Abstract
1. The effects of low concentrations of Cs+ (0·01-3mM) on the fully activated I-V relation īf(E) for the pace-maker current in calf Purkinje fibres have been investigated. The action of Cs+ is two-fold: in the negative region of the I-V curve Cs+ induces a channel blockade; on the other hand, at more positive potentials Cs+ can produce the opposite effect, i.e. a current increase.
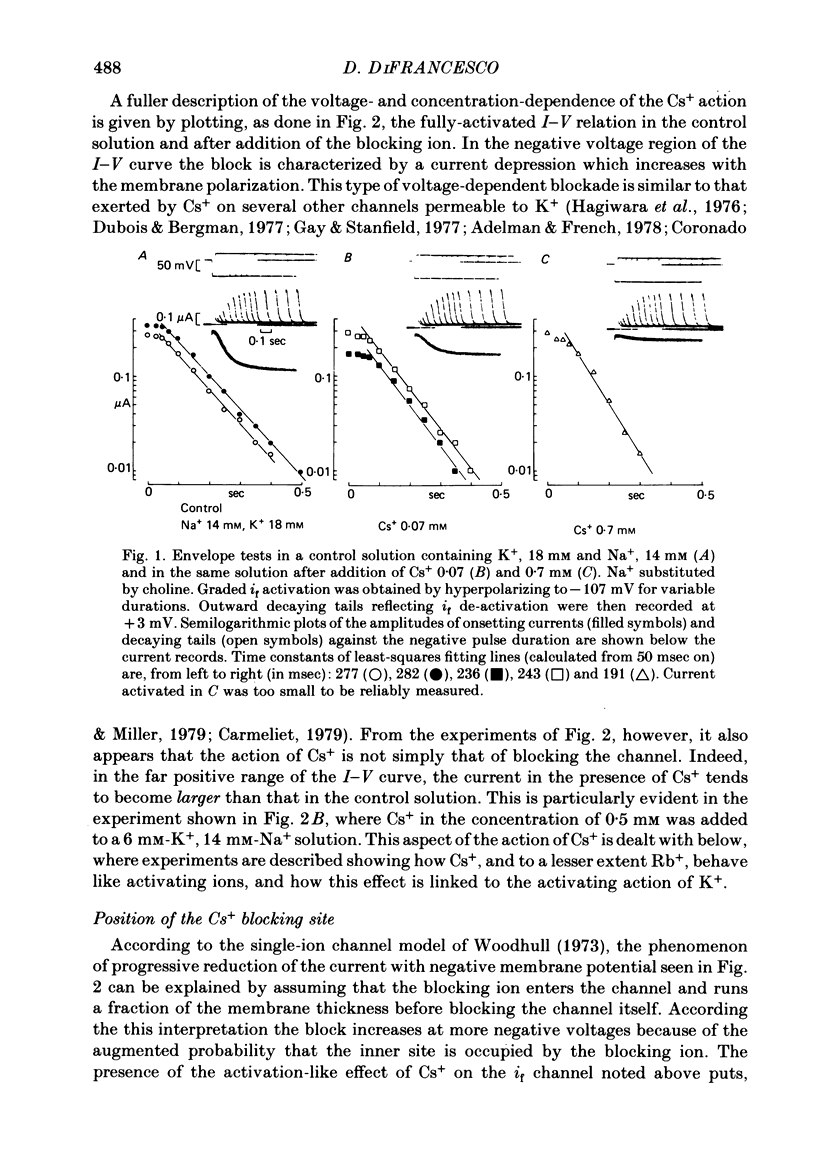
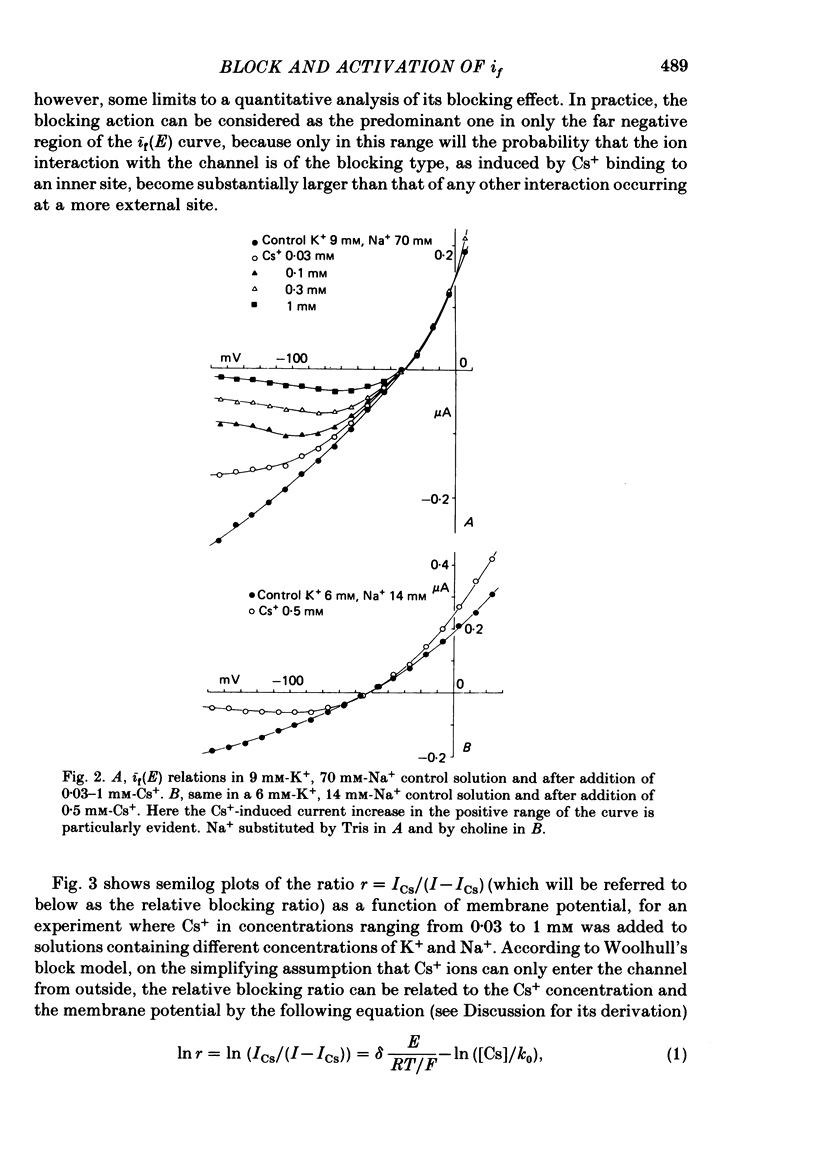
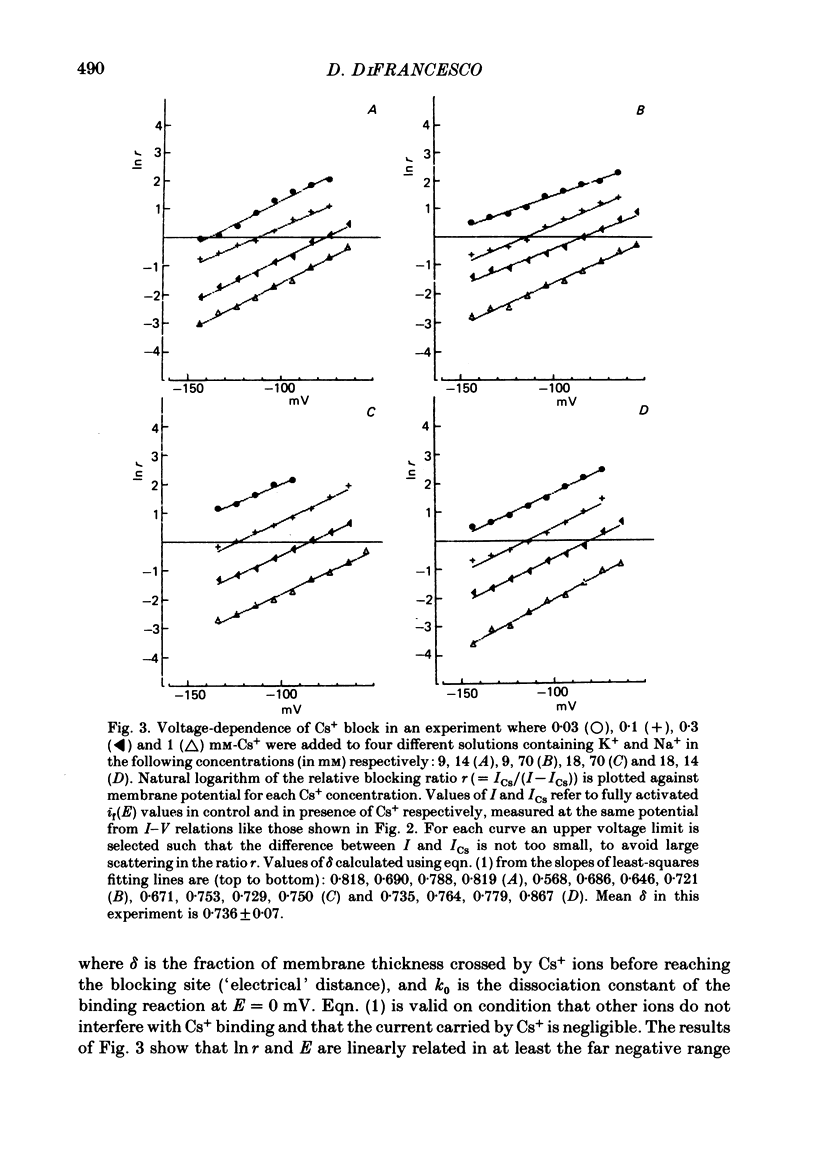
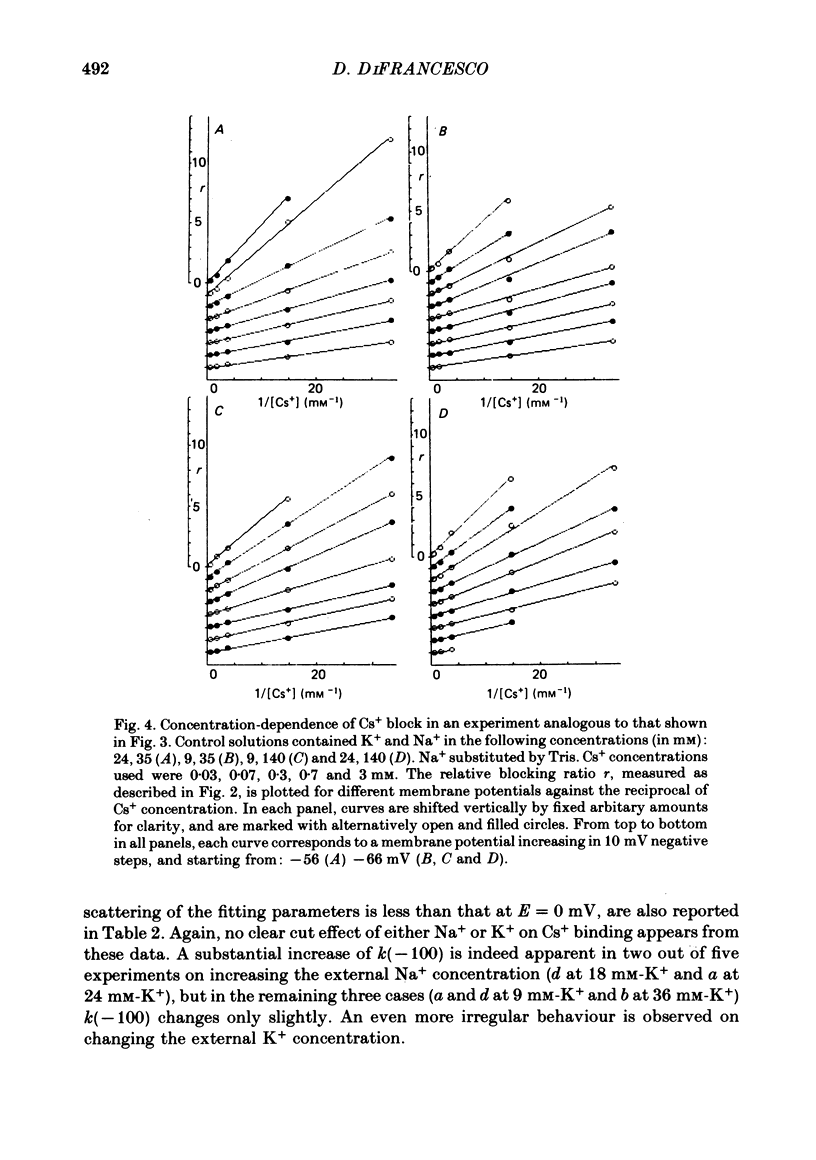
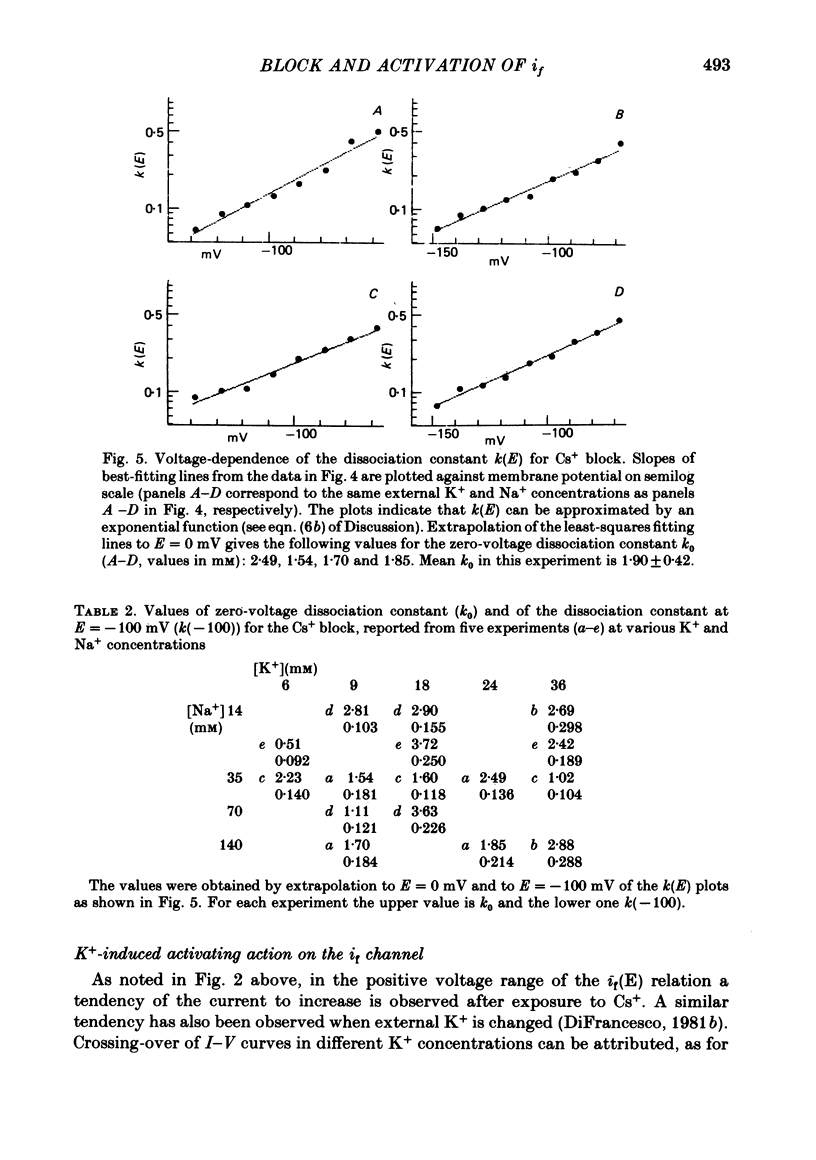
2. Cs+-induced blockade is concentration- and voltage-dependent, as observed on other cation channels. Data in the far negative voltage range (about - 150 to - 50 mV) can be fitted by a simple block model (Woodhull, 1973), which gives a mean value of 0·71 for the fraction of membrane thickness (δ) crossed by Cs+ ions before reaching the blocking site. The value of δ does not appear to be affected by either external Na or external K concentrations. Values for the dissociation constant of the blocking reaction at E = 0 mV (k0) are found in the range 0·5-3·7 mM. In the positive region of the īf(E) relation the current depression caused by channel blockade vanishes. Unexpectedly, in this range the current can be observed to increase with Cs+, and īf(E) curves in different Cs+ concentrations show cross-over.
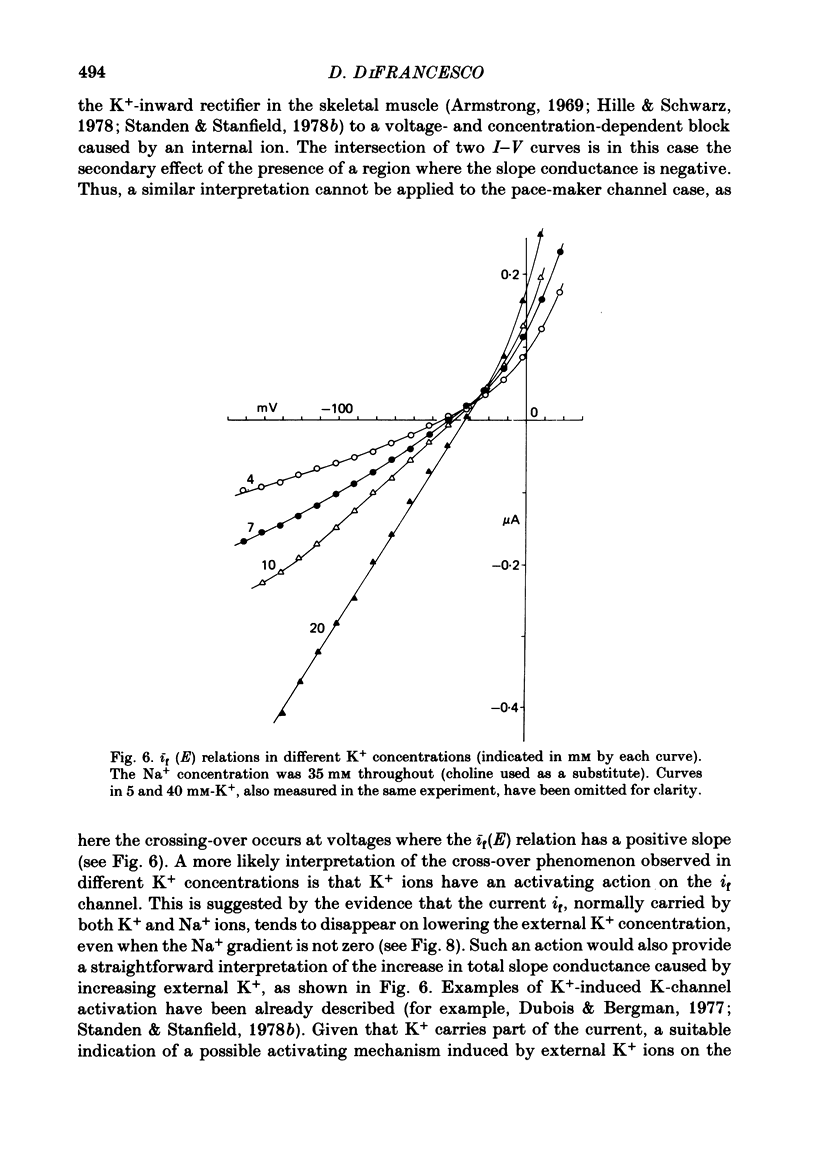
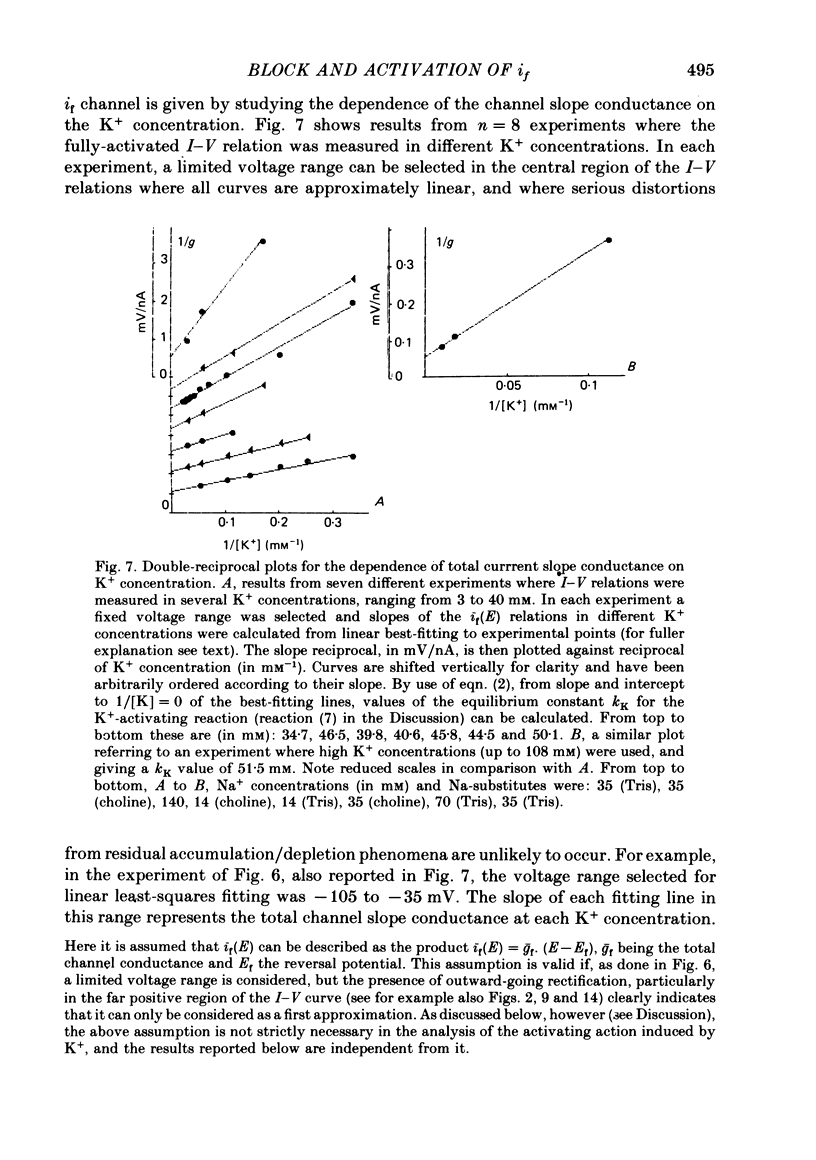
3. Changing external K+ also produces similar cross-over phenomena. Investigation of this effect reveals that the increase in slope of the I-V curve on raising the external K+ concentration follows Michaelis—Menten kinetics, and can be interpteted in terms of K+-induced channel activation. It is found that 44±6 mM-K+ half-saturates the channel activating reaction.
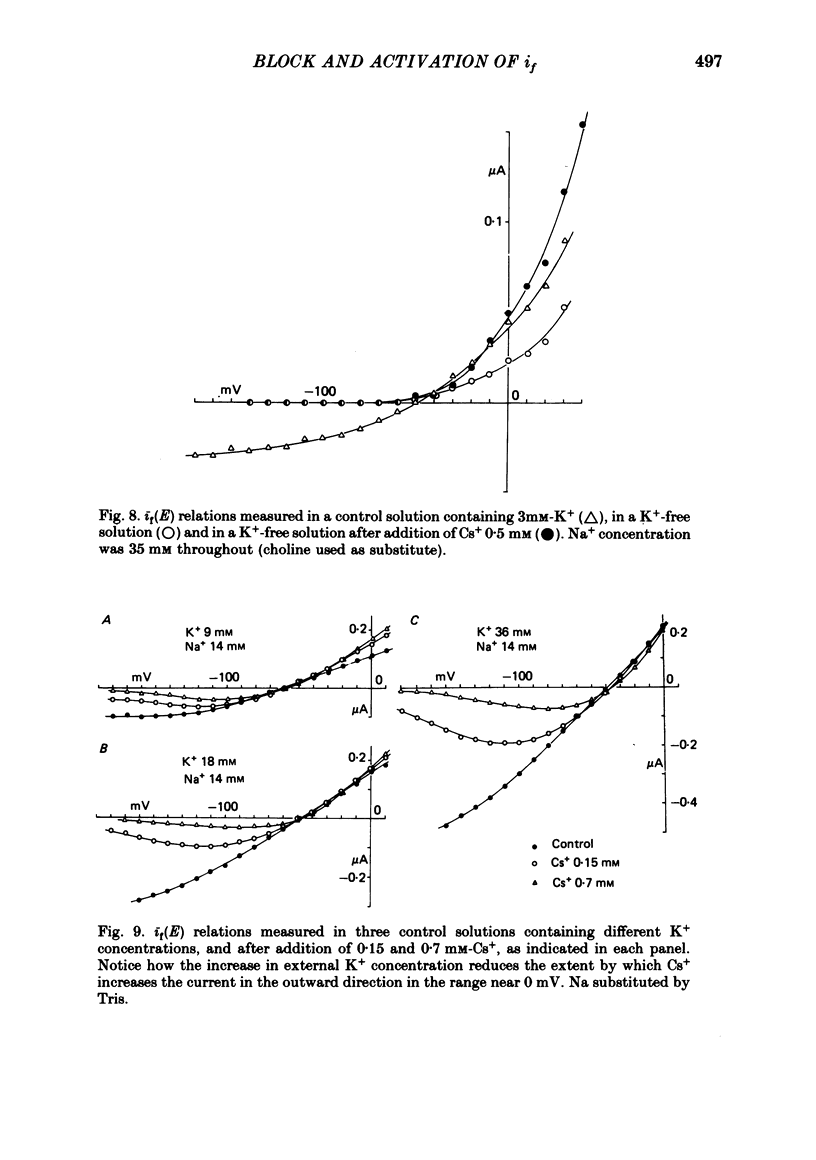
4. The Cs+-induced current increase is large in low-K+ solutions and vanishes in high-K+ solutions, suggesting a competition between Cs+ and K+ ions in their activating action. Increasing Na+ also limits the Cs+-induced current increase.
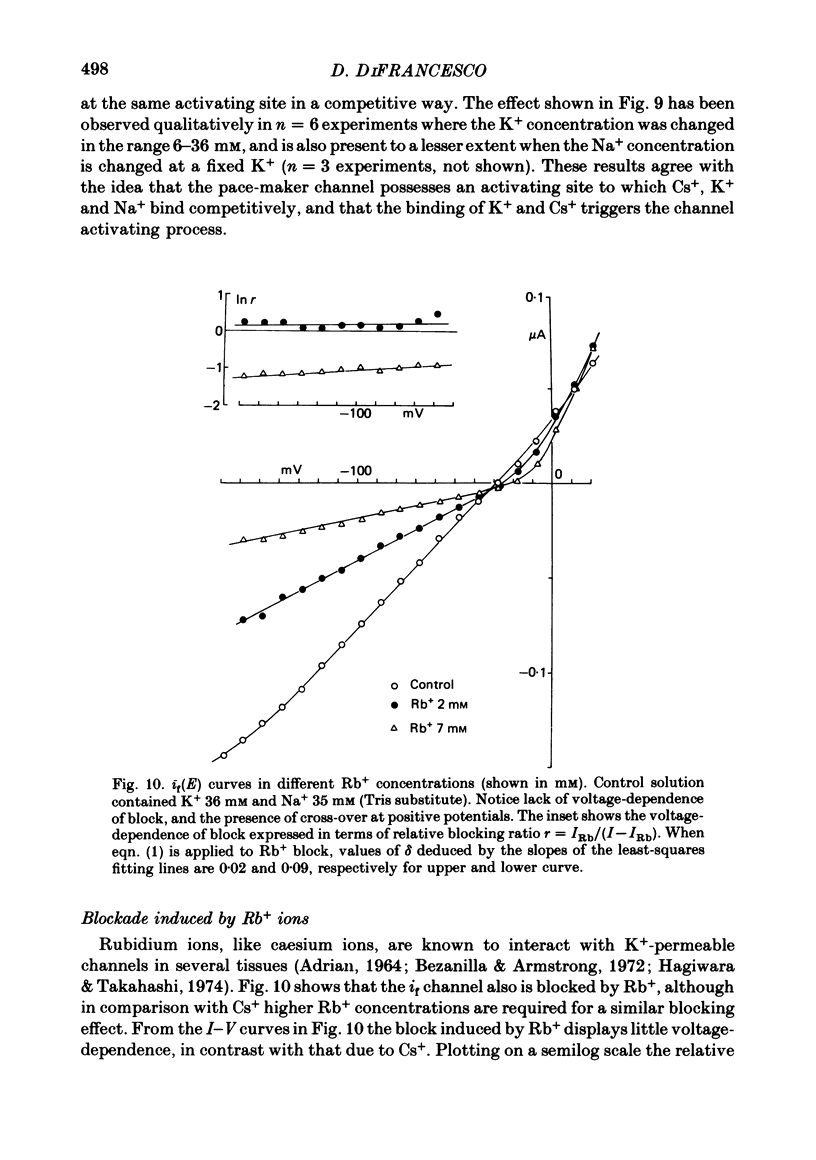
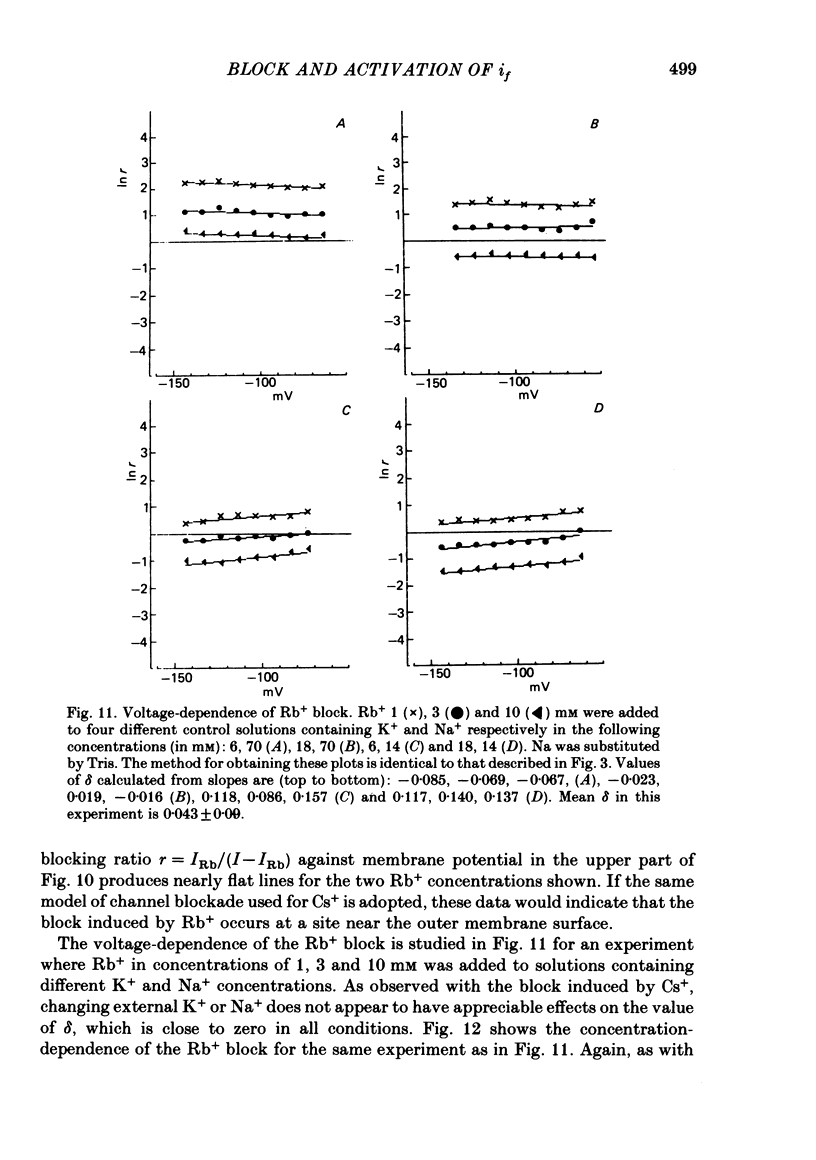
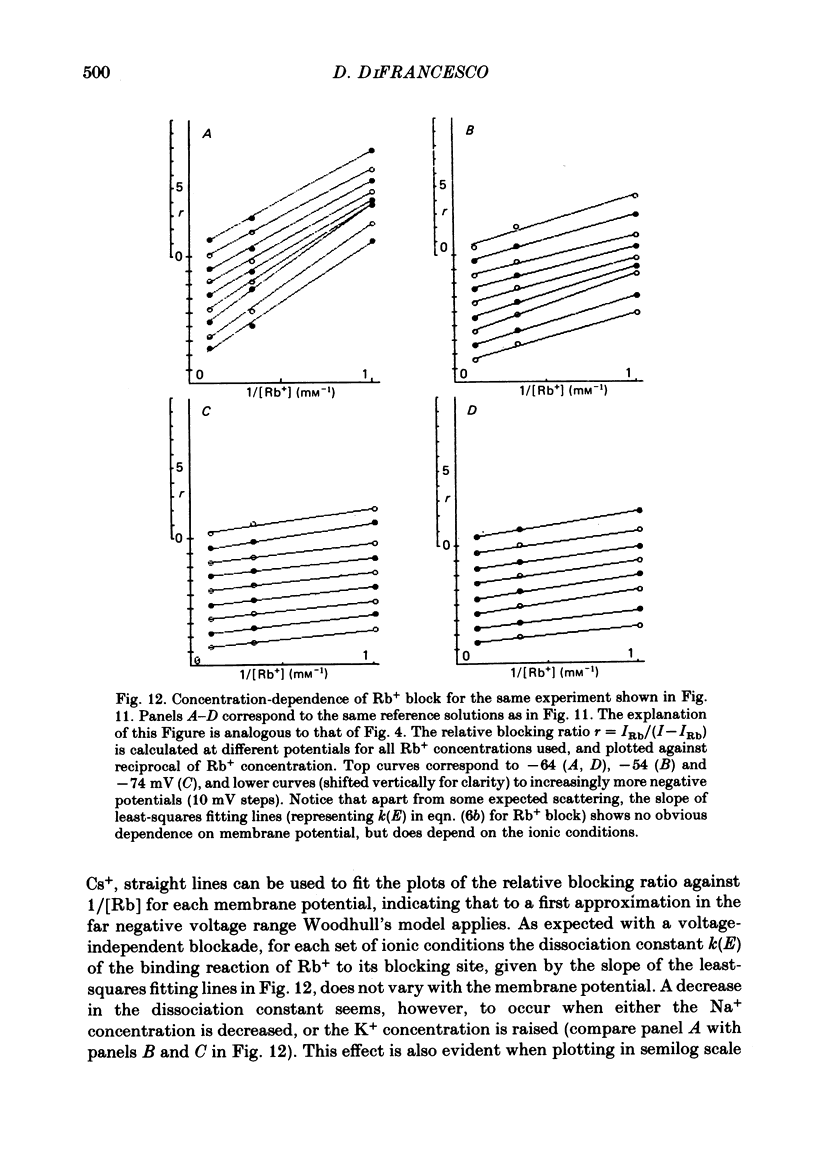
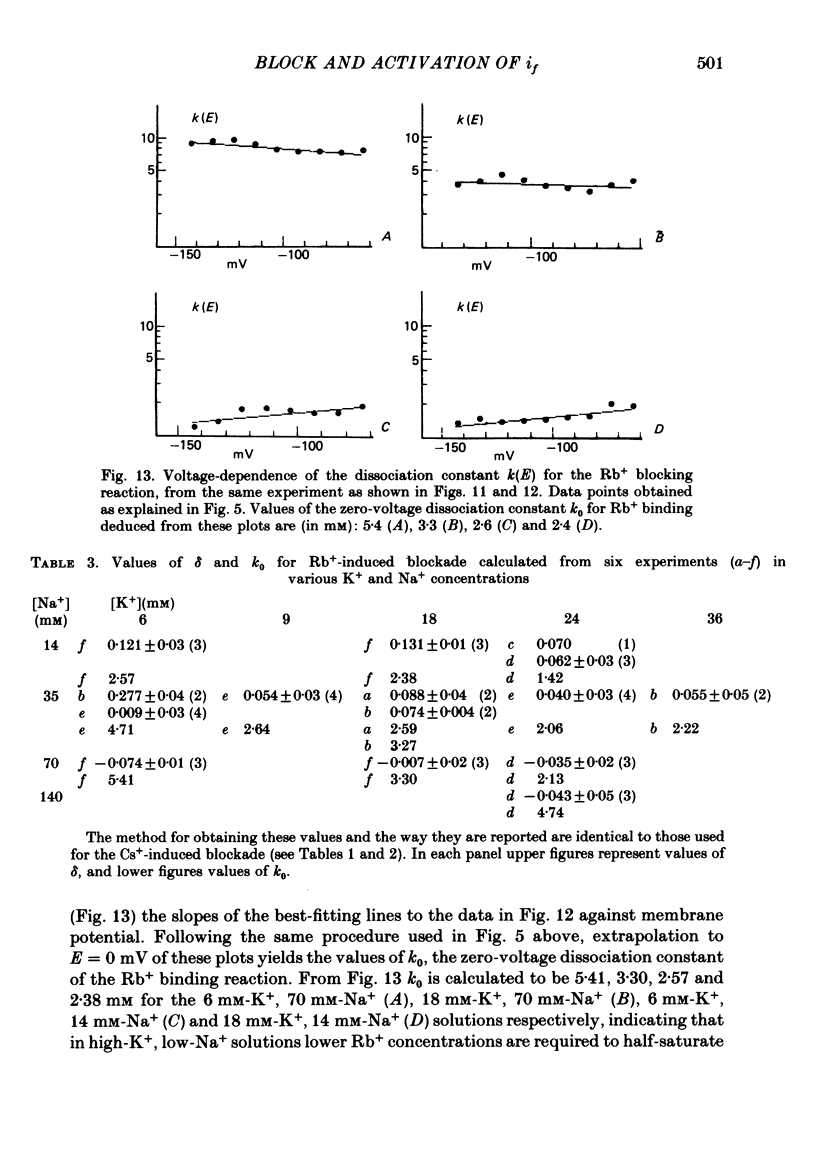
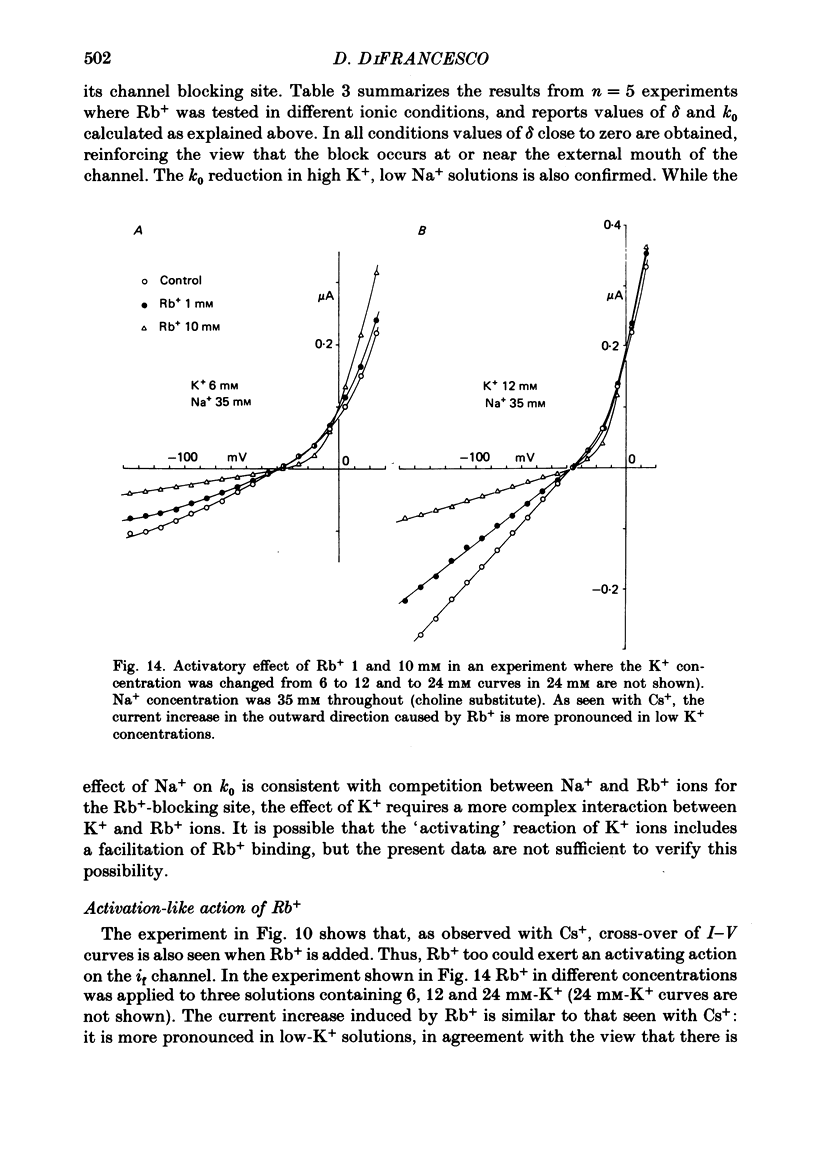
5. Rb+ also blocks the if channel, though less efficiently than Cs+. The block caused by Rb+ is, unlike that of Cs+, nearly voltage-independent, and is explained by assuming that the blocking reaction occurs near the external mouth of the channel (mean value of δ is 0·05). The zero-voltage dissociation constant (k0) of the Rb+-blocking reaction ranges between 1·4 and 5·4 mM, and is lower in low-Na+, high-K+ solutions.
6. A possible characterization of the if channel which explains these results includes an inner `blocking' site, to which external Cs+ ions bind, blocking the channel, and a more external `activatory' site, to which K+, Cs+, Rb+ and possibly Na+ ions bind. Binding of K+ to this site induces a current increase either by modulating the channel, or actually by opening the channel itself. A similar mechanism can apply to Cs+ and to Rb+ binding.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, French R. J. Blocking of the squid axon potassium channel by external caesium ions. J Physiol. 1978 Mar;276:13–25. doi: 10.1113/jphysiol.1978.sp012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol. 1969 Nov;54(5):553–575. doi: 10.1085/jgp.54.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Voltage dependent block of inward going rectification in cardiac Purkinje fibers by external Cs ions. Arch Int Pharmacodyn Ther. 1979 Dec;242(2):294–295. [PubMed] [Google Scholar]
- Ciani S., Krasne S., Hagiwara S. A model for the effects of potential and external K+ concentration on the Cs+ blocking of inward rectification. Biophys J. 1980 Apr;30(1):199–204. doi: 10.1016/S0006-3495(80)85089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. The steady-state potassium conductance of the Ranvier node at various external K-concentrations. Pflugers Arch. 1977 Aug 29;370(2):185–194. doi: 10.1007/BF00581693. [DOI] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Neher E. Ionic selectivity, saturation and block in gramicidin A channels: I. Theory for the electrical properties of ion selective channels having two pairs of binding sites and multiple conductance states. J Membr Biol. 1977 Mar 23;31(4):383–347. doi: 10.1007/BF01869414. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Inward rectification in skeletal muscle: a blocking particle model. Pflugers Arch. 1978 Dec 28;378(2):173–176. doi: 10.1007/BF00584452. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


