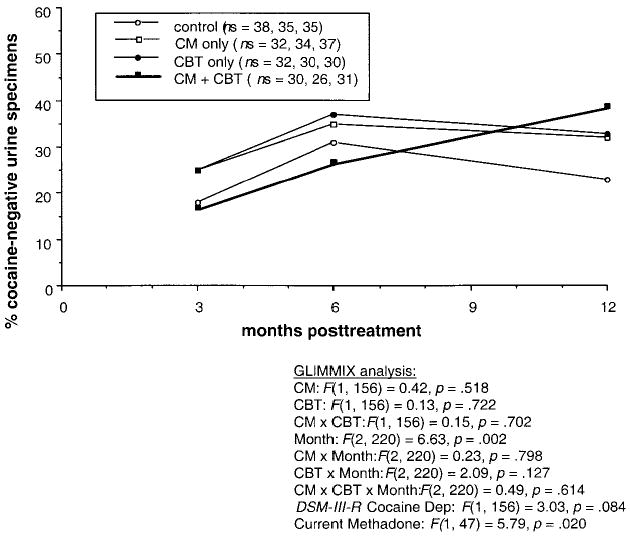
Percentage of participants who were cocaine abstinent at three follow-up visits occurring 3, 6, and 12 months after the final maintenance dose of methadone. Statistical results are from a generalized linear mixed model (SAS GLIMMIX macro) that does not require imputation of missing data. However, the data shown are actual percentages, with missing specimens ignored; in other words, the denominator at each time point is the number of specimens actually collected (see legend for ns). CM = contingency management; CBT = cognitive–behavioral therapy; DSM–III–R = Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987); Dep = dependence.
In seeming opposition to the urinalysis data, self-reported days of recent cocaine use tended to be higher in the two CBT groups (LSmean = 8.6, SEM = 1.0) than in the other two groups (LS-mean = 6.5, SEM = 0.9), although not significantly, F(1, 156) = 2.40, p = .123. Similarly, self-reported days of recent drug problems were higher in the two CBT groups (LSmean = 16.5, SEM = 1.2) than in the other two groups (LSmean = 13.0, SEM = 1.1), F(1, 156) = 5.03, p = .026. To explore this unexpected finding, we analyzed rates of nonreported use: Participants were classified as nonreporters if their urine was cocaine positive while they reported having used cocaine on 3 or fewer days out of the past 30. By this arbitrary criterion, participants in the social-support groups appeared more likely to be nonreporters (adjusted percentage: 28%, CL95: 19%–34%) than participants in the CBT groups (adjusted percentage: 19%, CL95: 11%–29%), although this was not significant, F(1, 156) = 1.95, p = .164. The strongest predictor of nonreporting, significant at trend level, was current use of methadone (adjusted percentage: 28%, CL95: 18%–40% for methadone users vs. 19%, CL95: 13%–27% for methadone nonusers), F(1, 47) = 3.07, p = .086.
Other ASI variables did not differ significantly among groups or across time, but there was a trend for the two CBT groups to report more days of paid employment in the past 30 (LSmean = 10.3, SEM = 1.1) than the other two groups (LSmean = 7.7, SEM = 1.0), F(1, 156) = 3.40, p = .067.
Use of other drugs, as assessed by qualitative urinalysis, did not differ significantly among groups or across time. However, self-reported days of recent heroin use tended to be higher in the two CBT groups (LSmean = 11.7, SEM = 1.0) than in the two social-support groups (LSmean = 9.3, SEM = 1.0), F(1, 156) = 3.08, p = .081. As with cocaine, this was not accompanied by a higher percentage of positive urines.
Discussion
The results support three major conclusions—one expected, one unexpected and intriguing, and one somewhat disappointing. First, as expected, CM rapidly and robustly reduced cocaine use. Second, the initial appearance of this effect was somehow briefly dampened if CM was augmented with CBT. Third, at a 12-month posttreatment follow-up, the augmentation appeared to have had the intended effect—a slowly emergent and enduring reduction of cocaine use—but the effect did not reach statistical significance except in exploratory post hoc contrasts.
Intervention and Maintenance Phases
Why the dampening of the initial increase in abstinence?
Our CM-only group showed a dramatic initial increase in cocaine-negative urines; this increase remained consistent through approximately 7 weeks of thrice-weekly testing and resembled the pattern of behavior seen in a previous study of CM for cocaine abstinence (Preston et al., 2001; see Abstinence group in Figure 1 of that article). Our combination group, despite receiving CM, did not show that initial increase. Yet, by the end of the intervention phase the CM-only group had shown a precipitous drop in abstinence rates, whereas the combination group appeared to maintain its modest treatment gains. A plausible, although speculative, explanation is that our CBT included exercises in which participants explicitly acknowledged their ambivalence about reducing cocaine use (e.g., “Advantages and Disadvantages of Cocaine Use,” the goal of which was to show that the disadvantages had come to outweigh the advantages), along with exercises in which it was implicit that lapsing is a normal event from which one can learn. This is not a criticism of CBT; on the contrary, the data in Figures 1 and 2 suggest that, for our combination group, “slow and steady won the race”—in other words, that the initial weeks of continuing to use cocaine while exploring the consequences of use prevented a precipitous loss of control in the following weeks and perhaps improved posttreatment outcome. We hasten to acknowledge again that the posttreatment effect reached significance only in post hoc contrasts.
Additional comments on the CBT intervention.
The lower attendance in CBT groups relative to social-support groups may have reflected aversion to the repetitiveness of our CBT intervention, in which the same exercises were done every week. This was necessary because the material taught was a form of skill learning. In any case, our CBT attendance rates compare favorably with those of Carroll, Rounsaville, Gordon, et al. (1994), in whose study participants missed an average of 4.8 out of 12 sessions (vs. 4.2 out of 12 in our study). One difference we should note is that Carroll, Rounsaville, Gordon, et al. administered CBT in an individual rather than a group setting. We chose the group setting to exploit some of the advantages of group therapy, such as having participants discuss with each other a variety of recent experiences (representing different degrees of success) and doing peer role play exercises. (Anecdotally, we noted that some participants kept filled-out paperwork from CBT exercises in their pockets throughout the week so they could refer to it when they experienced craving.) Participants’ ratings of group helpfulness suggested that they viewed CBT as worthwhile.
Posttreatment Follow-Up
At the 12-month follow-up, the rank order of treatment success (as measured by cocaine-negative urine) was exactly as hypothesized: Either treatment seemed better than neither, and the combination seemed best. Yet the effect was small. One reason for this is suggested by the exit-assessment questionnaires, in which most participants, regardless of group-therapy assignment, reported using similar strategies (e.g., avoiding drug cues) to reduce cocaine use. Some informal relapse prevention training probably occurred in the unmonitored individual-counseling sessions. Participants’ individual counselors (who also ran the social-support groups) were deliberately not given information about the CBT techniques and might not have realized when their own individual-counseling styles overlapped with the content of CBT groups. This aspect of the study design reflected our having sought to test whether CBT has effects detectable above those of good standard treatment.
A more general reason for participants’ continued cocaine use is that, as also shown by the exit data, participants had not come seeking treatment for cocaine abuse; most had come because they were tired of waking up with heroin withdrawal symptoms. Adverse effects of cocaine are rarely so salient; one study suggested that the health consequences of cocaine use are time lagged by 6 years (Chen, Scheier, & Kandel, 1996). Perhaps it is not surprising that most participants did not view cocaine as a major problem drug despite 12 weeks of therapy in which counselors reminded participants that “this group is about cocaine.” It is especially interesting that cocaine use during treatment was lower among participants who, at intake, had met DSM–III–R criteria for cocaine dependence. These criteria were assessed through self-report in a structured interview, so participants who met criteria may have been those with more insight into the adverse effects of cocaine on their lives. Our results suggest that even after intensive intervention, cocaine-using methadone maintenance patients resist the idea that their cocaine use is a major problem.
Nonetheless, exploratory analyses did suggest an ongoing posttreatment reduction of cocaine use in participants who had received CBT. There was also evidence that CBT enhanced participants’ forthcomingness about cocaine-related problems and increased some aspects of psychosocial functioning, such as employment. These findings are consistent with those of the studies that we had sought to replicate and extend (Carroll et al., 2000; Carroll, Rounsaville, Nich, et al., 1994; O’Malley et al., 1996).
Limitations of our study include participant attrition during treatment and some loss to follow-up after treatment. Neither of these differed across groups, and both were comparable to what is typically seen in this study population; in fact, the amount of loss to follow-up compares favorably to what was encountered in past studies from our own clinic (Preston, Umbricht, & Epstein, 2002) and others (Carroll, Rounsaville, Nich, et al., 1994). We used statistical methods that would reduce the impact of missing data. The mixed models used here, which were developed for longitudinal studies with missing data (Gibbons, Hedeker, Waternaux, & Davis, 1988; Hu, Goldberg, Hedeker, Flay, & Pentz, 1998), obviate the need for arbitrary and potentially biased methods of missing-data imputation, instead using data-driven estimation based on information from every participant who contributes data for at least one time point (Nich & Carroll, 1997). Because we could not assume that our missing data were missing at random, we combined this approach with the use of pattern-mixture models, in which participants’ patterns of missing data are dummy coded and controlled for (Hedeker & Gibbons, 1997). Thus, if drug-positive urine specimens had been higher or lower among participants who dropped out, this variance would be attributed to dropout, not CM or CBT. Obviously, no analytic strategy is a substitute for having the data. Also, pattern-mixture modeling was not practical for the follow-up data, because almost all participants missed at least one follow-up and, moreover, 32 contributed no follow-up data at all. We think it is reassuring to note that the follow-up sample did not differ from the total sample in terms of cocaine use during treatment, and if participants with poor posttreatment outcomes were more likely to miss follow-up visits, there is no reason to suspect that this would occur differentially across experimental groups.
During active treatment, cocaine use was reduced by CM and not by CBT. CBT seemed to dampen the initial effect of CM; however, CBT also seemed to lead to a slowly emerging posttreatment reduction in cocaine use. This reached statistical significance only in exploratory contrasts, but a case can be made for its clinical significance: It is remarkable that a psychotherapeutic intervention administered only 12 times across 12 weeks produced changes in behavior that were even faintly detectable over the “noise” of other life events 1 year later. Future work in cocaine-using methadone maintenance patients should include intensified efforts to help them recognize the short- and long-term consequences of continued cocaine use.
Contributor Information
David H Epstein, National Institute on Drug Abuse.
Wesley E Hawkins, Florida Atlantic University.
Lino Covi, National Institute on Drug Abuse.
Annie Umbricht, Sinai Hospital.
Kenzie L Preston, National Institute on Drug Abuse.
References
- American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders (3rd ed., rev.). Washington, DC: Author.
- Bigelow GE, Brooner RK, Silverman K. Competing motivations: Drug reinforcement vs. non-drug reinforcement. Journal of Psychopharmacology. 1998;12:8–14. doi: 10.1177/026988119801200102. [DOI] [PubMed] [Google Scholar]
- Carroll, K. M. (2000). A cognitive–behavioral approach: Treating cocaine addiction (NIH Publication No. 00-4308). Retrieved April 2, 2002, from http://www.nida.nih.gov/TXManuals/CBT/CBT1.html
- Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine–alcohol users: Sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Archives of General Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Chen K, Scheier LM, Kandel DB. Effects of chronic cocaine use on physical health: A prospective study in a general population sample. Drug and Alcohol Dependence. 1996;43:23–37. doi: 10.1016/s0376-8716(96)01285-9. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum.
- Covi L, Hess JM, Schroeder JR, Preston KL. Psychotherapy dosage effects in cocaine abusers. Journal of Substance Abuse Treatment. 2002;23:191–197. doi: 10.1016/s0740-5472(02)00247-7. [DOI] [PubMed] [Google Scholar]
- Delucchi KL, Jones RT, Batki SL. Measurement properties of quantitative urine benzoylecgonine in clinical trials research. Addiction. 1997;92:297–302. [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Waternaux C, Davis JM. Random regression models: A comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology Bulletin. 1988;24:438–443. [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
- Helzer J, Croughan J, Robins L, Ratcliff K. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacology Biochemistry and Behavior. 1997;57:419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg FE, Badger GJ. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150:1218–1224. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg FE, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins, S. T., & Wong, C. J. (1998). Treating cocaine abuse: What does research tell us? In S. T. Higgins & J. L. Katz (Eds.), Cocaine abuse: Behavior, pharmacology, and clinical applications (pp. 343–361). San Diego, CA: Academic Press.
- Higgins ST, Wong CJ, Badger GJ, Haug Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. American Journal of Epidemiology. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal, and coping. New York: Springer.
- Lewinsohn PM, Steinmetz JL, Antonuccio D, Teri L. Group therapy for depression: The Coping with Depression course. International Journal of Mental Health. 1984;13:8–33. [Google Scholar]
- Littel, R. C., Milliken, G. A., Stroup, W. W., & Wolfinger, R. D. (1996). SAS system for mixed models. Cary, NC: SAS Publications.
- Marlatt, G. A., & Gordon, J. R. (Eds.). (1985). Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press.
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don’t: A comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. Journal of Consulting and Clinical Psychology. 1997;65:252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Change G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Archives of General Psychiatry. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Abstinence reinforcement maintenance contingency and one-year follow-up. Drug and Alcohol Dependence. 2002;67:125–137. doi: 10.1016/s0376-8716(02)00023-6. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong C, Epstein DH. Reinforcement of stepwise decreases in cocaine use. Journal of Consulting and Clinical Psychology. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, Stitzer ML. The Brief Abstinence Test: Voucher-based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- SAS Institute. (1997). The MIXED procedure. In SAS/STAT software: changes and enhancements through Release 6.12 (pp. 571–701). Cary, NC: Author.
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of reinforcement of cocaine abstinence in methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Zachary, R. A. (1986). Shipley Institute of Living Scale: Revised manual. Los Angeles: Western Psychological Services.


