Abstract
1. The responses of Purkinje cells to short duration (pulse) ionophoretic applications of L-aspartate (L-asp), L-glutamate (L-glu), N-methyl DL-aspartate (NMDLA) and quisqualic acid in their dendritic fields were studied in vitro on sagittal slices of lobules IX and X of the adult rat cerebellum.
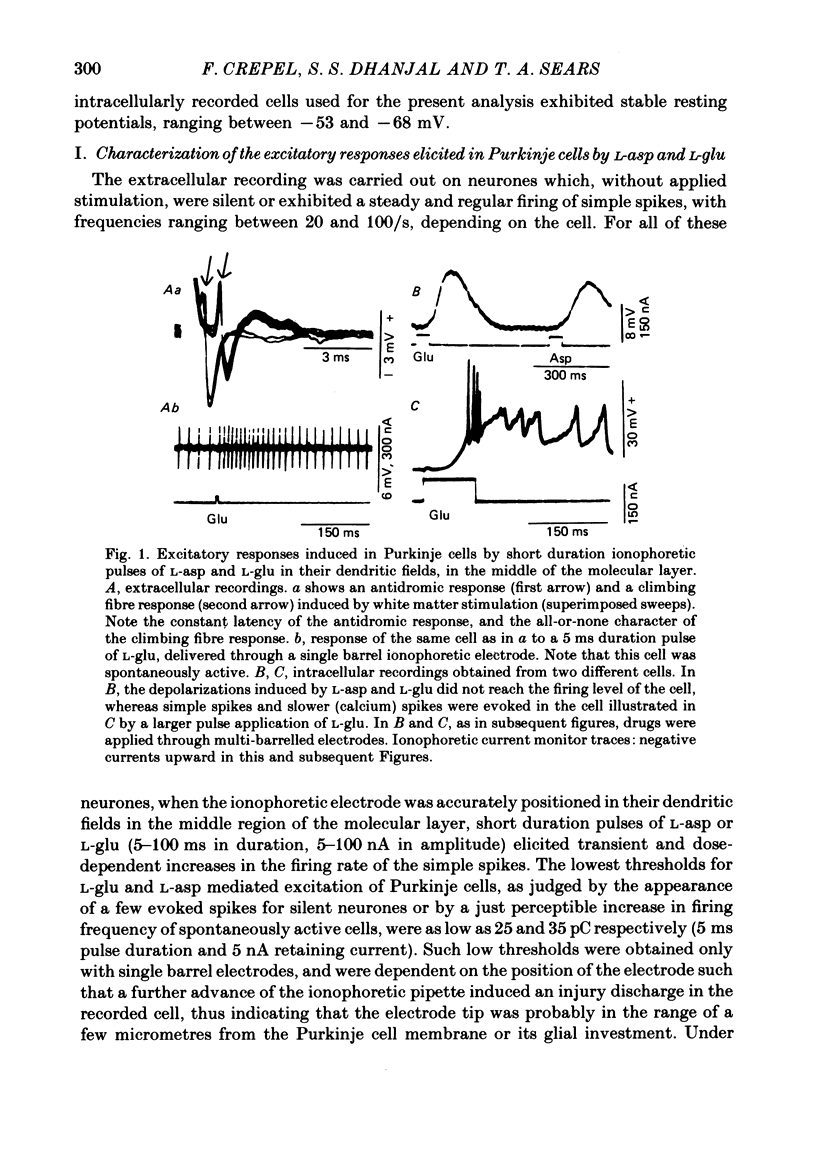
2. Pulse application of L-asp or L-glu evoked transient and dose-dependent increases in the firing rate of the simple spikes recorded extracellularly as single units. When the ionophoretic electrode was positioned in the dendritic field of the Purkinje cells, the lowest thresholds for L-glu and L-asp mediated excitations of the cells were as low as 25 and 35 pC respectively, with a latency for maximal responses as brief as 7 ms.
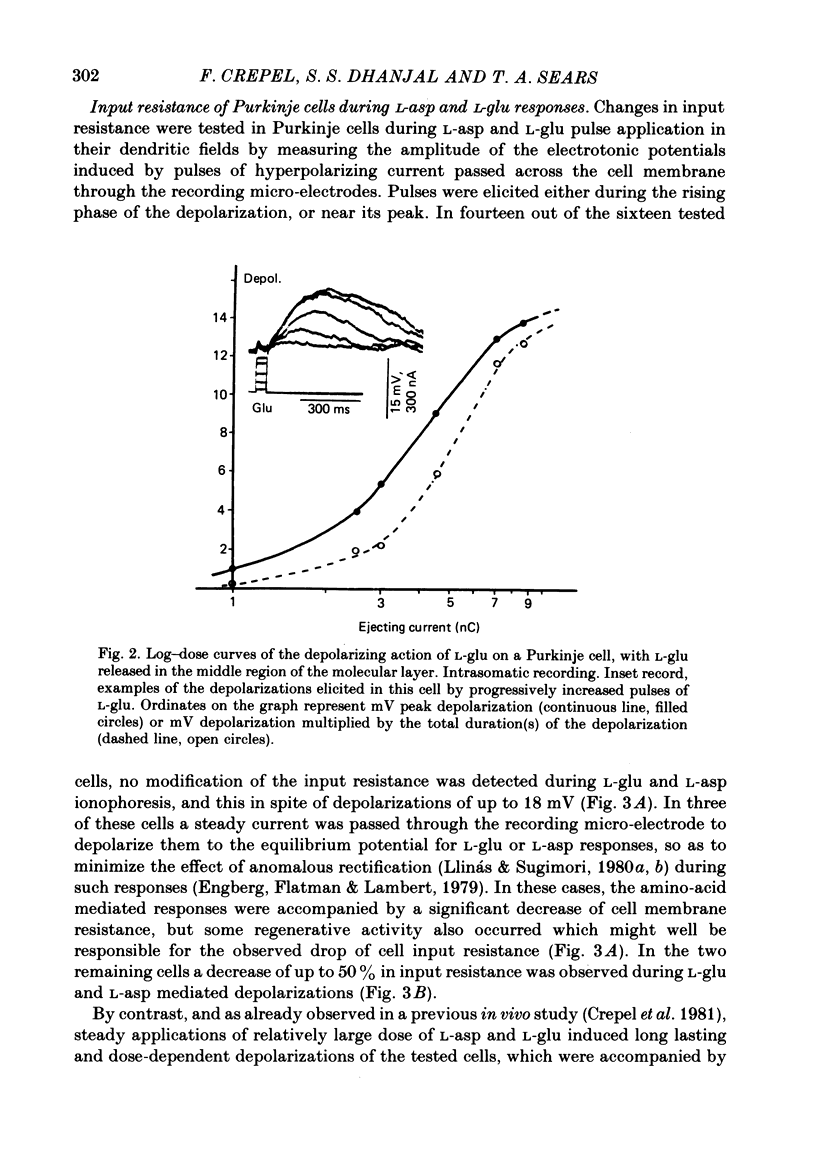
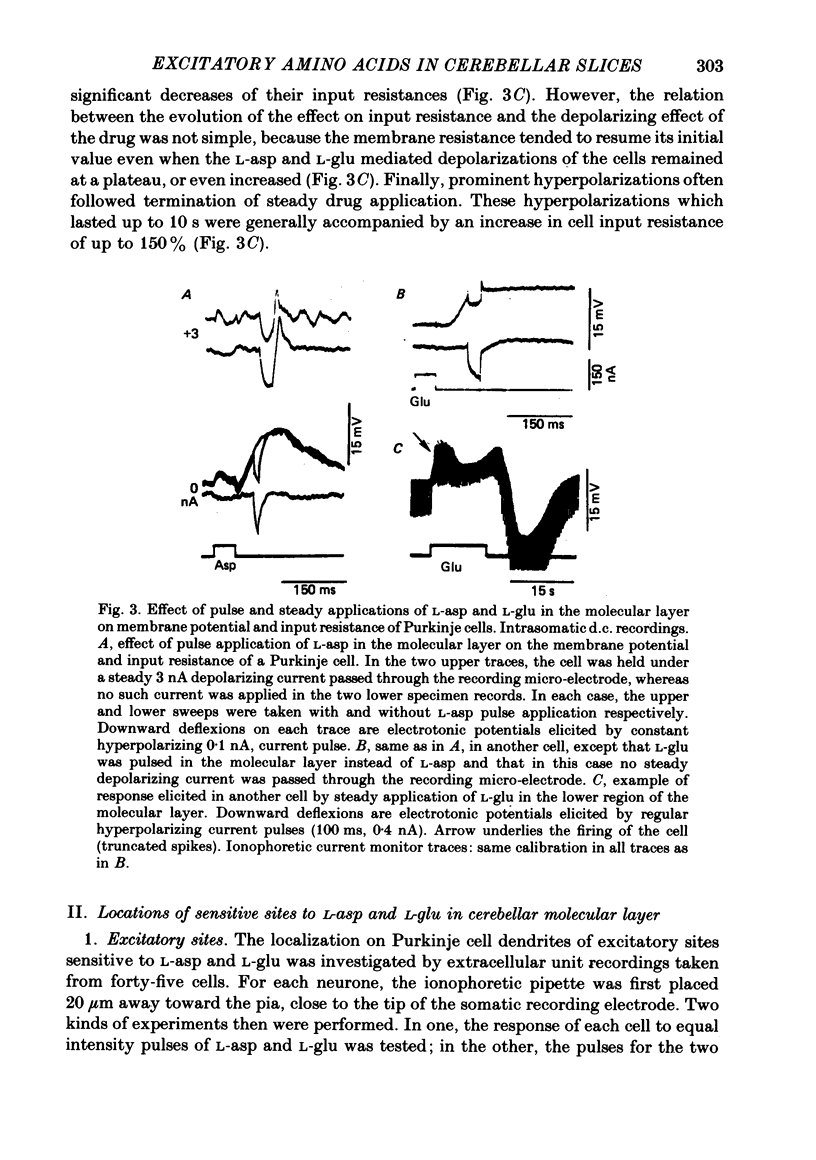
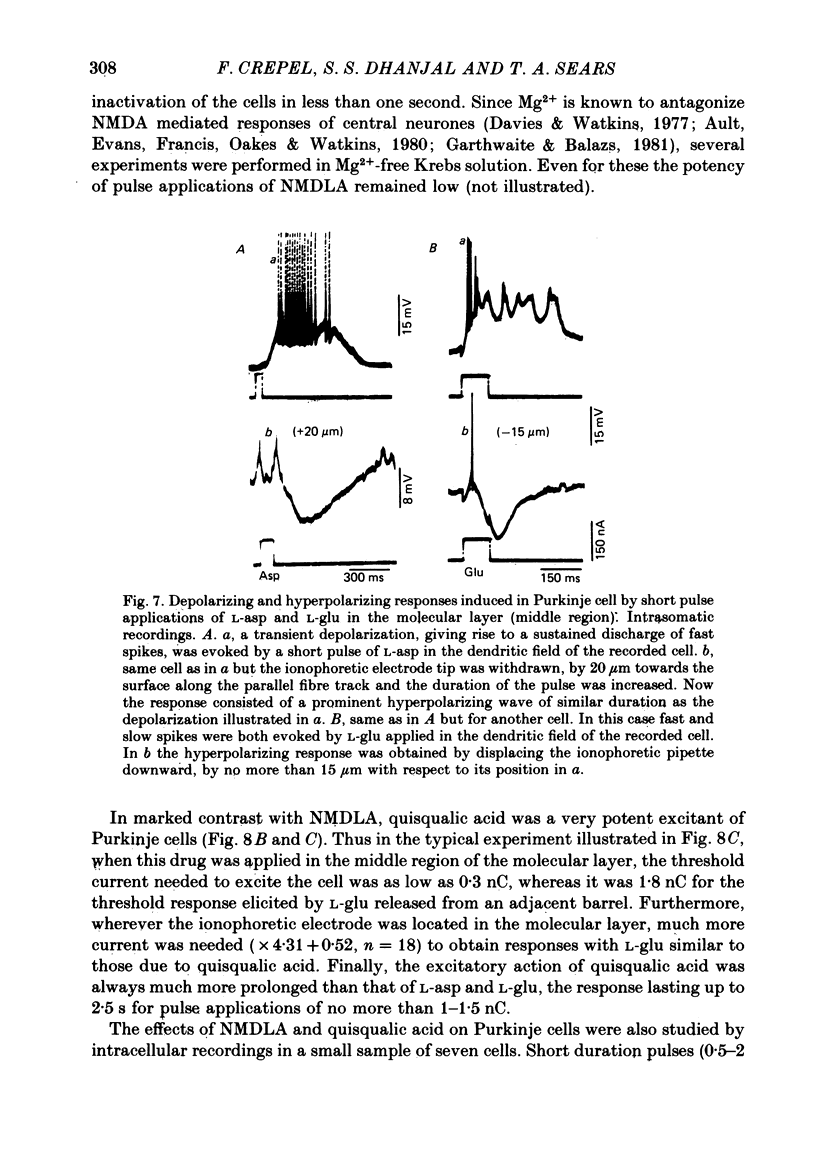
3. In intracellular recordings these excitatory responses consisted of depolarizations of up to 18 mV in amplitude and with depolarizing slopes up to 0·52 mV/ms. They were generally unaccompanied by changes in cell input resistance in contrast to the marked decrease which occurred in response to steady applications of large doses of L-asp and L-glu.
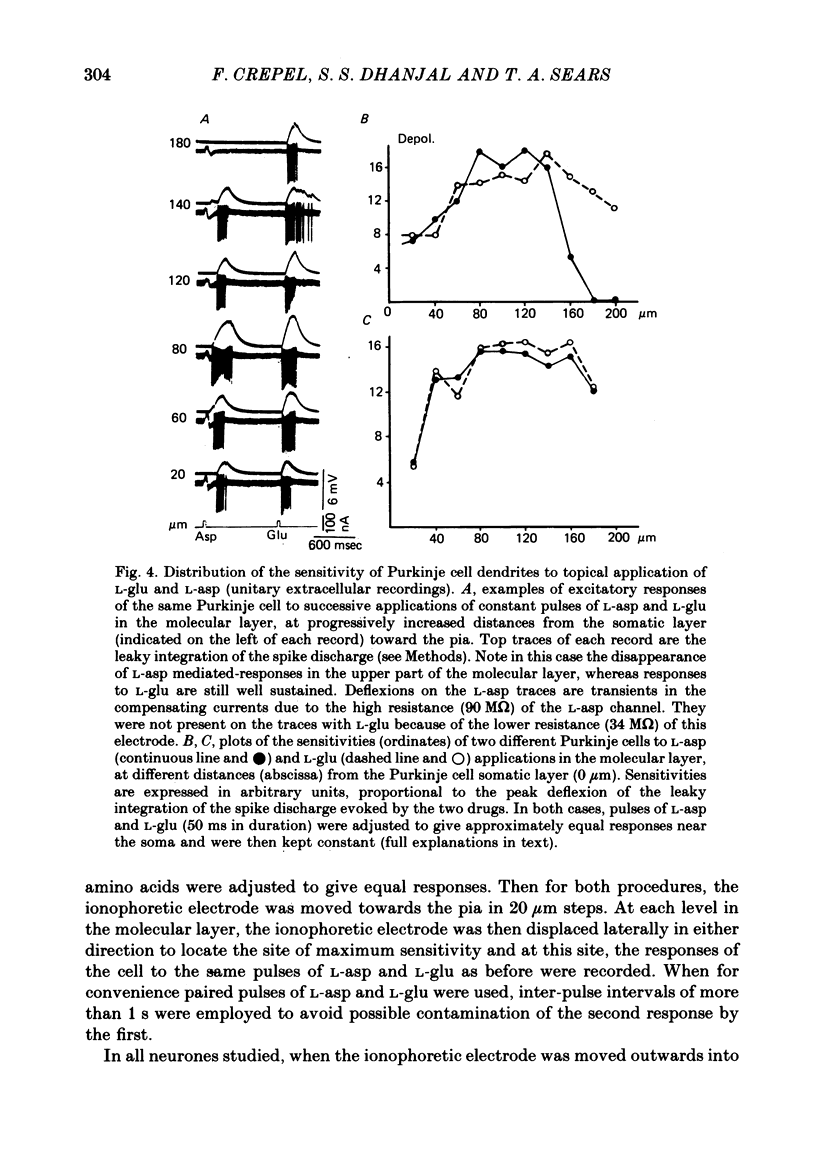
4. The spatial distribution of the excitatory sites confirmed that the dendritic sensitivity to L-glu was greater than that of the soma and showed that the same was true for L-asp. In 34% of cells the sensitivity for L-asp declined markedly in the upper region of the molecular layer, whereas it remained high for L-glu; no such differential sensitivity was detected in the remaining 66% of cells.
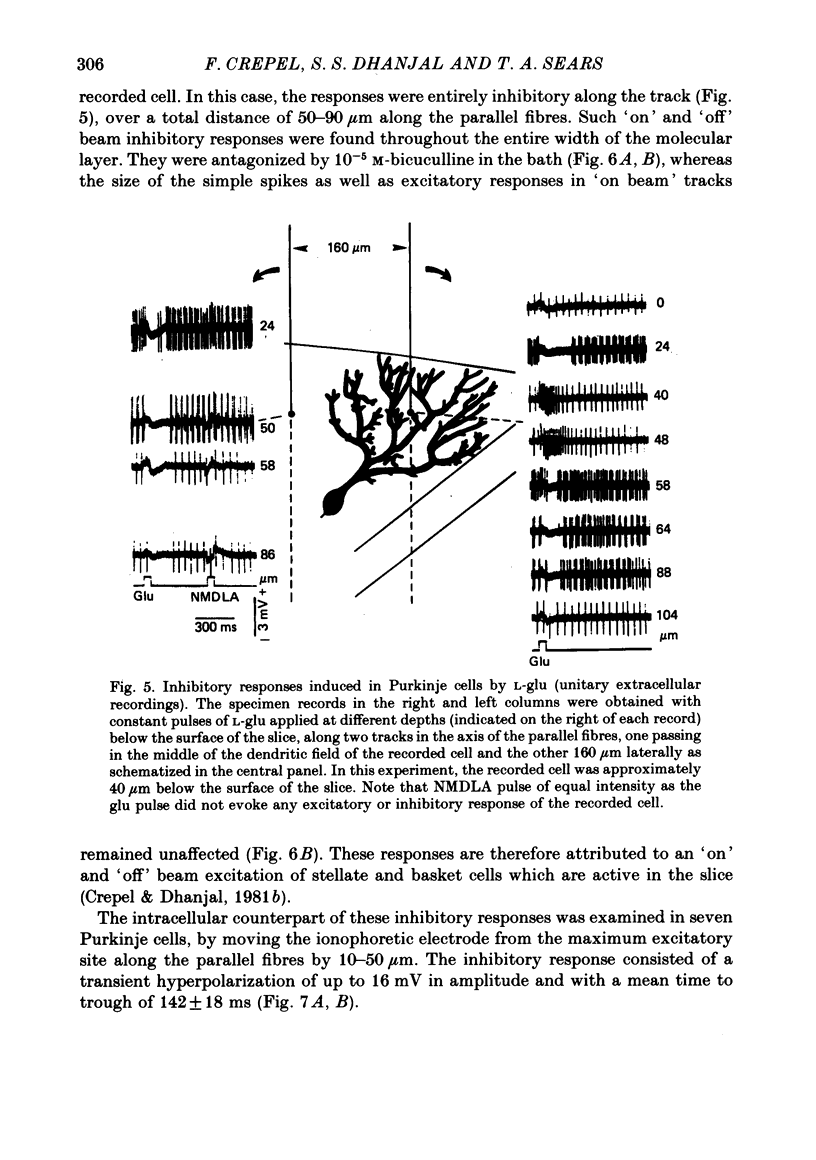
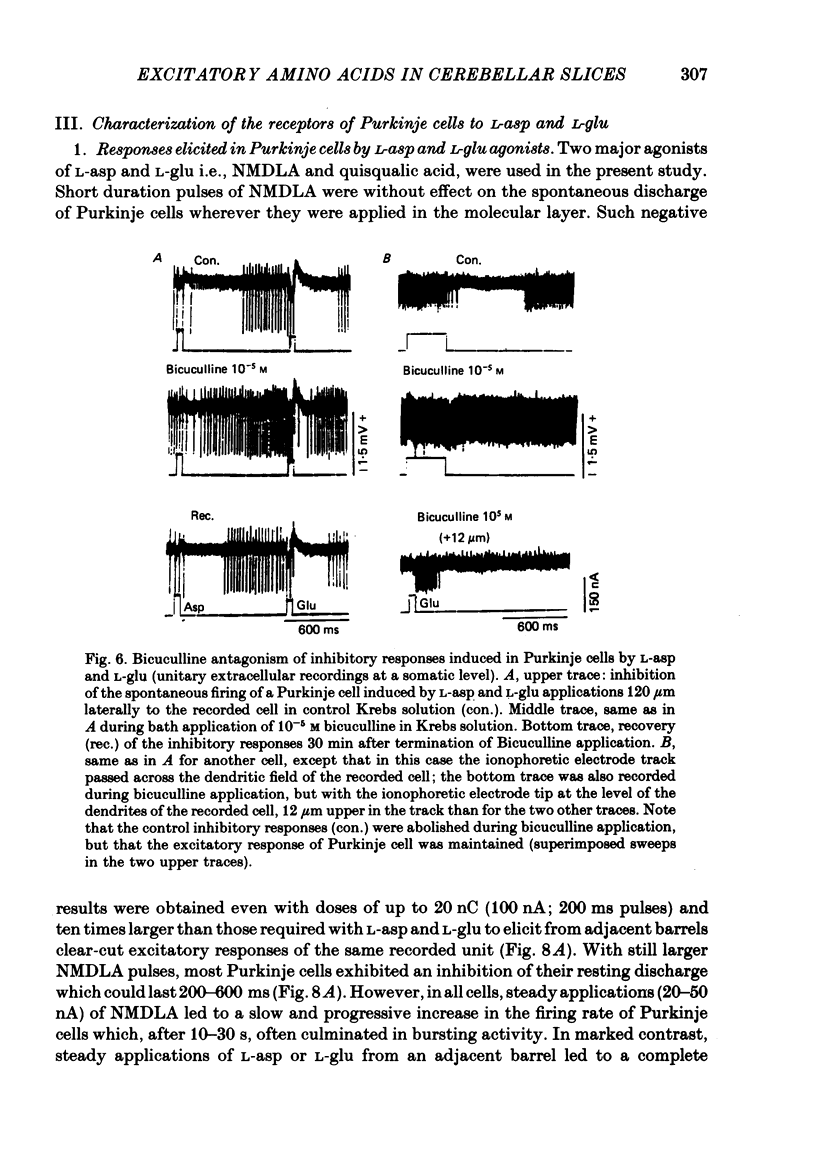
5. Inhibitory responses, antagonized by 10-5 M-bicuculline in the bath, were also induced in Purkinje cells by L-glu and L-asp when the ionophoretic electrode was withdrawn from the excitatory sites by as little as 8 μm and up to 40 μm upward or downward along the track of parallel fibres or positioned as far as 250 μm laterally.
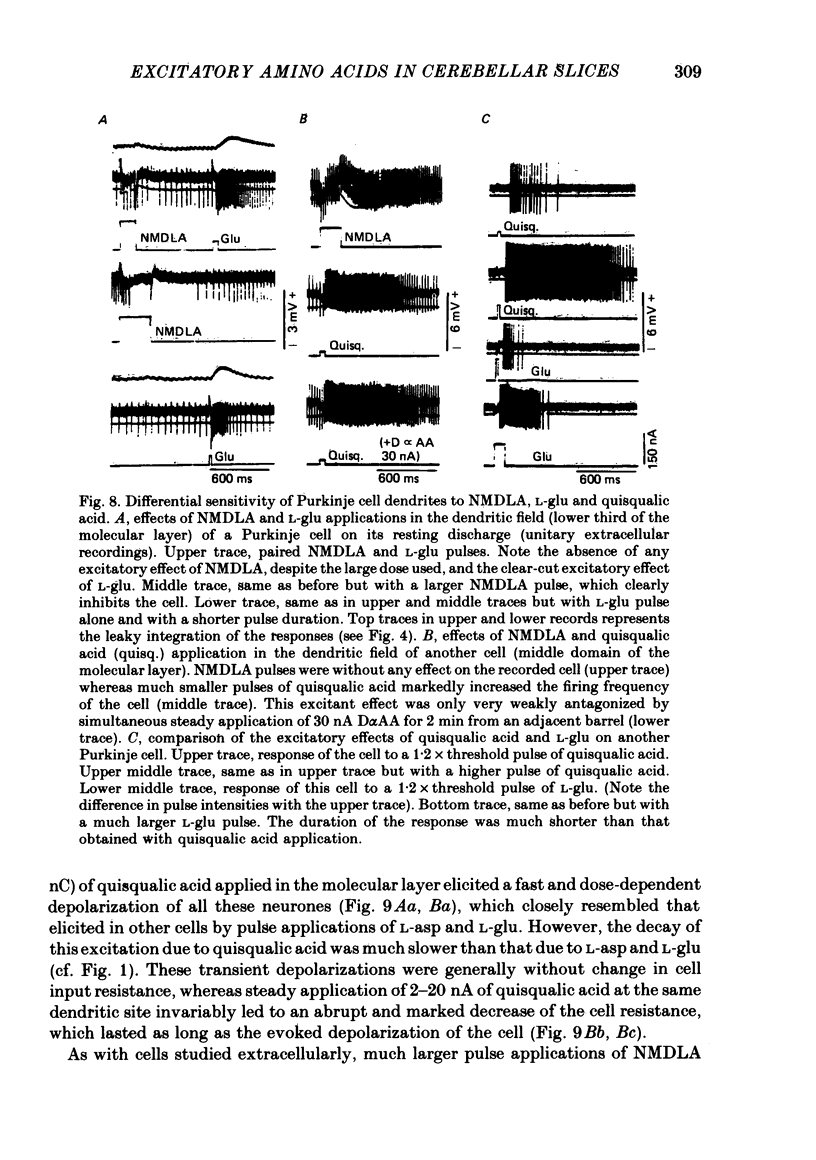
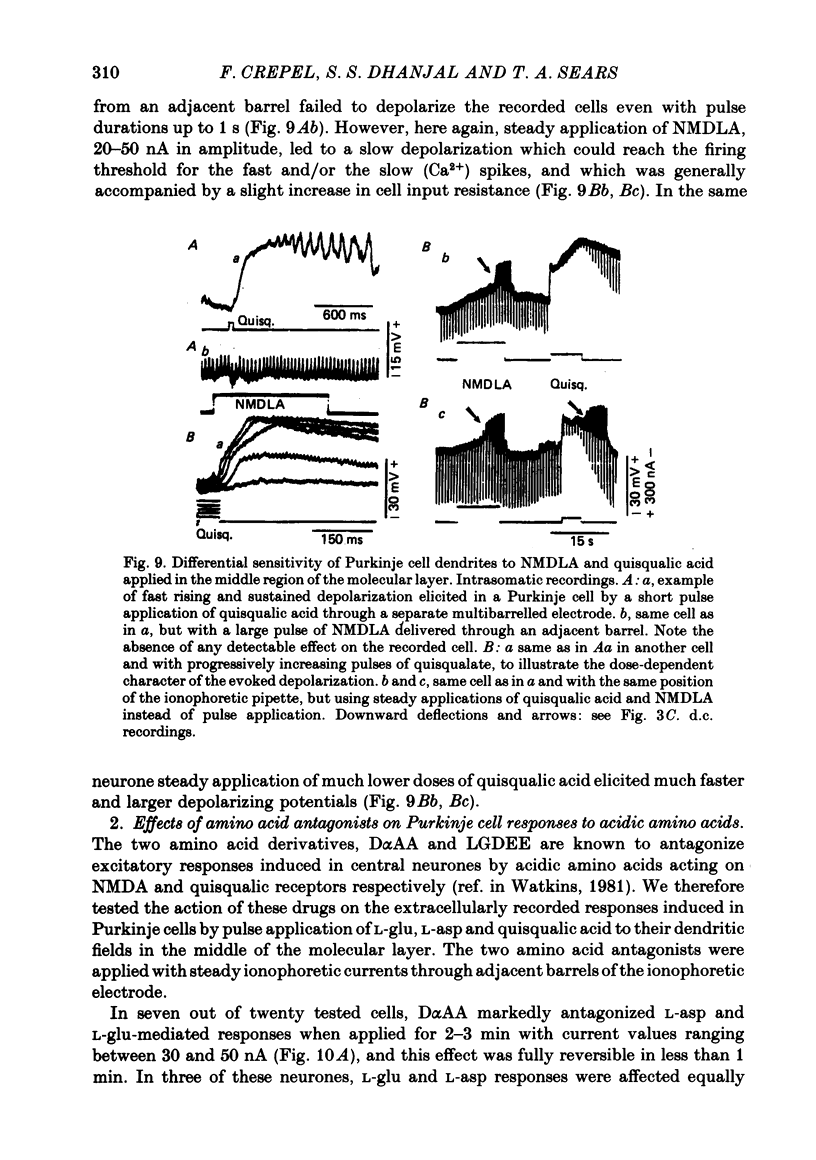
6. Whenever it was applied in the molecular layer, the pulse application of NMDLA elicited no excitatory response in Purkinje cells recorded extra or intracellularly. However, slow depolarizations accompanied by a slight increase in cell input resistance were obtained with steady applications of 20-50 nA of the drug for 20-30 s.
7. In contrast, pulse application of quisqualic acid appeared to have the same type of fast excitatory effect on Purkinje cells as L-asp and L-glu, but its potency was greater and its action more prolonged. Furthermore, its steady application led to an abrupt and marked decrease in cell membrane resistance.
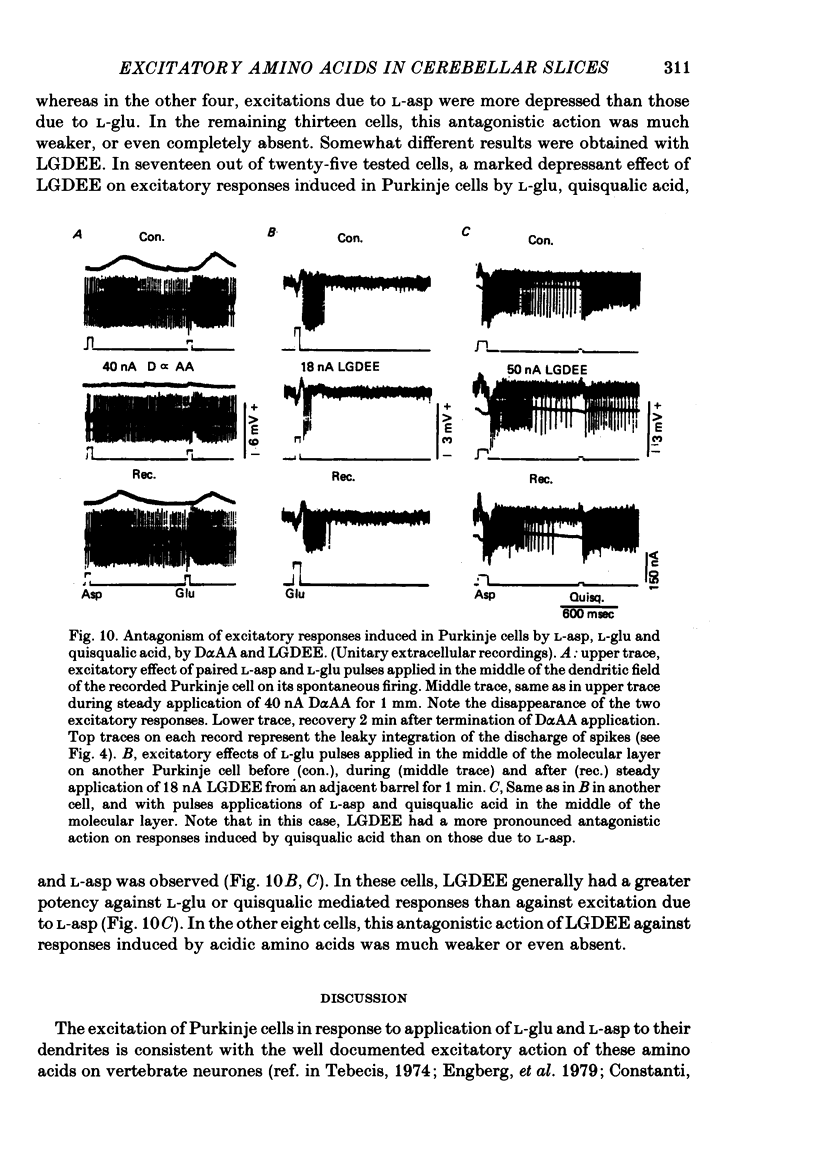
8. The excitatory effects of L-asp, L-glu and quisqualic acid were antagonized by L-glutamic acid diethyl ester more consistently than by D-α-aminoadipate, suggesting together with previous observations that L-asp and L-glu act on Purkinje cells via quisqualic acid rather than via NMDLA receptors.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Chujo T., Yamada Y., Yamamoto C. Sensitivity of Purkinje cell dendrites to glutamic acid. Exp Brain Res. 1975 Sep 29;23(3):293–300. doi: 10.1007/BF00239741. [DOI] [PubMed] [Google Scholar]
- Constanti A., Connor J. D., Galvan M., Nistri A. Intracellularly-recorded effects of glutamate and aspartate on neurones in the guinea-pig olfactory cortex slice. Brain Res. 1980 Aug 18;195(2):403–420. doi: 10.1016/0006-8993(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Constanti A., Krnjević K., Nistri A. Intraneuronal effects of inhibitory amino acids. Can J Physiol Pharmacol. 1980 Feb;58(2):193–204. doi: 10.1139/y80-032. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R., Voorhoeve P. E., Wilson V. J. Acetylcholine sensitivity of cerebellar neurones in the cat. J Physiol. 1966 Sep;186(1):139–165. doi: 10.1113/jphysiol.1966.sp008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N., Pumain R. Amino acids and neurotransmission in rat cerebellar cortex. Adv Biochem Psychopharmacol. 1981;29:295–299. [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Garthwaite J. Morphological and electrophysiological characteristics of rat cerebellar slices maintained in vitro. J Physiol. 1981 Jul;316:127–138. doi: 10.1113/jphysiol.1981.sp013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Differentiation of kainate and quisqualate receptors in the cat spinal cord by selective antagonism with gamma-D(and L)-glutamylglycine. Brain Res. 1981 Feb 9;206(1):172–177. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Effect of magnesium ions on the responses of spinal neurones to excitatory amino acids and acetylcholine. Brain Res. 1977 Jul 15;130(2):364–368. doi: 10.1016/0006-8993(77)90284-0. [DOI] [PubMed] [Google Scholar]
- Diamond J., Huxley A. F. The activation and distribution of GABA and L-glutamate receptors on goldfish Mauthner neurones: an analysis of dendritic remote inhibition. J Physiol. 1968 Feb;194(3):669–723. doi: 10.1113/jphysiol.1968.sp008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Dingledine R., Kelly J. S. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res. 1981 Feb 23;207(1):109–127. doi: 10.1016/0006-8993(81)90682-x. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The action of N-methyl-D-aspartic and kainic acids on motoneurones with emphasis on conductance changes [proceedings]. Br J Pharmacol. 1978 Nov;64(3):384P–385P. [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Balazs R. Excitatory amino acid-induced changes in cyclic GMP levels in slices and cell suspensions from the cerebellum. Adv Biochem Psychopharmacol. 1981;27:317–326. [PubMed] [Google Scholar]
- Geller H. M., Woodward D. J. Responses of cultured cerebellar neurons to iontophoretically applied amino acids. Brain Res. 1974 Jul 5;74(1):67–80. doi: 10.1016/0006-8993(74)90112-7. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Hicks T. P., McLennan H., Richardson T. L., Wheal H. V. The excitation of mammalian central neurones by amino acids. J Physiol. 1979 Jan;286:29–39. doi: 10.1113/jphysiol.1979.sp012605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H., Provini L. Depression of cerebellar Purkinje cells by microiontophoretic application of GABA and related amino acids. Brain Res. 1970 Dec 1;24(2):293–304. doi: 10.1016/0006-8993(70)90108-3. [DOI] [PubMed] [Google Scholar]
- Kehoe J. S. Electrogenic effects of neutral amino acids on neurons of Aplysia californica. Cold Spring Harb Symp Quant Biol. 1976;40:145–155. doi: 10.1101/sqb.1976.040.01.016. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. D., Flatman J. A., Engberg I. Actions of excitatory amino acids on membrane conductance and potential in motoneurones. Adv Biochem Psychopharmacol. 1981;27:205–216. [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Glutamate and synaptic excitation of reticulospinal neurones of lamprey. J Physiol. 1979 Aug;293:417–433. doi: 10.1113/jphysiol.1979.sp012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Quastel J. H. Spontaneous action potentials in isolated guinea-pig cerebellar slices: effects of amino acids and conditions affecting sodium and water uptake. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):83–90. doi: 10.1098/rspb.1973.0032. [DOI] [PubMed] [Google Scholar]
- Padjen A. L., Smith P. A. Possible role of divalent cations in amino acid responses of frog spinal cord. Adv Biochem Psychopharmacol. 1981;29:271–280. [PubMed] [Google Scholar]
- Schousboe A., Hertz L. Role of astroglial cells in glutamate homeostasis. Adv Biochem Psychopharmacol. 1981;27:103–113. [PubMed] [Google Scholar]
- Segal M. The actions of glutamic acid on neurons in the rat hippocampal slice. Adv Biochem Psychopharmacol. 1981;27:217–225. [PubMed] [Google Scholar]
- Siggins G. R., Oliver A. P., Hoffer B. J., Bloom F. E. Cyclic adenosine monophosphate and norepinephrine: effects on transmembrane properties of cerebellar Purkinje cells. Science. 1971 Jan 15;171(3967):192–194. doi: 10.1126/science.171.3967.192. [DOI] [PubMed] [Google Scholar]
- Sonnhof U., Bührle C. An analysis of glutamate-induced ion fluxes across the membrane of spinal motoneurons of the frog. Adv Biochem Psychopharmacol. 1981;27:195–204. [PubMed] [Google Scholar]
- Stone T. W. Glutamate as the neurotransmitter of cerebellar granule cells in the rat: electrophysiological evidence. Br J Pharmacol. 1979 Jun;66(2):291–296. doi: 10.1111/j.1476-5381.1979.tb13678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Oster-Granite M. L., Herndon R. M., Snyder S. H. Glutamic acid: selective depletion by viral induced granule cell loss in hamster cerebellum. Brain Res. 1974 Jun 14;73(1):1–13. doi: 10.1016/0006-8993(74)91002-6. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


