Abstract
1. Intracellular recordings were made from guinea-pig myenteric neurones in vitro.
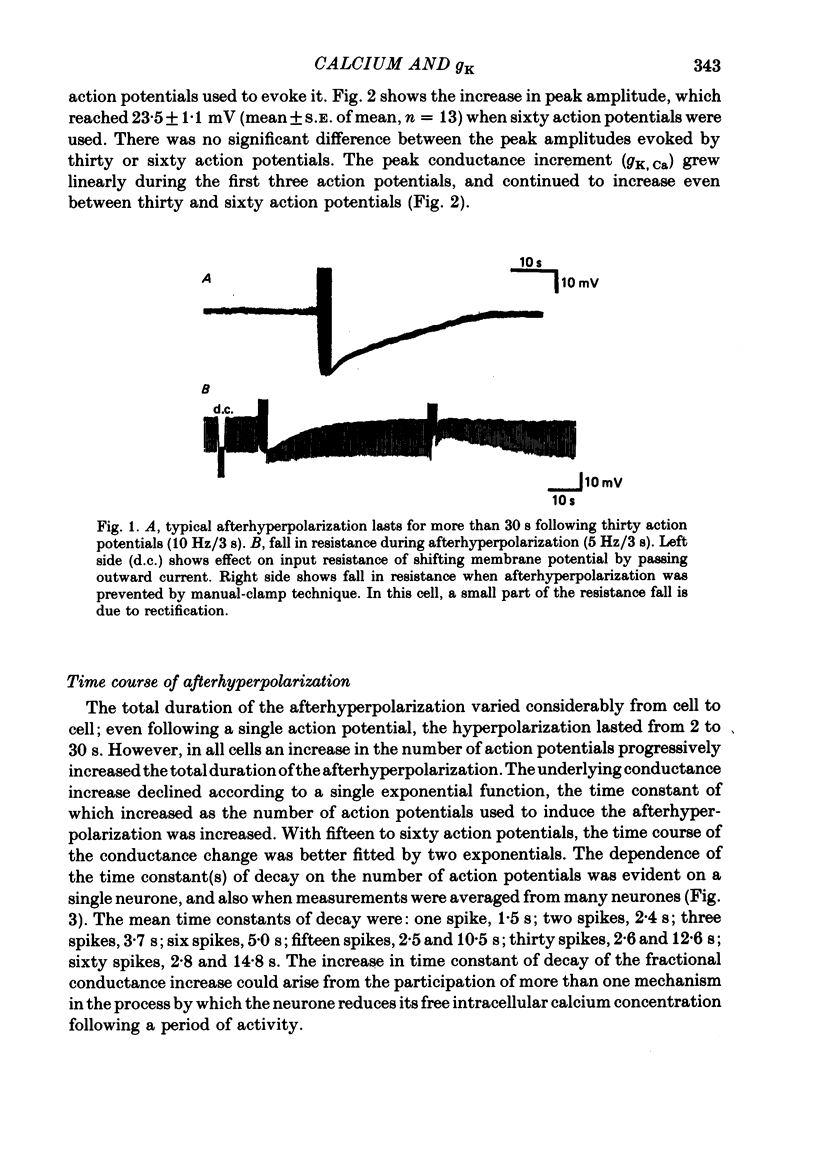
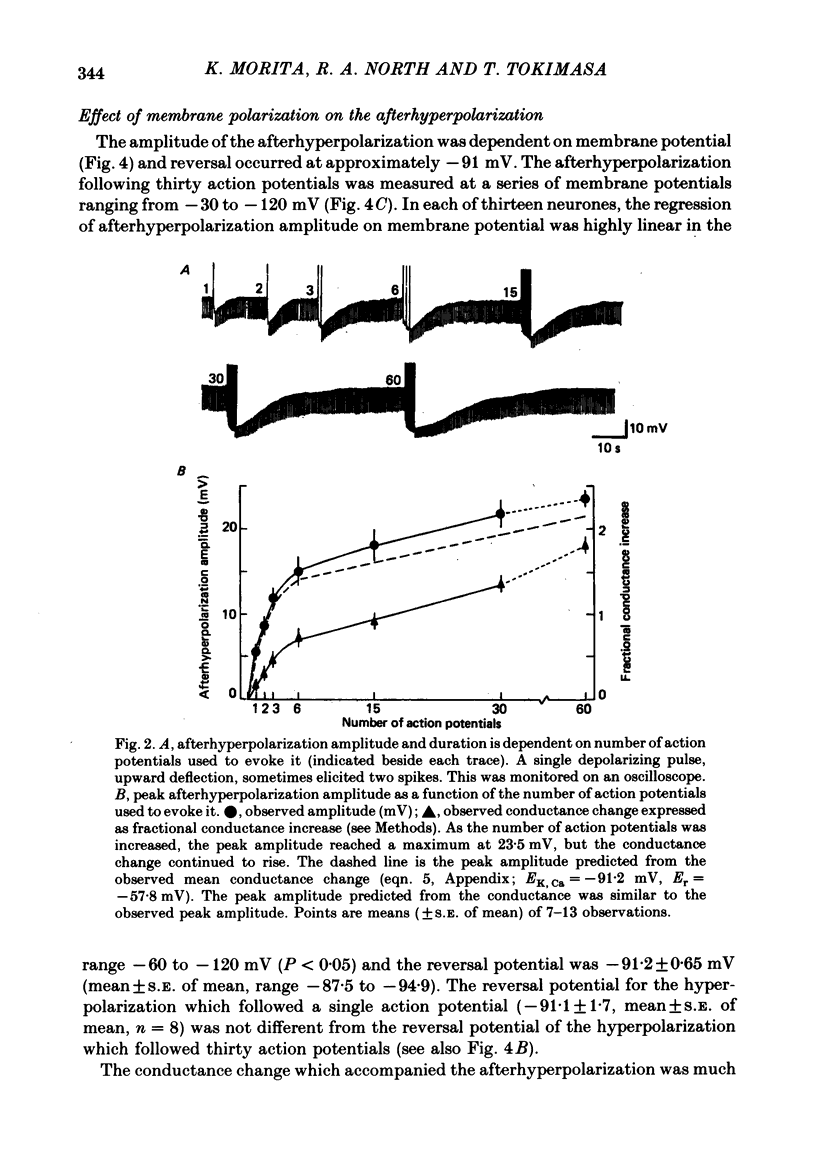
2. From one to sixty action potentials were followed by an afterhyperpolarization, the amplitude and duration of which increased with the number of preceding action potentials.
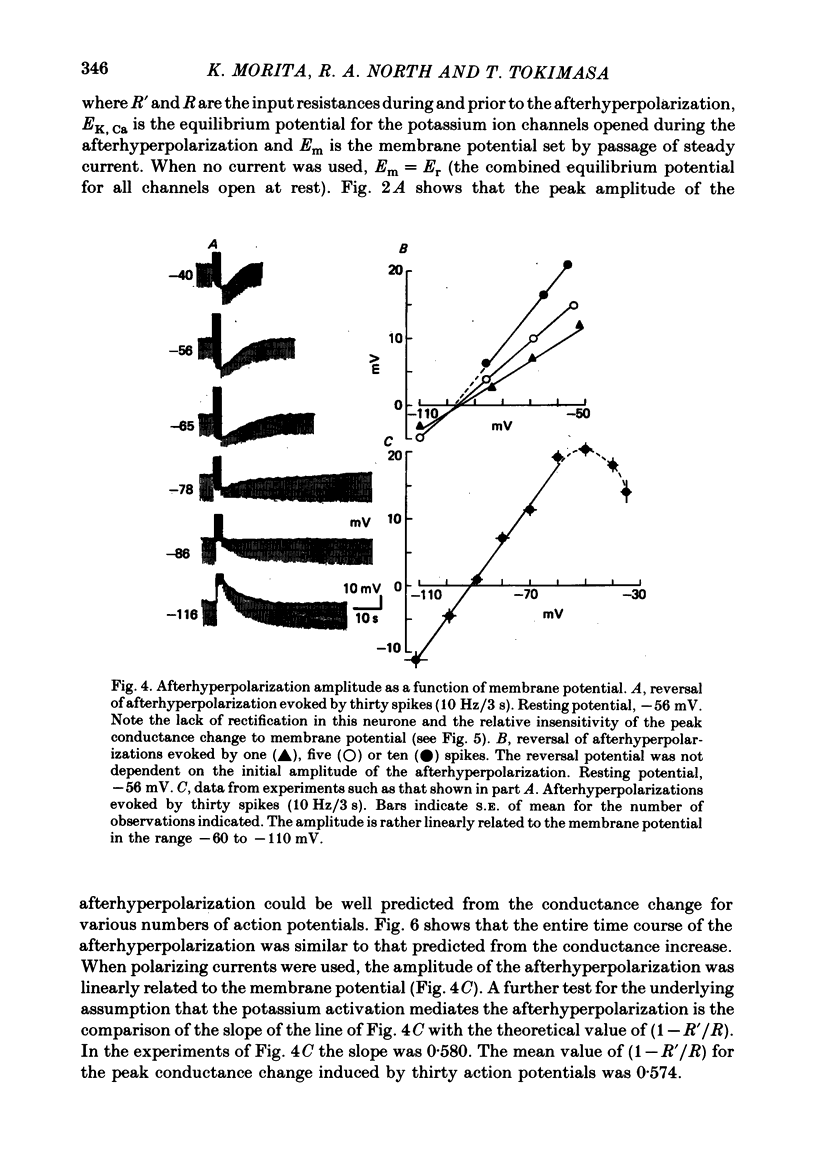
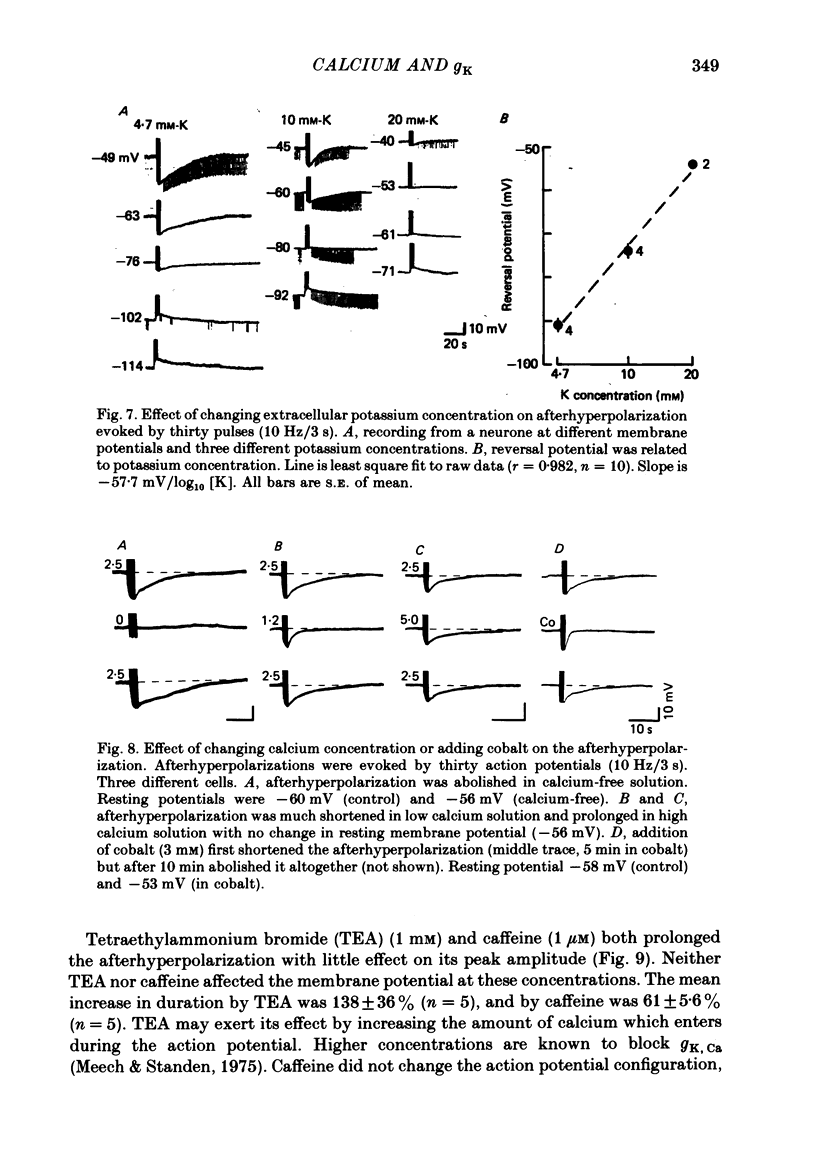
3. The afterhyperpolarization reversed its polarity at a membrane potential of -91 mV. This value changed by 58 mV when the potassium concentration of the perfusing solution was changed ten-fold.
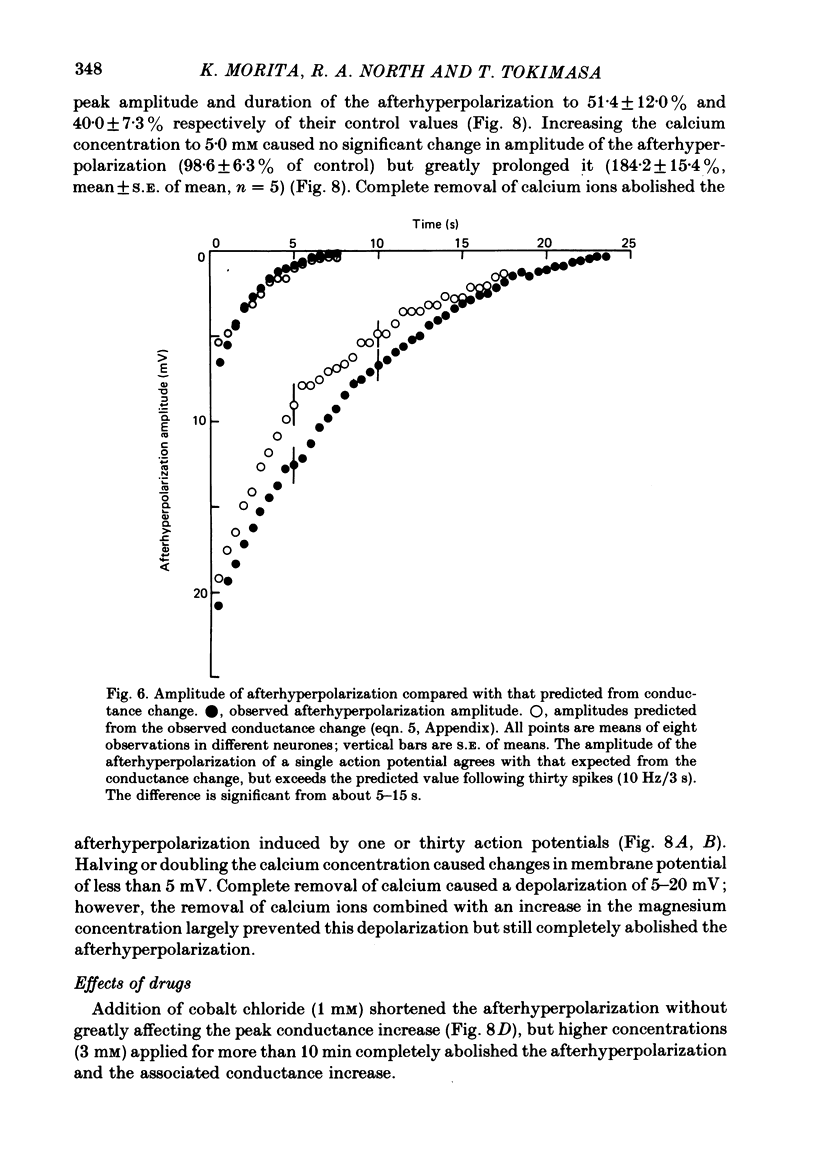
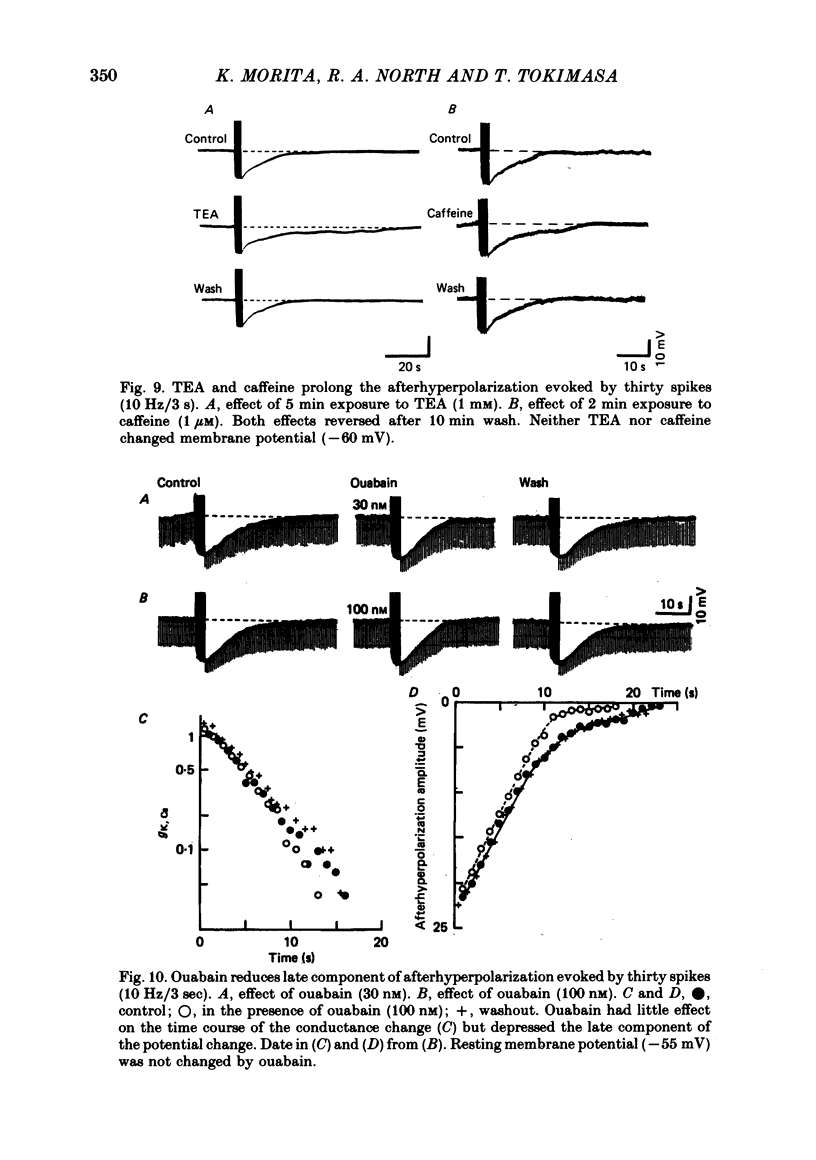
4. The afterhyperpolarization was abolished in calcium-free solutions. It was shortened in low calcium (1·2 mM) solutions and prolonged in solutions which contained high (5·0 mM) calcium concentrations, TEA (1 mM) or caffeine (1 μM).
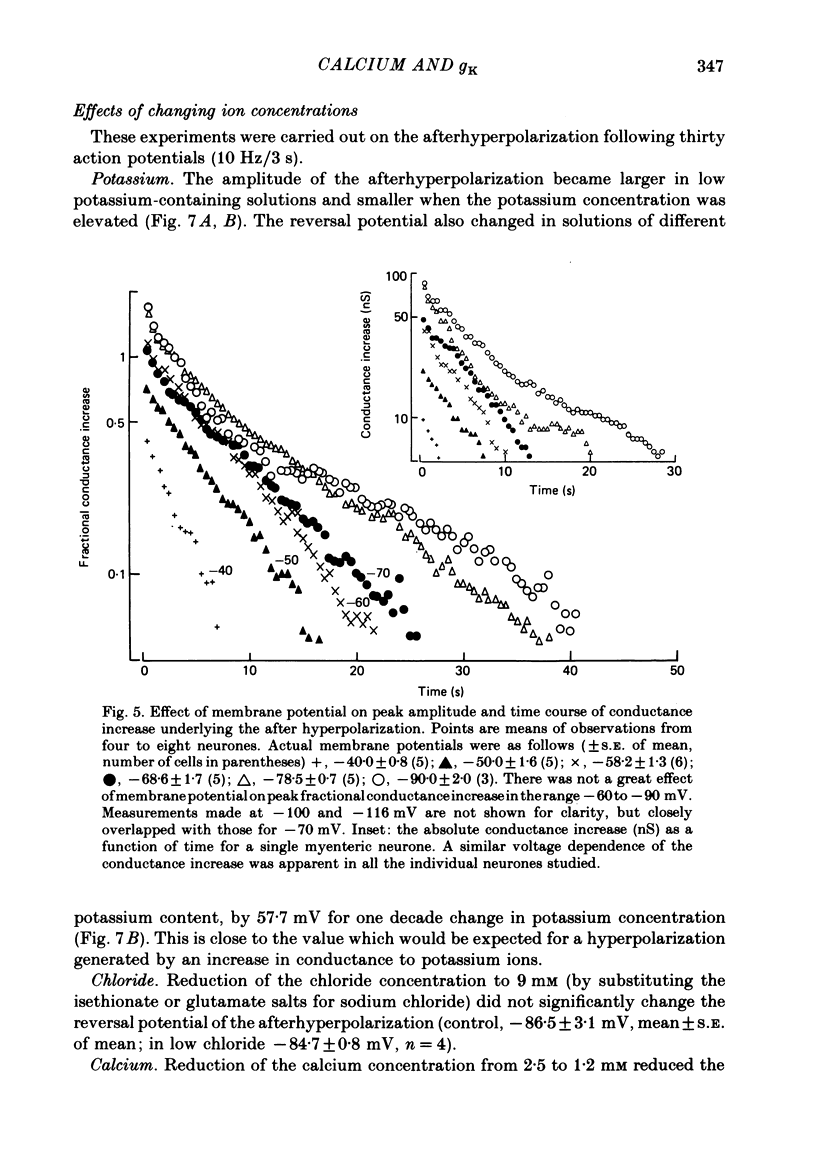
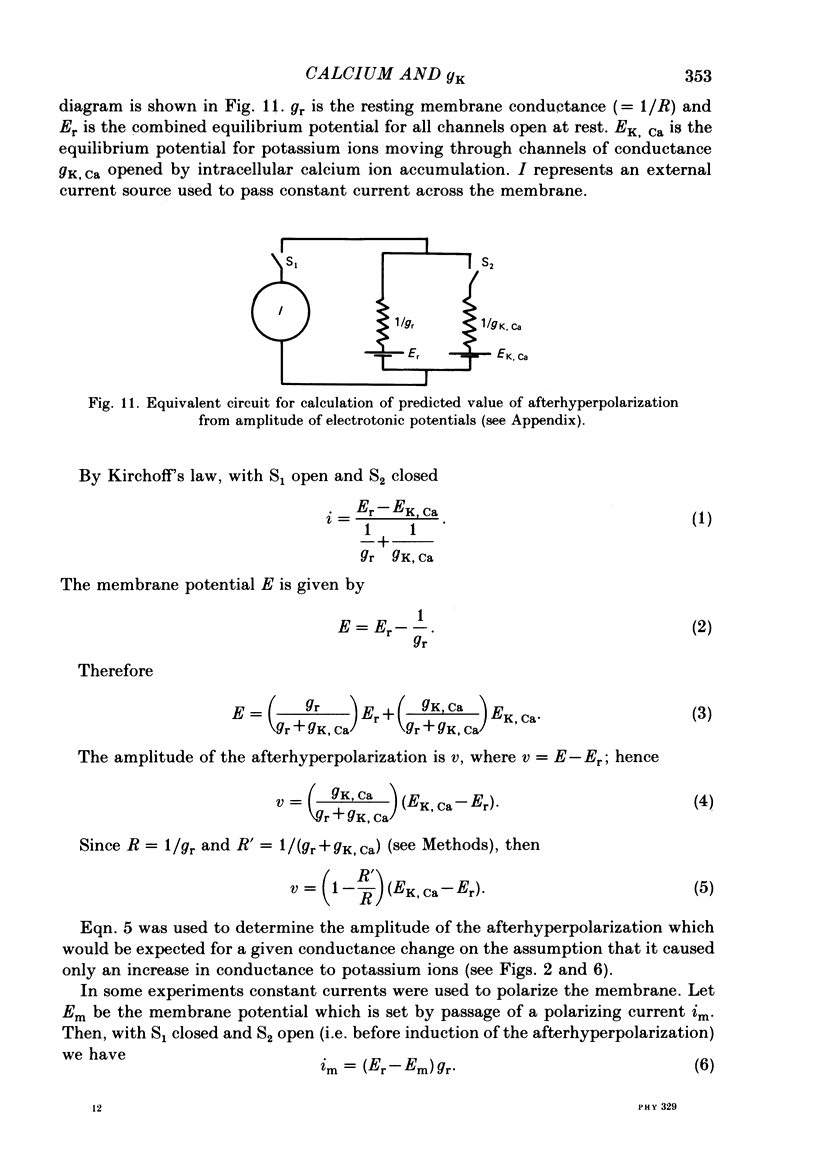
5. The conductance increase during the afterhyperpolarization (gK, Ca) was calculated from the amplitude of electrotonic potentials, taking advantage of the lack of membrane rectification in the range -60 to -90 mV. Peak gK, Ca increased as the number of action potentials was increased, but was relatively independent of membrane potential in this range.
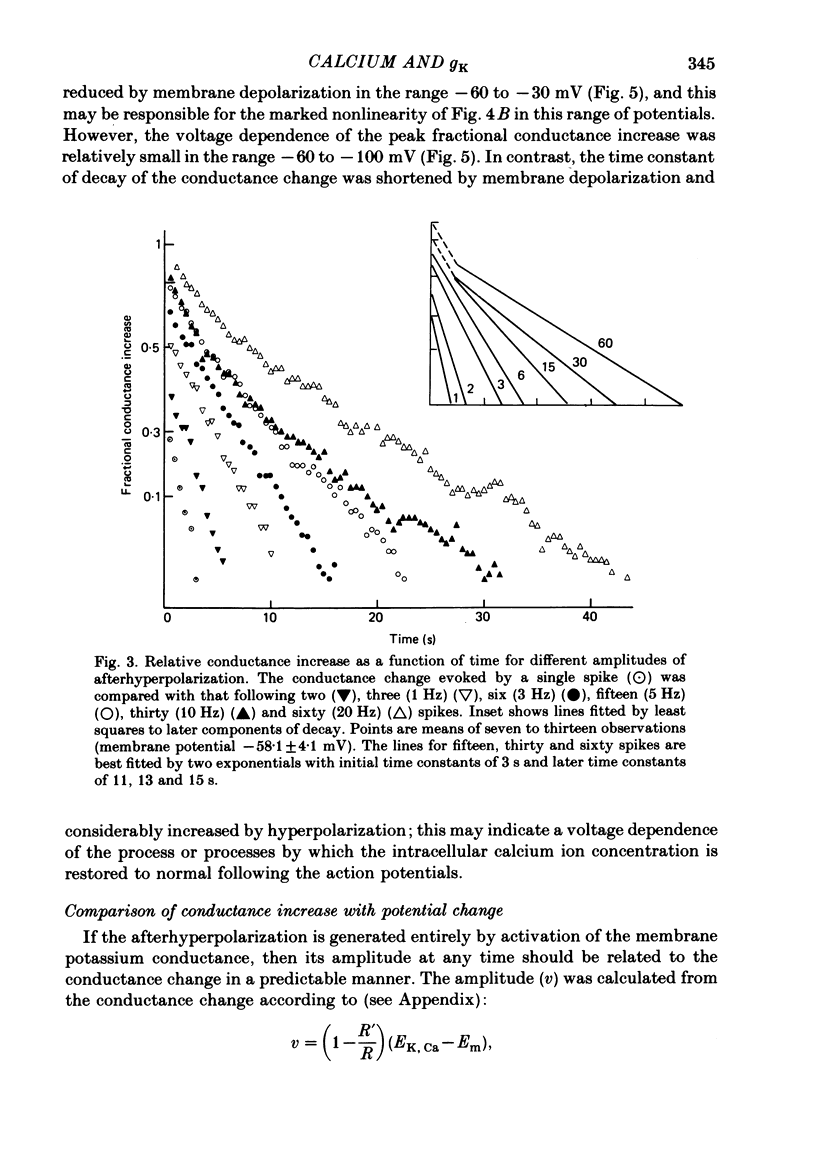
6. gK, Ca declined with a time course which was single exponential (time constant 1·5-5 s) following one to six action potentials, and double exponential (time constants about 3 and 12 s) following fifteen to sixty action potentials.
7. It is concluded that the calcium which enters the neurone during the action potential elevates the membrane potassium conductance. The time course of this conductance increase probably reflects the free intracellular calcium concentration, and therefore describes the calcium sequestration or extrusion process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Potassium conductance and internal calcium accumulation in a molluscan neurone. J Physiol. 1980 Nov;308:287–313. doi: 10.1113/jphysiol.1980.sp013472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Spence I. Calcium action potentials in mammalian peripheral neurones. Nat New Biol. 1973 May 9;243(123):54–56. [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience. 1981;6(10):1943–1951. doi: 10.1016/0306-4522(81)90034-8. [DOI] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. The calcium-dependent slow after-hyperpolarization in myenteric plexus neurones with tetrodotoxin-resistant action potentials. Br J Pharmacol. 1973 Dec;49(4):709–711. doi: 10.1111/j.1476-5381.1973.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Morita K., North A. Opiates and clonidine prolong calcium-dependent after-hyperpolarizations. Nature. 1981 Nov 12;294(5837):162–163. doi: 10.1038/294162a0. [DOI] [PubMed] [Google Scholar]


