Abstract
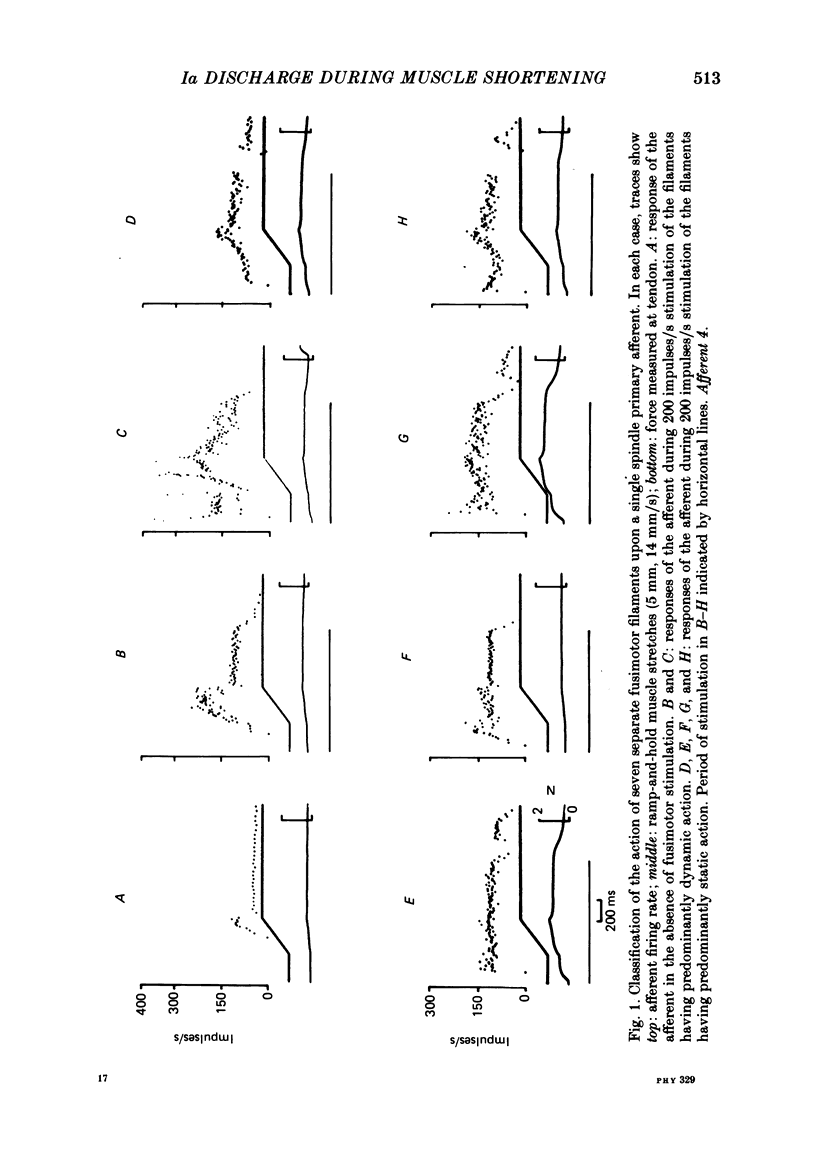
1. In barbiturate-anaesthetized cats, the L7 and S1 dorsal and ventral roots were dissected to isolate functionally single afferents identified as primary endings of soleus muscle spindles, and motor filaments which exerted a fusimotor action on the afferents with limited action on extrafusal muscle. Up to seven filaments, with an action on a given primary ending, could be isolated and each was classified as exerting either a predominantly dynamic or static action.
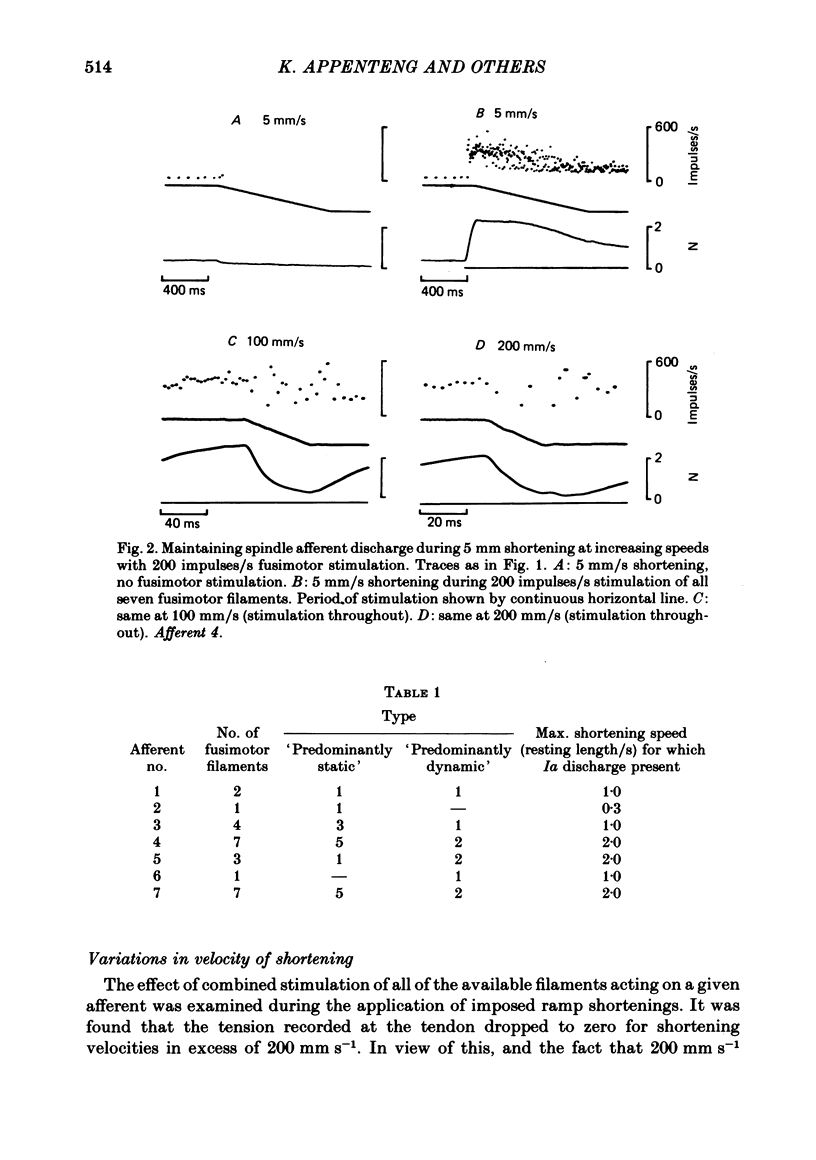
2. Combined stimulation of these filaments, at rates up to 200 impulses/s could maintain afferent firing during muscle shortenings at speeds up to 200 mm/s.
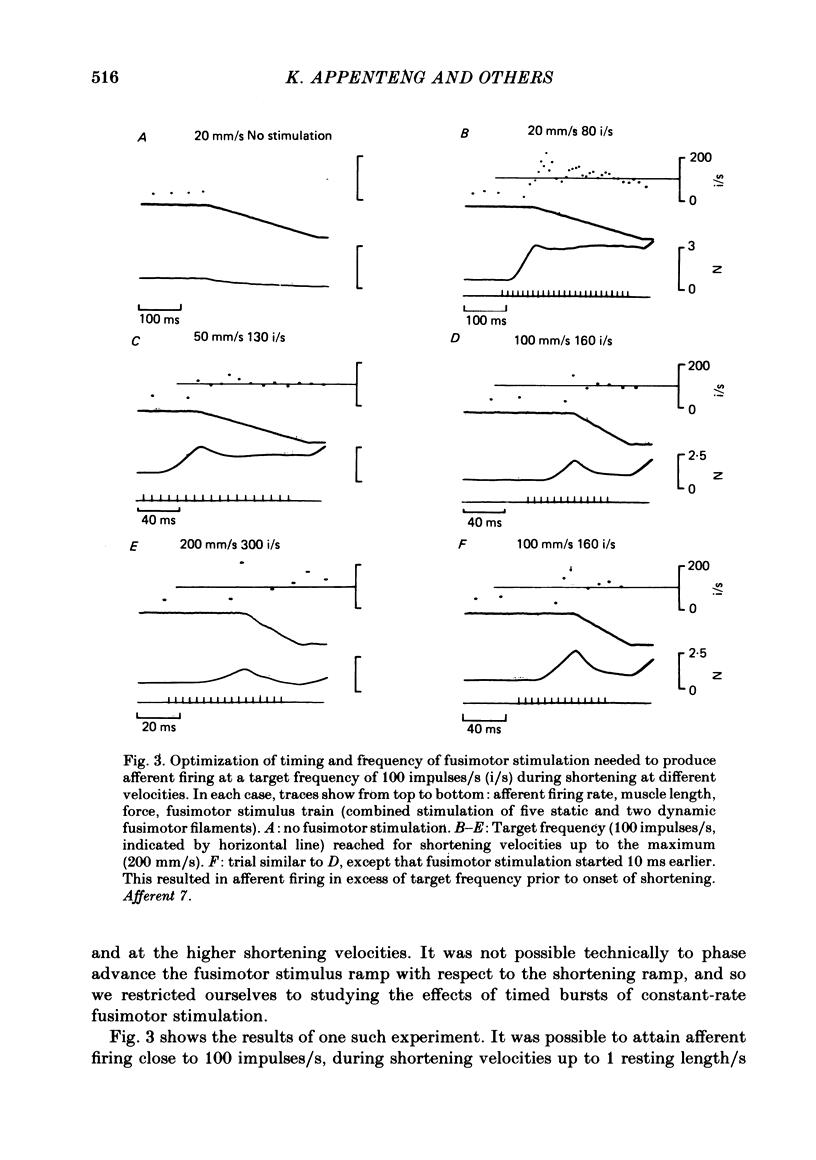
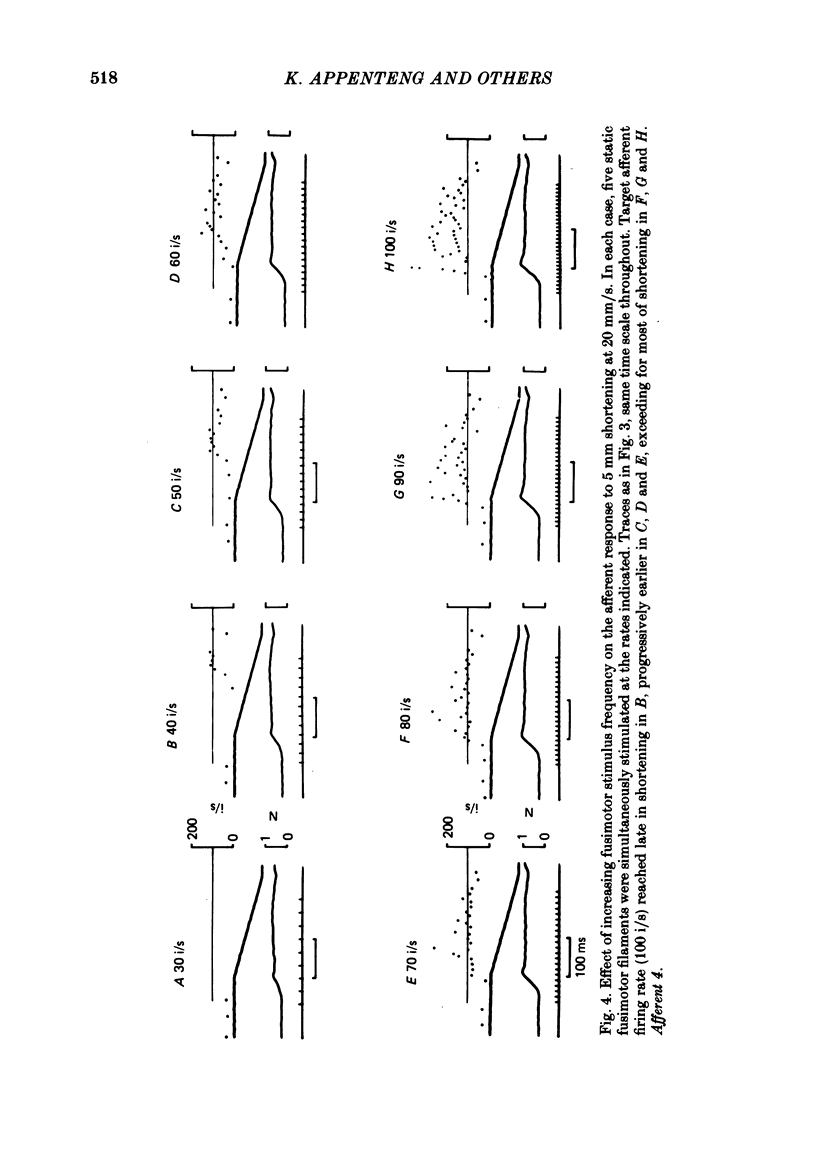
3. Fusimotor stimulation could also maintain afferent firing at a target frequency of 100 impulses/s during muscle shortenings up to 200 mm/s. The timing, in relation to the onset of shortening, and the rates of fusimotor stimulation were found to be critical in achieving the target frequency.
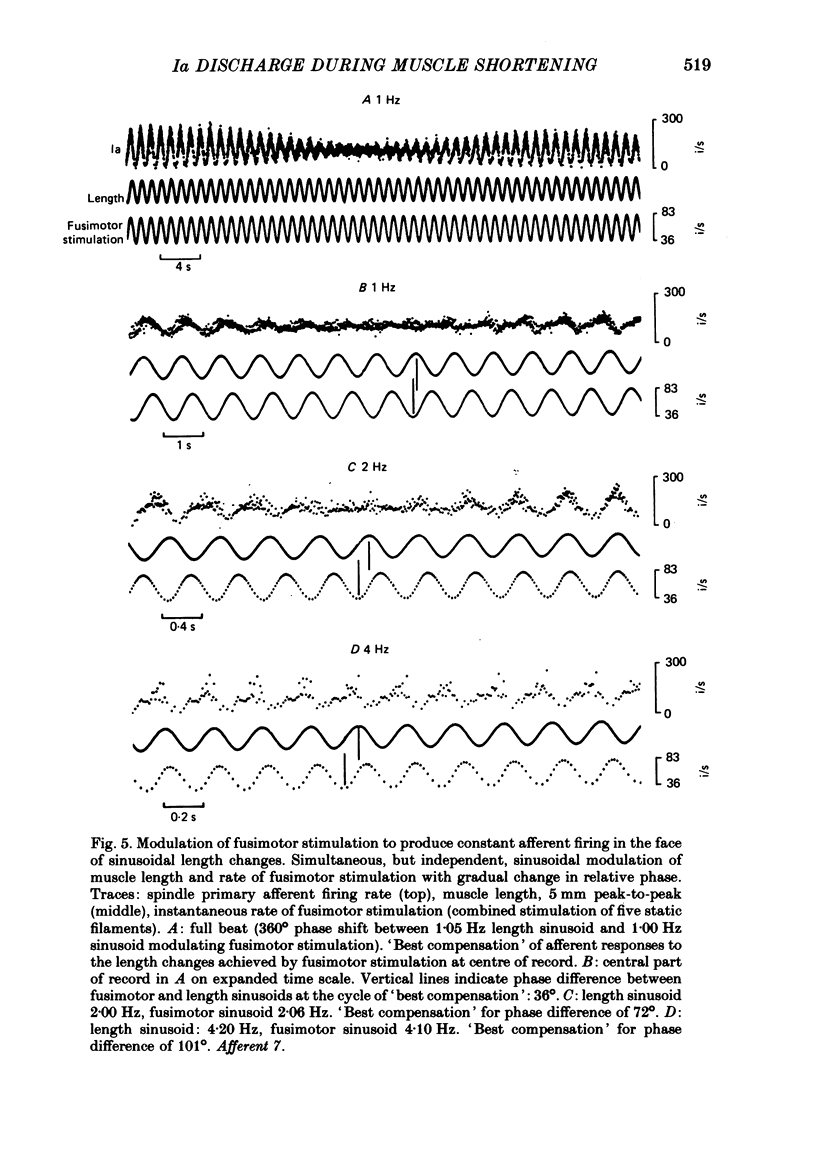
4. Sinusoidal modulation of the frequency of fusimotor stimulation was used to study the conditions required to achieve constant afferent firing in the face of imposed sinusoidal length changes.
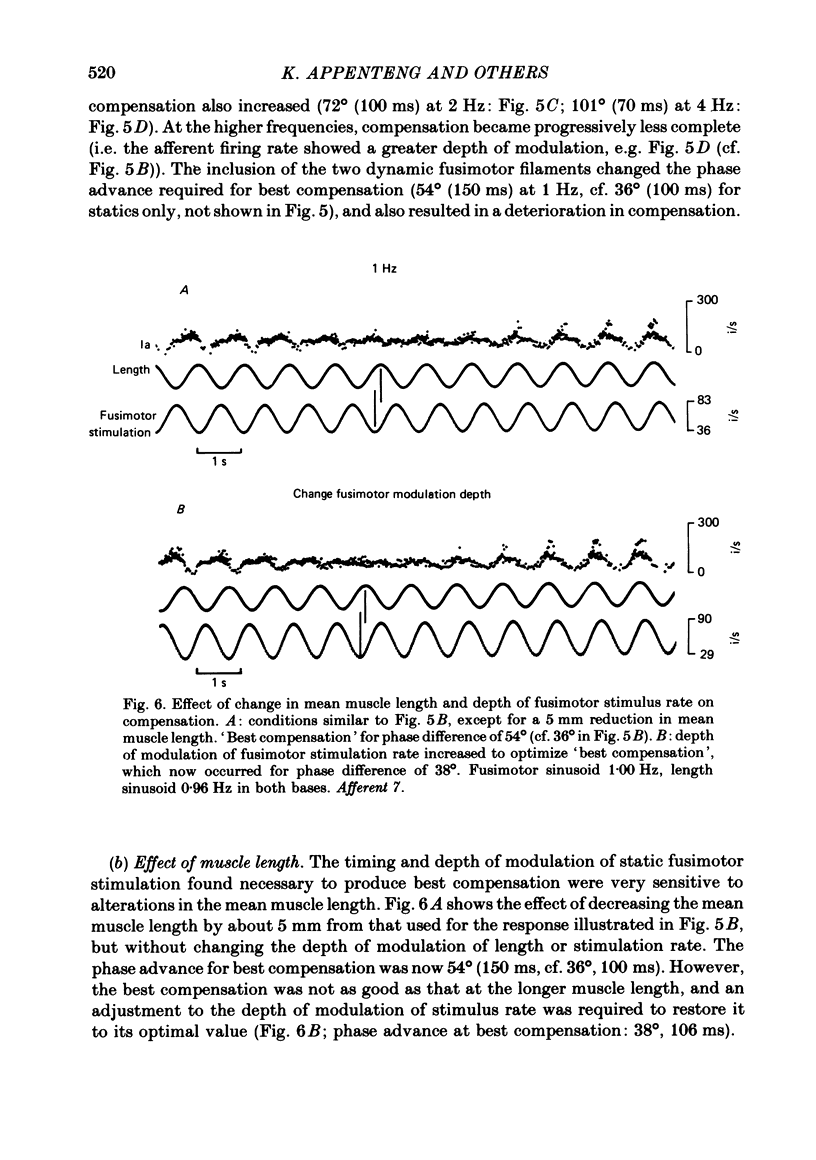
5. For given depths of modulation, the phase advance of fusimotor stimulation needed to produce minimum modulation of afferent firing (best compensation) increased with increasing frequency of the sinusoids. The compensation deteriorated with an increase in the frequency of the sinusoids and a change in the mean muscle lengths, although in some cases it could be restored by adjustments to the depth of modulation of fusimotor rate. This suggests that for movements of varying speeds and amplitudes, settings which are appropriate for shortening at a given velocity and mean muscle length, do not apply if either of these two variables are altered.
6. These findings demonstrate that the fusimotor system is potentially capable of eliciting constant afferent firing as envisaged in the `servo-assistance' hypothesis (Matthews, 1964, 1972; Stein, 1974). This, and the fact that constant afferent firing is not seen during normal unobstructed shortenings at velocities greater than 0·2 resting length/s (Prochazka, 1981), are used to argue that it is by choice rather than necessity that `servo-assistance' (as defined above) is not employed during normal movements. However, servo-assistance of a different form (involving modulated spindle afferent feed-back from both agonists and antagonists) remains a viable alternative.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Lawrence D. G., Matthews P. B. Static fusimotor fibres and the position sensitivity of muscle spindle receptors. Brain Res. 1969 Jun;14(1):173–187. doi: 10.1016/0006-8993(69)90038-9. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978 Apr;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Harrison L. M., Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol. 1975 Dec;253(2):565–582. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Jami L., Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975 Jul;249(1):153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Matthews P. B., Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol. 1977 Jul;268(3):827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y. Proportion of muscles spindles supplied by skeletofusimotor axons (beta-axons) in peroneus brevis muscle of the cat. J Neurophysiol. 1975 Nov;38(6):1390–1394. doi: 10.1152/jn.1975.38.6.1390. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., Luschei E. S. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975 May;38(3):560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Stretch receptor discharges during muscle contraction. J Physiol. 1951 Apr;113(2-3):298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977 Jun;267(3):839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Vallbo A. B. The responses of muscle spindle afferents during voluntary tracking movements in man. Load dependent servo assistance? Brain Res. 1979 Apr 27;166(2):401–404. doi: 10.1016/0006-8993(79)90227-0. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Muscle spindle responses to concomitant variations in lenght and in fusimotor activation. Acta Physiol Scand. 1968 Sep-Oct;74(1):153–165. doi: 10.1111/j.1748-1716.1968.tb04224.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Proske U. The effect of muscle length and rate of fusimotor stimulation on the frequency of discharge in primary endings from muscle spindles in the cat. J Physiol. 1972 May;222(3):511–535. doi: 10.1113/jphysiol.1972.sp009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. P., Smith A. M., Sessle B. J., Murakami T. Activity of trigeminal alpha- and gamma-motoneurons and muscle afferents during performance of a biting task. J Neurophysiol. 1979 May;42(3):710–725. doi: 10.1152/jn.1979.42.3.710. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Muscle spindle function during normal movement. Int Rev Physiol. 1981;25:47–90. [PubMed] [Google Scholar]
- Prochazka A., Stephens J. A., Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979 Feb;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M. H., Thach W. T. Alpha-gamma dissociation during slow tracking movements of the monkey's wrist: preliminary evidence from spinal ganglion recording. Brain Res. 1980 Nov 24;202(1):213–216. [PubMed] [Google Scholar]
- Sjöström A., Zangger P. ALPHA-GAMMA-Linkage in the spinal generator for locomotion in the cat. Acta Physiol Scand. 1975 May;94(1):130–132. doi: 10.1111/j.1748-1716.1975.tb05869.x. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Betts B., Edgerton V. R., Zernicke R. F. Rapid ankle extension during paw shakes: selective recruitment of fast ankle extensors. J Neurophysiol. 1980 Mar;43(3):612–620. doi: 10.1152/jn.1980.43.3.612. [DOI] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- Taylor A. Muscle receptors in the control of voluntary movement. Paraplegia. 1972 Feb;9(4):167–172. doi: 10.1038/sc.1971.28. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hulliger M. Independence of skeletomotor and fusimotor activity in man? Brain Res. 1981 Oct 26;223(1):176–180. doi: 10.1016/0006-8993(81)90819-2. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Proske U. Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. J Neurophysiol. 1981 Aug;46(2):250–259. doi: 10.1152/jn.1981.46.2.250. [DOI] [PubMed] [Google Scholar]


