Abstract
TAK1 mitogen-activated protein kinase kinase kinase participates in the Interleukin-1 (IL-1) signaling pathway by mediating activation of JNK, p38, and NF-κB. TAK1-binding protein 2 (TAB2) was previously identified as an adaptor that links TAK1 to an upstream signaling intermediate, tumor necrosis factor receptor-associated factor 6 (TRAF6). Recently, ubiquitination of TRAF6 was shown to play an essential role in the activation of TAK1. However, the mechanism by which IL-1 induces TRAF6 ubiquitination remains to be elucidated. Here we report that TAB2 functions to facilitate TRAF6 ubiquitination and thereby mediates IL-1-induced cellular events. A conserved ubiquitin binding domain in TAB2, the CUE domain, is important for this function. We also found that TAB2 promotes the assembly of TRAF6 with a downstream kinase, IκB kinase (IKK). These results show that TAB2 acts as a multifunctional signaling molecule, facilitating both IL-1-dependent TRAF6 ubiquitination and assembly of the IL-1 signaling complex.
Introduction
The cytokine IL-1 plays a pivotal role in promoting the inflammatory response to infection and injury. IL-1 binding to its cell surface receptor drives an intracellular signaling pathway that leads to activation of two transcription factors, NF-κB and AP-1 (homo- or hetero-dimer consisting of Jun, Fos, or ATF family proteins) (Dinarello, 1996). These transcription factors in turn activate the expression of target genes involved in inflammatory responses. Activation of NF-κB involves the degradation of the inhibitor of NF-κB (IκBs) subunits (Ghosh et al., 1998; Li and Verma, 2002). In un-stimulated cells, NF-κB is sequestered in the cytoplasm in complexes containing IκBs. In response to IL-1 stimulation, these IκBs are phosphorylated by a kinase complex consisting of two kinase subunits, IKKα and IKKβ, and a regulatory subunit, NEMO/IKKγ. Phosphorylation tags the IκBs for proteasome-mediated degradation, which allows NF-κB to be released and translocated to the nucleus, where it transcriptionally upregulates a number of target genes. Another transcription factor involved in the IL-1 response, AP-1, is activated through a mitogen-activated protein kinase (MAPK) cascade consisting of three kinases, MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK) and MAPK (Pearson et al., 2001). IL-1 stimulation activates a subset of MAPKs involved in the stress response: predominantly c-Jun N-terminal kinase (JNK) and p38. Several MAPKKKs have been reported to be responsible for IL-1-induced activation of MAPK cascade, and MKK3, 4, 6, 7 have been shown to be specifically involved in the activation of JNK and p38. TAK1 appears to play a major role in the activation of both JNK/p38 and IKK in response to IL-1 (Ninomiya-Tsuji et al., 1999). The evidence that TAK1 plays an essential role in IL-1 signaling pathway is several-fold: (i) Small interfering RNA (siRNA) inhibition of TAK1 expression was reported to abolish the IL-1 response (Takaesu et al., 2003): (ii) TAK1 was found in a biochemical fraction essential for in vitro IKK activation (Wang et al., 2001): (iii) a specific inhibitor of TAK1, 5Z-7-oxozeaenol, was shown to block IL-1-induced activation of JNK/p38 and IKK (Ninomiya-Tsuji et al., 2003).
Recent studies have revealed the mechanism by which TAK1 is activated upon IL-1 stimulation. There are two important events preceding IL-1-induced TAK1 activation. One is the formation of a complex between TAK1 and TRAF6 (Ninomiya-Tsuji et al., 1999). This TAK1-TRAF6 interaction is mediated by TAB2 (Takaesu et al., 2000; Takaesu et al., 2001). Another event is the K63-linked ubiquitination of TRAF6 (Wang et al., 2001). TRAF6 possesses an E3 ubiquitin ligase activity in its RING finger domain, and is self-ubiquitinated in a K63-linked manner. K63-linked ubiquitination has been implicated in modifying protein function, whereas the classical K48-linked ubiquitination targets proteins for degradation (Weissman, 2001). Ubiquitination-dependent activation of the TRAF6 complex leads to activation of IKK and the MAPK cascade. However, the mechanisms by which TRAF6 autoubiquitination is induced and how the TRAF6 complex engages IKK activation are currently unclear.
In the present study, we attempted to evaluate the role of TAB2 using TAB2 knockout mouse embryonic fibroblasts. We found that TAB2 is required for IL-1-induced TRAF6 ubiquitination. We also found that TAB2 facilitates the assembly of TRAF6 with IKK. Our data presented here show that TAB2 acts as a multifunctional protein that facilitates TRAF6 ubiquitination and assembly of an IL-1 signaling complex containing TRAF6, TAK1 and IKK.
Results
TAB2 is required for IL-1-induced TRAF6 ubiquitination
To evaluate the function of TAB2 in the IL-1 signaling pathway, we analyzed IL-1-induced activation of TAK1 in mouse embryonic fibroblasts (MEF) from TAB2-deficient mice. Previous studies have demonstrated that TAB2 is essential for embryonic development (Sanjo et al., 2003). TAB2−/− mice display fetal liver degeneration and die at embryonic day 12.5. We established several lines of immortalized MEFs from wild type and TAB2−/− embryos by standard 3T3 methods. We treated wild-type (TAB2+/+) MEF and TAB2-deficient (TAB2−/− #1 and #2) MEF lines with IL-1, and tested for IL-1 responsiveness. We have previously shown that activation of TAK1 coincides with its autophosphorylation, as evidenced by mobility shift in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Kishimoto et al., 2000). TAK1 showed a migration shift at 5 min after IL-1 stimulation in wild-type MEFs, whereas the migration shift was significantly reduced in both TAB2−/− MEFs (Figure 1). These results suggest that TAK1 is not activated in TAB2−/− MEFs, consistent with a previous study utilizing primary TAB2−/− MEFs (Sanjo et al., 2003).
Figure 1. TAB2 is required for IL-1-induced TRAF6 ubiquitination.
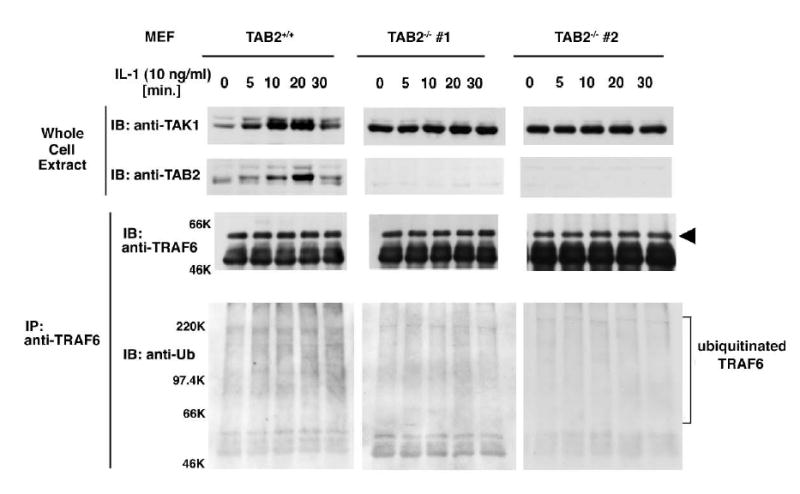
Wild-type (TAB2+/+) and two lines of TAB2-deficient (TAB2−/− #1 and #2) MEFs were treated with 10 ng/ml IL-1 for the indicated times. Whole cell extracts were subjected to immunoblotting with anti-TAK1 and anti-TAB2 antibody. Cell extracts were also immunoprecipitated with anti-TRAF6. The immunoprecipitated TRAF6 was detected by immunoblotting with anti-TRAF6 and also with anti-Ubiquitin.
IL-1 stimulation has been shown to induce ubiquitination of TRAF6 (Wang et al., 2001). We therefore examined the effect of TAB2 deficiency on IL-1-induced ubiquitination of TRAF6. TRAF6 was immunoprecipitated and the ubiquitinated form was detected with anti-ubiquitin antibody (Figure 1). In wild-type MEFs, a smear of very high molecular weight material representing ubiquitinated TRAF6 was found to increase upon IL-1 treatment. In contrast, no increase in ubiquitinated TRAF6 was observed in either of the TAB2−/− MEFs. These results indicate that TAB2 is required for IL-1-induced TRAF6 ubiquitination.
TRAF6 possesses E3 ubiquitin ligase activity (Wang et al., 2001) and is autoubiquitinated when overexpressed. However, under physiological conditions, this ubiquitination is regulated by IL-1 stimulation, as shown above. Our results raised the possibility that TAB2 functions as a trigger of IL-1-dependent TRAF6 ubiquitination. We therefore examined whether overexpression of TAB2 induced TRAF6 ubiquitination. HA-tagged TAB2 was co-expressed with Myc-tagged ubiquitin in 293 cells. Endogenous TRAF6 was immunoprecipitated, and ubiquitinated TRAF6 was detected by immunoblotting with anti-Myc (Figure 2). We found that endogenous TRAF6 was highly ubiquitinated in the presence of exogenously expressed TAB2. This result suggests that TAB2 has the ability to induce TRAF6 ubiquitination.
Figure 2. TAB2 induces TRAF6 ubiquitination.
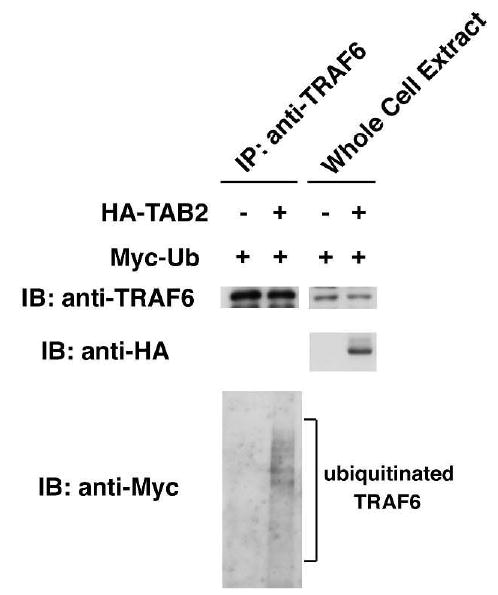
293 cells were transiently transfected with Myc-Ub in combination with empty vector (−) or with expression vector for HA-TAB2. Cell extracts were immunoprecipitated with anti-TRAF6. Immunoprecipitated endogenous TRAF6 was detected by immunoblotting with anti-TRAF6 (top panel). Immunoprecipitates were also immunoblotted with anti-Myc in order to detect ubiquitinated TRAF6 (bottom panel). In the middle panel, whole cell extracts (WCE) were immunoblotted with anti-HA to detect HA-TAB2.
The TAB2 CUE domain recognizes monoubiquitin
Protein ubiquitination and ubiquitin-dependent biological processes have been shown to depend upon a number of ubiquitin-binding domains in proteins, including the coupling of ubiquitin conjugation to ER degradation (CUE) domain (Hicke and Dunn, 2003). The CUE domain is structurally characterized as a monoubiquitin-recognizing motif, and has been implicated in facilitating covalent binding of ubiquitin to the target (Biederer et al., 1997; Prag et al., 2003; Shih et al., 2003). The N-terminal region of TAB2 contains a conserved CUE domain sequence that includes FP residues essential for ubiquitin binding (Figure 3A) (Prag et al., 2003; Shih et al., 2003). This suggested the possibility that TAB2 may bind to ubiquitin. We therefore examined potential interaction between the TAB2 CUE domain and monoubiquitin using a yeast two-hybrid system (Figure 3B, left panel). We used TRAF6 as a positive control binding partner, since we previously demonstrated that TAB2 directly interacts with TRAF6 (Takaesu et al., 2001). We found that TAB2 was able to bind to ubiquitin. To examine whether the TAB2 CUE domain mediated ubiquitin binding, we generated the mutants TAB2(F20A) and TAB2(F20D), in which a phenylalanine in the FP motif of the TAB2 CUE domain was replaced with alanine or aspartic acid, respectively (Figure 3A). These mutants were also tested for their abilities to recognize ubiquitin (Figure 3B, middle and right panels). We found that binding to ubiquitin was significantly impaired in both mutants, whereas binding to TRAF6 remained intact. These results suggest that TAB2 binds to ubiquitin via its CUE domain.
Figure 3. TAB2 contains a CUE domain that recognizes monoubiquitin.
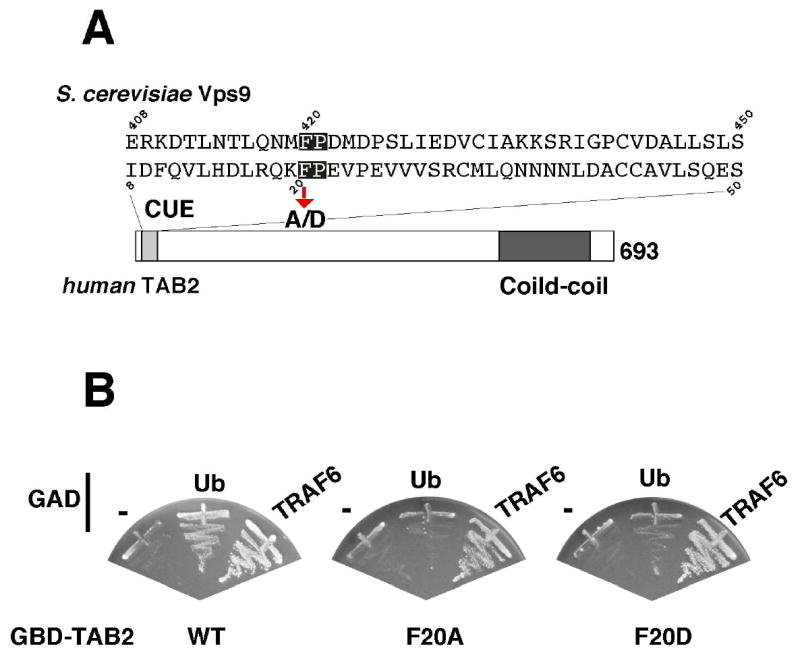
(A) Alignment of the amino acid sequences of the CUE domain from S. cerevisiae Vps9 and human TAB2. The critical FP motif for ubiquitin recognition is highlighted. We mutated phenylalanine 20 to alanine (F20A) or aspartic acid (F20D).
(B) The ability of TAB2 (wild type, F20A and F20D) to recognize ubiquitin was examined in the yeast two-hybrid system. Yeast strain PJ69-4A was transformed with the indicated combinations of Gal4 DNA-binding domain (GBD)-fused with TAB2 and Gal4 activation domain (GAD)-fused constructs. The transformants were streaked on selection plates lacking histidine (and containing 3 mM 3-AT). Growth on the selection plate indicates the interaction between the GAD- and the GBD-fused proteins.
The effect of TAB2 CUE mutations on NF-κB activation
Next we asked whether the TAB2 CUE domain is required for TAB2 function. To this end, we examined activation of NF-κB using an NF-κB-dependent reporter construct. We transfected 293 cells with an NF-κB reporter plasmid in combination with expression vectors encoding HA-tagged wild-type TAB2, TAB2(F20A), TAB2(F20D) or TAB2ΔCUE (deletion of the entire CUE domain) (Figure 4). Overexpression of wild-type TAB2 in 293 cells gave about a 10x increase in NF-κB reporter activity, whereas when TAB2ΔCUE, TAB2(F20A) or TAB2(F20D) was overexpressed, activity increased only 2-to 4-fold. To verify the expression of wild-type TAB2 and the mutants, we immunoblotted cell extracts with anti-HA (Figure 4, upper panel). we observed no prominent difference in the amounts of TAB2 expressed in each case. Therefore, we concluded that TAB2 CUE mutants were impaired in their ability to activate NF-κB. These results demonstrate that the TAB2 CUE domain is important for TAB2-dependent cellular responses.
Figure 4. CUE domain is involved in TAB2-induced NF- κ B activation.
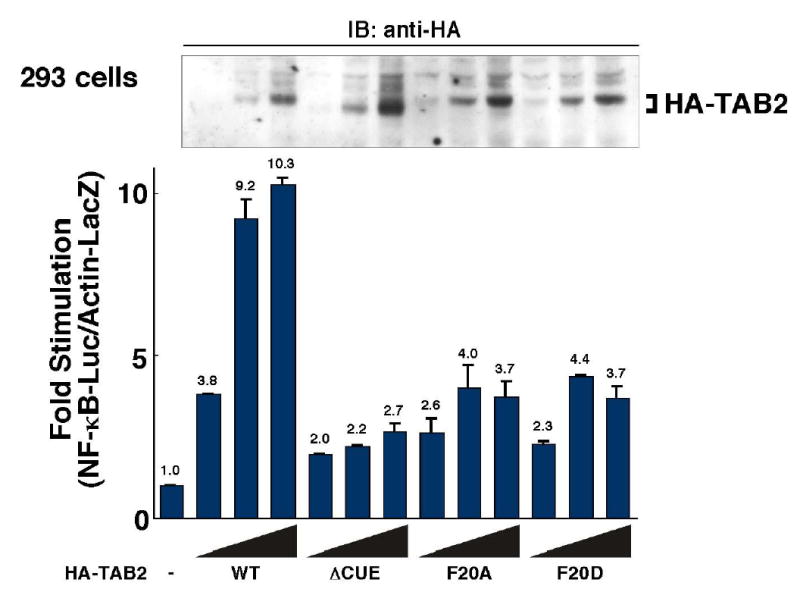
293 cells were transiently transfected with the reporter vector Ig-κ-luciferase and pAct-β-Gal in combination with empty vector (−) or various amounts of expression vectors for HA-TAB2 (WT, ΔCUE, F20A, and F20D). Luciferase activities were determined and normalized to the levels of β-galactosidase activity. Results are expressed as the fold-increase in luciferase activity relative to the cells transfected with empty vector. In upper panel, cell extracts were immunoblotted with anti-HA to confirm the expression levels of HA-tagged wild-type and mutant TAB2 proteins.
TAB2 engages TRAF6 with IKK
We have previously demonstrated that TAB2 functions as an adaptor between TRAF6 and TAK1 (Takaesu et al., 2000). Overexpression of TAB2 can drive interaction of TAK1 with TRAF6 independent of IL-1, leading to the activation of TAK1 and IKK. This suggested the possibility that TAB2 may also mediate the interaction of the active TAK1-TRAF6 complex with IKK. To test this possibility, we examined whether overexpression of TAB2 could induce the association of IKK with TRAF6 (Figure 5A). When 293 cells were transfected with an expression vector for TAB2, and proteins from the cell lysates were immunoprecipitated with anti-IKKα, we could detect co-precipitation of TRAF6 and TAB2 (Figure 5A). We found that overexpression of TAB2 enhanced co-precipitation of TRAF6 with IKKα. This suggests that TAB2 facilitates the interaction between TRAF6 and IKK.
Figure 5. TAB2 recruits IKK to the TRAF6 complex.
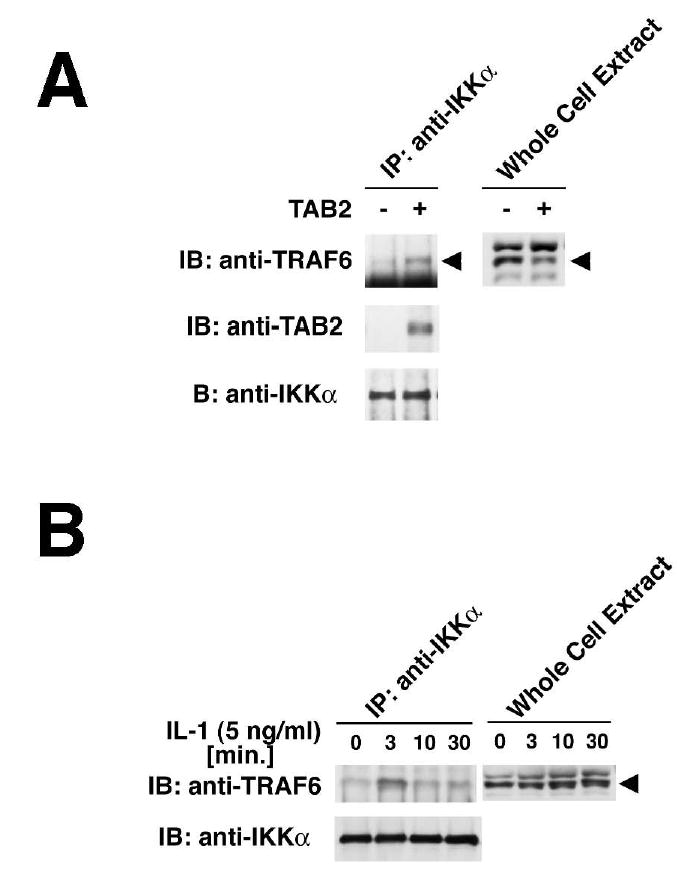
(A) 293 cell were transiently transfected with plasmid encoding TAB2, and whole cell extracts were immunoprecipitated with anti-IKKα antibody. The precipitates were separately immunoblotted with anti-TRAF6, anti-TAB2 and anti-IKKα. Whole cell extracts were also immunoblotted with anti-TRAF6.
(B) 293 IL-1RI cells were treated with IL-1 for the indicated times, and whole cell extracts were immunoprecipitated with anti-IKKα followed by immunoblotting with anti-TRAF6 and anti-IKKα. Whole cell extracts were also immunoblotted with anti-TRAF6.
We have previously shown that endogenous TAB2 and TAK1 transiently interact with TRAF6, reaching a peak at 2–5 min after IL-1 stimulation (Takaesu et al., 2001). We therefore tested for IL-1-dependent interaction of TRAF6 and IKK (Figure 5B). 293 IL- 1RI cells were treated with IL-1 and lysates were subjected to co-precipitation as above. We found that IKK interacted with TRAF6 in a transient manner at 3 min after IL-1 stimulation, suggesting that the TRAF6-TAB2-TAK1 complex interacts with IKK. Taken together, these data suggest that TAB2 may mediate assembly of the IL-1 signaling complex.
To further examine this complex formation, we employed immunofluorescent staining of TRAF6, TAB2 and IKKα. We transfected HeLa cells with expression vectors for TRAF6, TAB2 and IKKα and visualized their localization with Cy2- and/or Cy3-conjugated antibodies. TRAF6 and IKK were seen to localize in the cytoplasm and exhibit diffused staining, whereas TAB2 displayed a punctate pattern (Figure 6A). TAB2 staining did not overlap with mitochondria (data not shown). This suggests that TAB2 may localize in/on the endosome. When TRAF6 was co-expressed together with TAB2, TRAF6 also showed a punctate staining pattern and co-localized with TAB2 (Figure 6B). When IKKα was co-expressed with TRAF6 and TAB2, it showed overlapping staining with TRAF6 in some punctate clusters, although the predominant IKKα staining was diffuse (Figure 6C). These results suggest that TAB2 mediates assembly of a signaling complex containing TRAF6 and IKK in the cytoplasm.
Figure 6. TRAF6 and IKK α co-localize in the presence of TAB2.
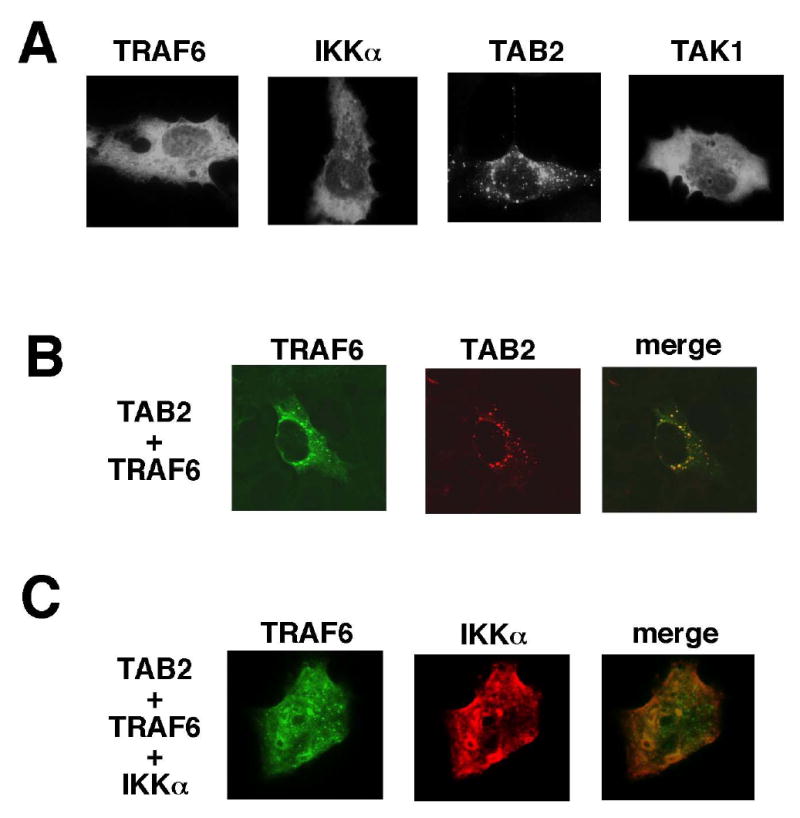
(A) HeLa cells were transiently transfected with plasmids encoding HA-TRAF6, IKKα, TAB2 and TAK1. After transfection at 36 hrs, cells were fixed and incubated with anti-HA, anti-IKKα, anti-TAB2 and anti-TAK1, followed by Cy2-conjugated anti-mouse or Cy3-conjugated anti-rabbit secondary antibody. Cells were examined by fluorescent microscopy.
(B) HeLa cells were transfected with plasmids encoding Flag-TRAF6 and TAB2. Those proteins were visualized using anti-Flag and anti-TAB2 primary antibodies.
(C) HeLa cells were transfected with plasmids encoding HA-TRAF6, IKKα, and TAB2. HA-TRAF6 and IKKα proteins were visualized using anti-HA and anti-IKKα primary antibodies.
Discussion
We have previously demonstrated that TAB2 links TAK1 to TRAF6 and mediates activation of TAK1 (Takaesu et al., 2000). Chen and colleagues have independently identified TAB2 as a component in a biochemical fraction that is essential for TRAF6-dependent IKK activation (Deng et al., 2000; Wang et al., 2001). They have also shown that TRAF6 ubiquitination is essential for TAK1 activation (Kanayama et al., 2004; Wang et al., 2001). In the present study, we discovered some novel functions of TAB2, viz. TAB2 binds to ubiquitin and promotes TRAF6 ubiquitination in IL-1 stimulated cells. Furthermore, we found that TAB2 facilitates assembly of TRAF6 with IKK. These results demonstrate that TAB2 is a multifunctional scaffold/mediator in the IL-1 signaling pathway. In agreement with this, earlier genetic studies utilizing TAB2 knockout mice revealed that TAB2 is essential for IL-1-induced TAK1 activation (Sanjo et al., 2003). However, these studies have also demonstrated that TAB2 is not essential for IL-1-induced activation of JNK, p38 or NF-κB in MEFs (Sanjo et al., 2003). Moreover, the transcriptional activation of IL-1 target genes is intact in TAB2−/− MEFs. These results strongly suggest that TAB2 is not an essential intermediate in the cellular response to IL-1. We assume that in the absence of TAB2 and consequent loss of TAK1 activation, those downstream events in the IL-1 pathway are induced through other signaling molecules. Such signaling molecules may include MEKK1 (Baud et al., 1999; Kopp et al., 1999; Lee et al., 1997), MEKK2 (Zhao and Lee, 1999), MEKK3 (Huang et al., 2004; Yang et al., 2001; Zhao and Lee, 1999) and NF-κB inducing kinase (Malinin et al., 1997), which have been previously implicated in IL-1-induced activation of JNK, p38 and NF-κB.
We and others have recently identified TAB3, a homologue of TAB2 that also associates with TAK1 (Cheung et al., 2004; Ishitani et al., 2003; Jin et al., 2004). Studies in cells treated with TAB2 and TAB3 siRNAs have revealed that TAB3 functions overlap those of TAB2 in HeLa cells (Ishitani et al., 2003; Kanayama et al., 2004). In contrast, the earlier and present studies utilizing TAB2 knockout cells suggest that TAB2 functions predominantly to activate TAK1 in MEF cells. TAB3 also contains a conserved CUE domain in its N-terminal region. Therefore, it is very likely that TAB3 also participates in TRAF6 ubiquitination in some cell types. Further genetic studies will be needed to address the exact roles of TAB2 and TAB3.
Experimental procedures
Cell Culture and Expression Vectors: 293 cells and mouse embryonic fibroblasts (MEF) were maintained in Dulbecco's modified Eagle’s medium supplemented with fetal calf serum (10%) at 37°C and 5% CO2. A mammalian expression vector encoding TAB2 (pCMV-HA-TAB2) was described previously (Takaesu et al., 2000). The TAB2ΔCUE mutant was generated by utilizing EcoRV site to remove sequences encoding amino acids 1–53 of TAB2 cDNA. TAB2(F20A) and TAB2(E20D) mutants were generated by PCR and verified by DNA sequencing. The expression vector for ubiquitin (pCDNA3.1-Myc-Ub) was a kind gift from Dr. Keiji Tanaka (The Tokyo Metropolitan Institute of Medical Science).
Antibodies and Immunoprecipitation: Polyclonal rabbit antibodies to TRAF6, TAK1, TAB1, and TAB2 have been described previously (Ninomiya-Tsuji et al., 1999; Takaesu et al., 2000). Polyclonal rabbit antibodies to JNK, p38, IκBα, IKKα and monoclonal antibodies to Myc and ubiquitin were purchased from Santa Cruz. Monoclonal antibodies to phospho-JNK and phospho-p38 were purchased from Cell Signaling. Monoclonal antibody to HA (HA. 11) was purchased from Covance. For immunoprecipitation, MEFs were plated (1 x 106) on 10 cm dishes 24 hrs prior to stimulation. Cells were starved in serum-free medium at 37°C for 3 hrs and stimulated with IL-1β (Roche). After stimulation, cells were washed once with ice-cold phosphate-buffered saline (PBS) and lysed in 0.5% Triton X-100 lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF and 20 mM aprotinin. Cellular debris was removed by centrifugation at 10,000 x g for 5 min. Proteins from cell lysates were immunoprecipitated with 1 μg of various antibodies and 15 μl of protein G-Sepharose (Pharmacia). For the transfection studies, 293 cells (1 x 106) were plated in 10 cm dishes, and transfected by the calcium phosphate precipitate method with a total of 15 μg DNA of various expression vectors . After incubation for 48 hrs, cells were lysed with 0.5% Triton X-100 lysis buffer.
Reporter Gene Assay: For the reporter gene assays, 293 cells (1.6 x 105 cells/well) were plated into 6-well (35 mm) plates. At 24 hrs after plating, cells were transfected with an Ig-κ-luciferase reporter plasmid and the indicated expression plasmids. A plasmid containing the β-galactosidase gene under the control of the β-actin promoter (pAct-β-Gal) was used for normalizing transfection efficiency.
Yeast two-hybrid analysis: The yeast strain PJ69-4A was transformed with yeast expression vectors encoding proteins fused in-flame to the Gal4 DNA-binding domain (GBD) or the Gal4 activation domain (GAD). The plasmid pACT2-wtUbi for expressing wild-type ubiquitin fused with Gal4-activation domain was a kind gift from Dr. Linda Hicke (Northwestern University). The interactions between the bait and prey proteins were evaluated by growth on medium lacking histidine and containing 3 mM 3-AT (histidine synthesis inhibitor).
Subcellular localization: HeLa cells, plated on chamber slides, were transfected by Transfast transfection reagent (Promega). After transfection at 36 hrs, cells were washed with PBS, fixed in 4% paraformaldehyde, and washed 2 times with PBS. The fixed cells were subsequently permeabilized with acetone for 2 min at room temperature and blocked with 10% serum for 30 min. The cells were then incubated with various combinations of anti-HA, anti-Flag, anti-IKKα, anti-TAB2 and anti-TAK1, followed by Cy2-conjugated anti-mouse and/or Cy3-conjugated anti-rabbit secondary antibody (Amersham), and examined by fluorescent microscopy (Olympus).
Acknowledgments
We thank K. Tanaka and L. Hicke for materials. This work was supported by special grants for CREST and Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan (KM), and by NIH grants GM068812 and AR050972 (JNT).
References
- Baud V, Liu ZG, Bennett B, et al. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Volkwein C, Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. doi: 10.1126/science.278.5344.1806. [DOI] [PubMed] [Google Scholar]
- Cheung PC, Nebreda AR, Cohen P. TAB3, a new binding partner of the protein kinase TAK1. Biochem J. 2004;378:27–34. doi: 10.1042/BJ20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yang J, Lin Y, et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Takaesu G, Ninomiya-Tsuji J, et al. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Klika A, Callahan M, et al. Identification of a human NF-κB-activating protein, TAB3. Proc Natl Acad Sci USA. 2004;101:2028–2033. doi: 10.1073/pnas.0307314101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama A, Seth RB, Sun L, et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- Kopp E, Medzhitov R, Carothers J, et al. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, et al. Activation of the IκB α kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Malinin NL, Boldin MP, Kovalenko AV, et al. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, et al. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kajino T, Ono K, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Prag G, Misra S, Jones EA, et al. Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell. 2003;113:609–620. doi: 10.1016/s0092-8674(03)00364-7. [DOI] [PubMed] [Google Scholar]
- Sanjo H, Takeda K, Tsujimura T, et al. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol. 2003;23:1231–1238. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Prag G, Francis SA, et al. A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J. 2003;22:1273–1281. doi: 10.1093/emboj/cdg140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G, Kishida S, Hiyama A, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Ninomiya-Tsuji J, Kishida S, et al. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol Cell Biol. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, et al. TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- Yang J, Lin Y, Guo Z, et al. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Lee FS. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-α and IκB kinase-β. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]


