Abstract
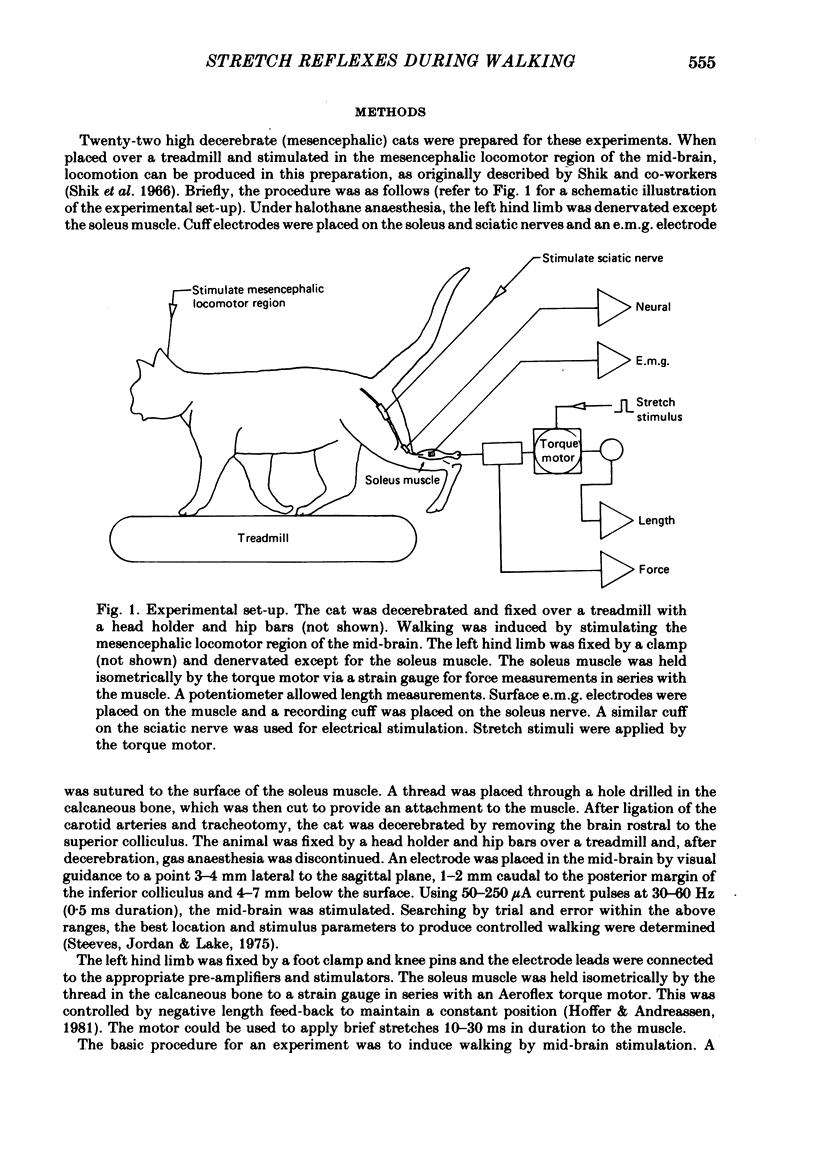
1. A cat preparation was used to study the modulation of stretch reflexes during locomotion. The brain stem was transected and locomotion was induced by electrical stimulation of the mesencephalic locomotor region below the level of transection. Three legs walked normally on a treadmill, while the fourth leg, which was denervated except for the soleus muscle, was held fixed. Brief length perturbations were applied to the soleus muscle at various phases of the stepping cycle.
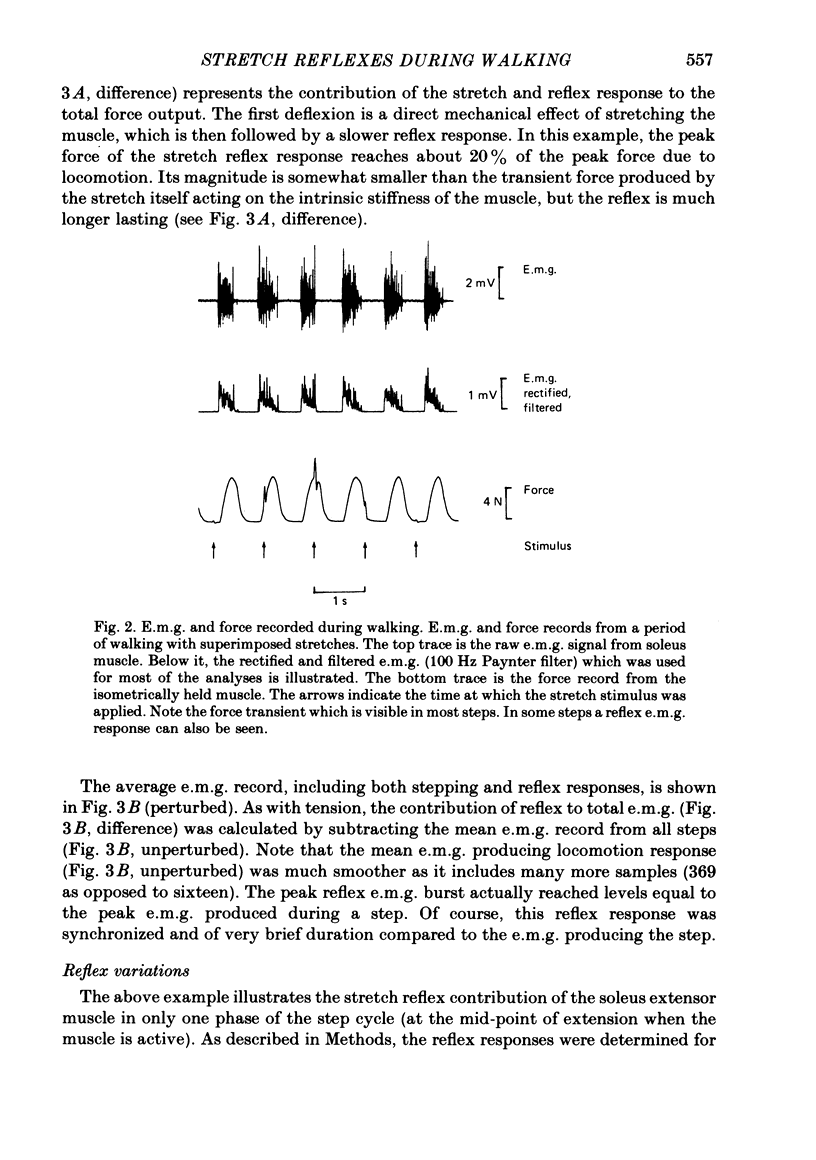
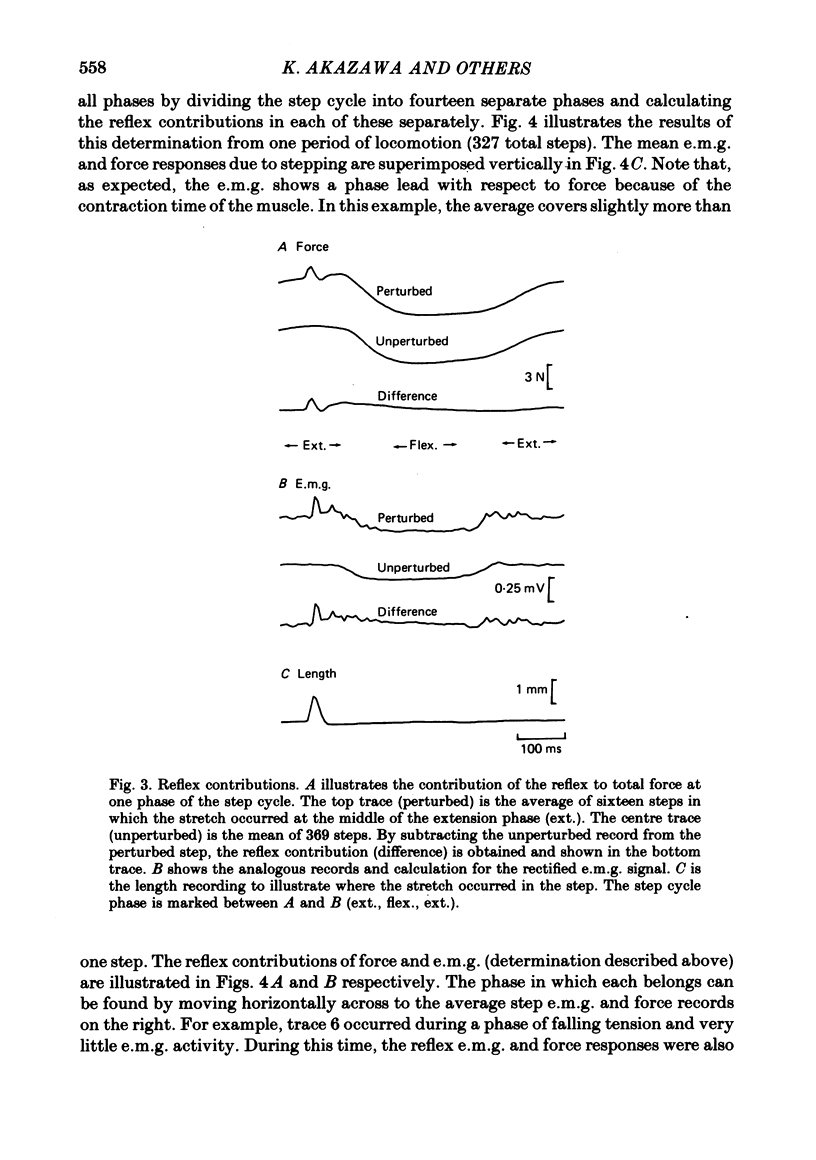
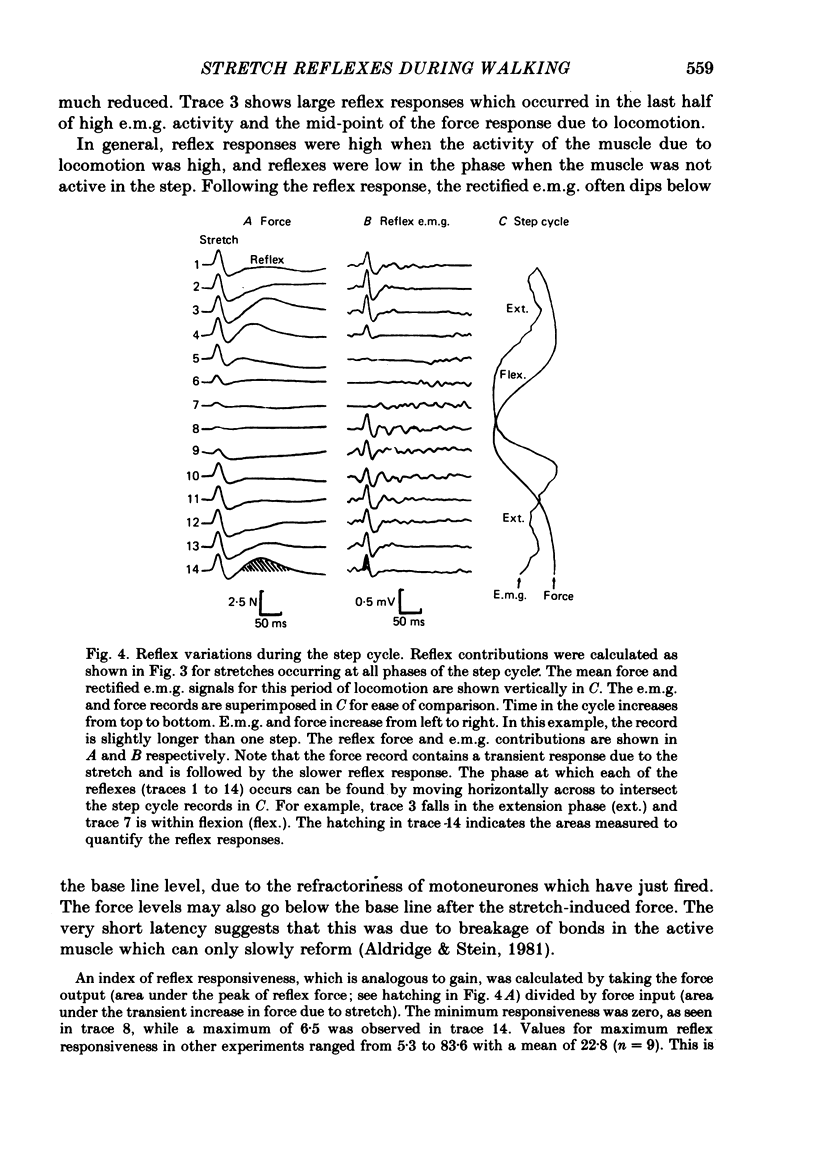
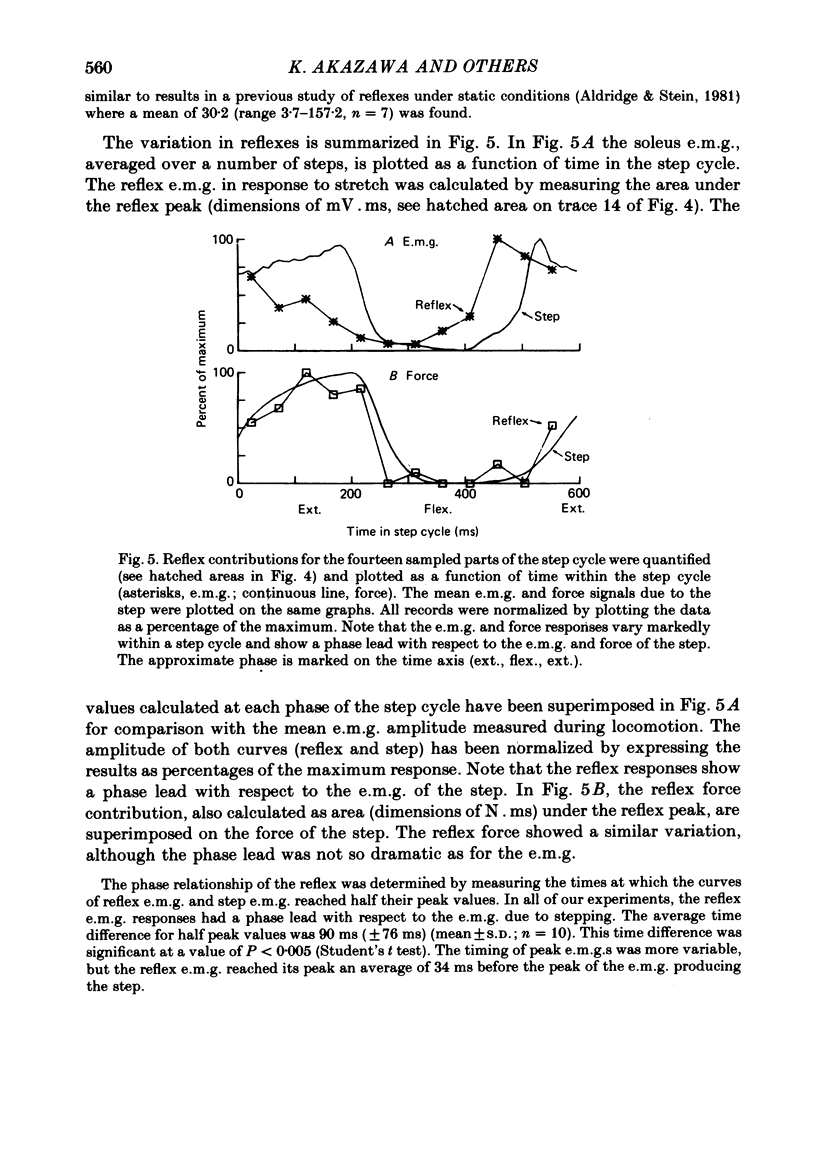
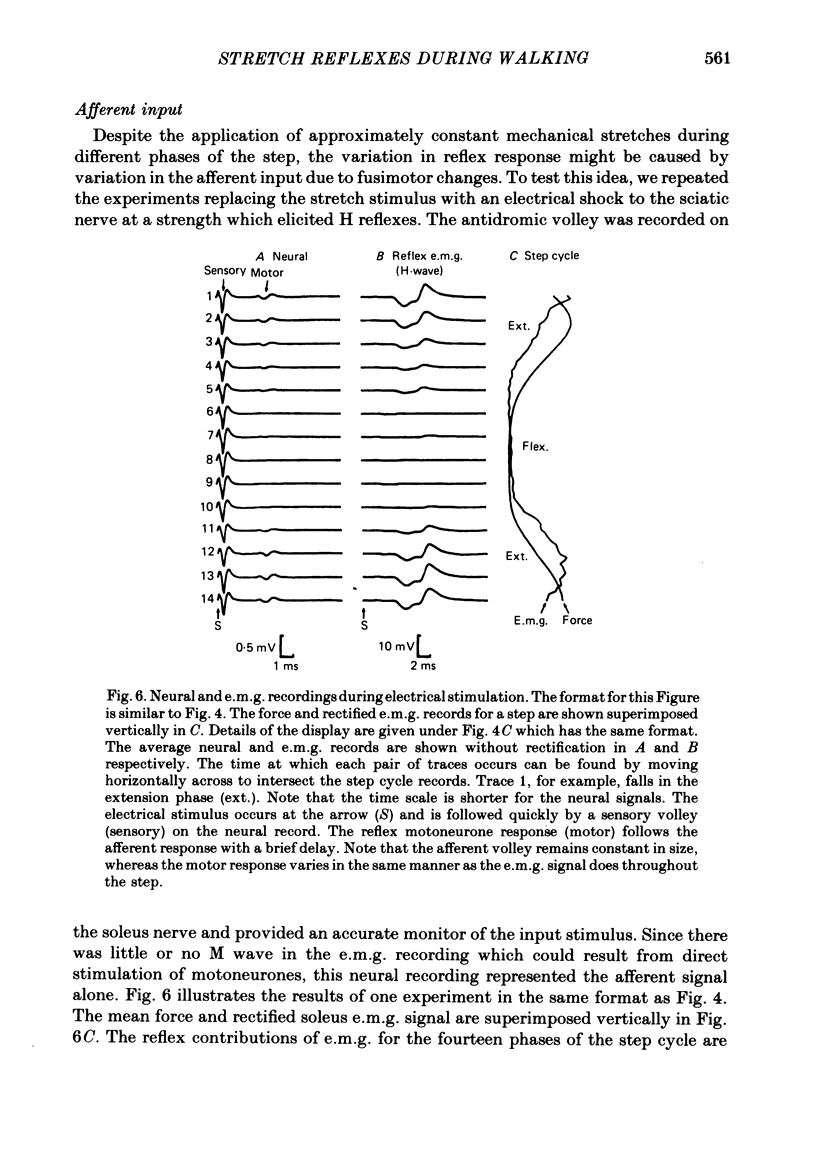
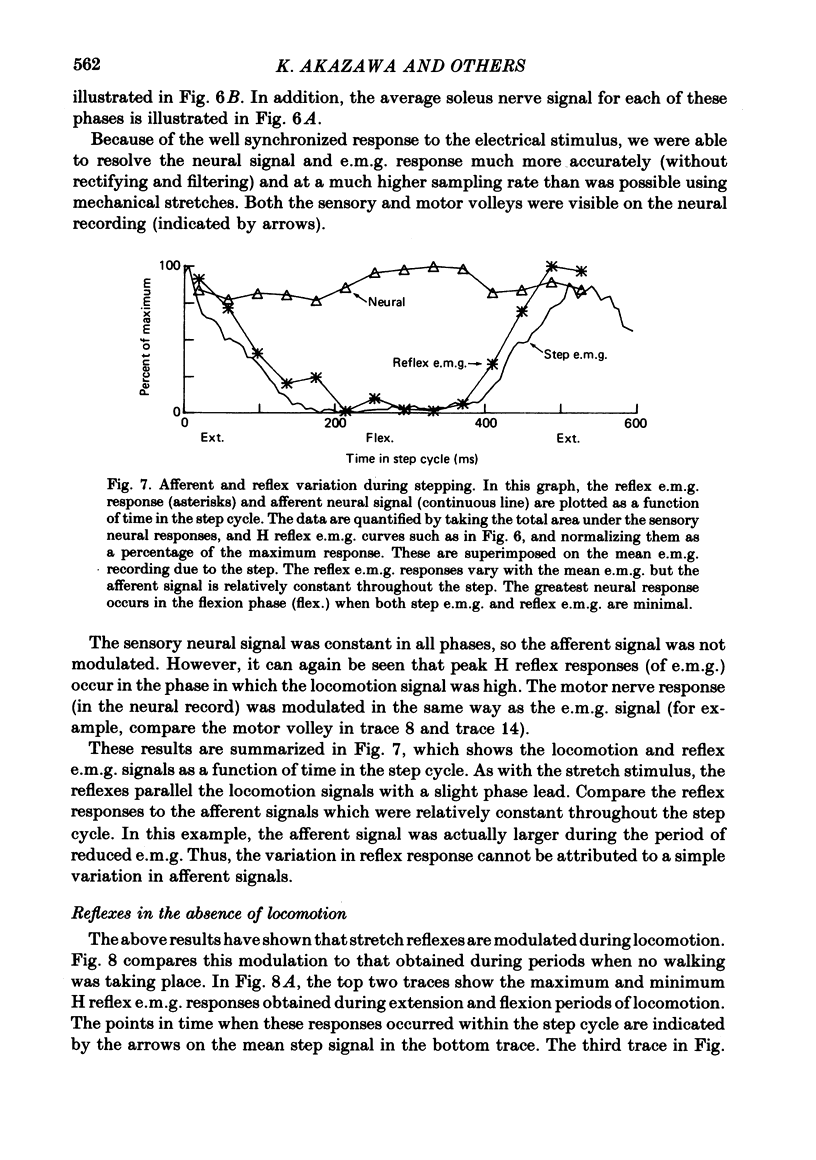
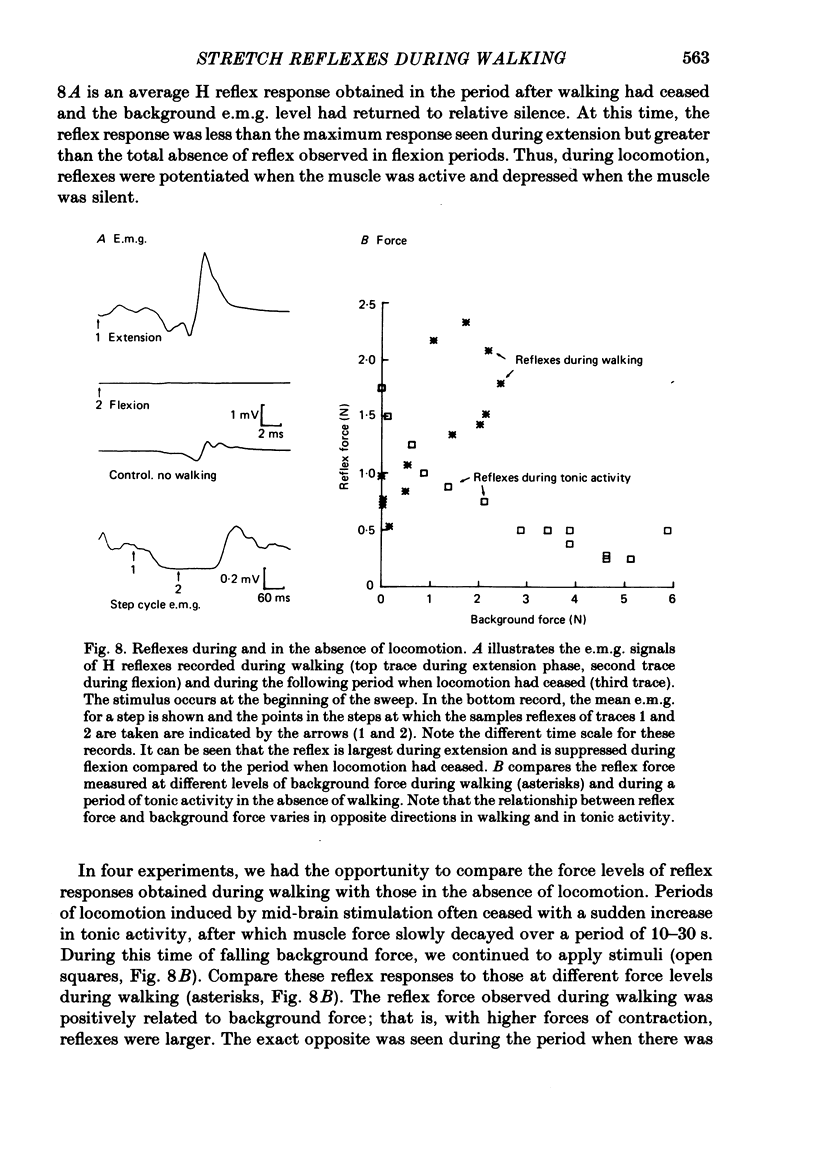
2. The stretch reflex in this muscle was deeply modulated during the step cycle, and reached its peak at or before the peak in soleus e.m.g. activity associated with the locomotion. A similar variation was observed when the sciatic nerve was stimulated electrically at a strength which elicited reflex activity (H wave), but did not directly elicit motor nerve activity (M wave). Variation in the reflex during electrical stimulation could not be accounted for by cyclic variation in fusimotor activity or in the afferent volley, but must be due to post-synaptic changes in α-motoneurones or in the presynaptic inputs to them.
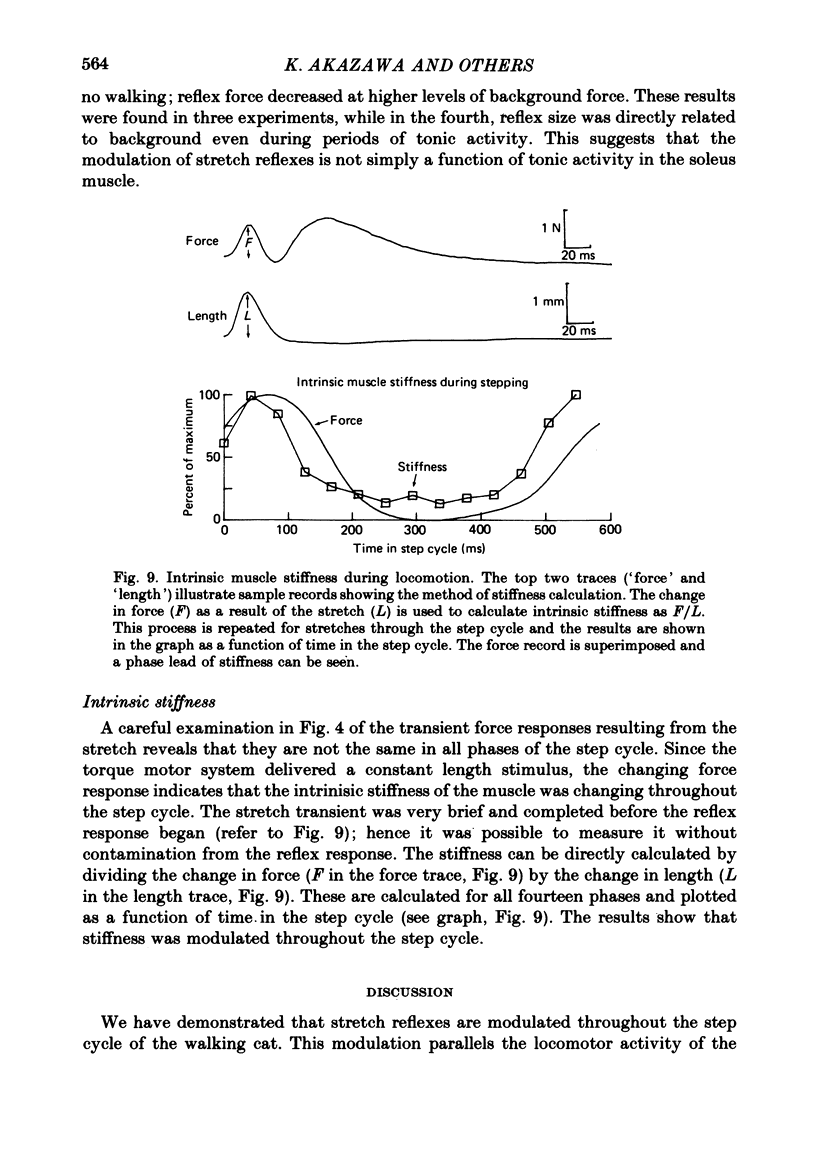
3. Large changes were also observed in intrinsic muscle stiffness during the step cycle. The maximum stiffness occurred near the time the limb would normally strike the ground during locomotion. A high stiffness would be useful in reducing the amount that the limb would yield under the weight of the body during the extension phase of the step cycle. The variation of the stretch reflex in parallel with stiffness suggests that reflexes could assist in this load compensation. The variation is not consistent with the idea that the stretch reflex is used to compensate for changes in intrinsic muscle properties, so that the total system behaves more like a spring of constant stiffness than does muscle alone.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J. W., Stein R. B. Nonlinear properties of stretch reflex studied in the decerebrate cat. J Neurophysiol. 1982 Feb;47(2):179–192. doi: 10.1152/jn.1982.47.2.179. [DOI] [PubMed] [Google Scholar]
- Andersson O., Forssberg H., Grillner S., Wallén P. Peripheral feedback mechanisms acting on the central pattern generators for locomotion in fish and cat. Can J Physiol Pharmacol. 1981 Jul;59(7):713–726. doi: 10.1139/y81-108. [DOI] [PubMed] [Google Scholar]
- Brown T. G. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol. 1914 Mar 31;48(1):18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelguen J. M. Static and dynamic fusimotor controls in various hindlimb muscles during locomotor activity in the decorticate cat. Brain Res. 1981 May 25;213(1):83–97. doi: 10.1016/0006-8993(81)91249-x. [DOI] [PubMed] [Google Scholar]
- Duysens J., Pearson K. G. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976 Jan 26;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Duysens J., Stein R. B. Reflexes induced by nerve stimulation in walking cats with implanted cuff electrodes. Exp Brain Res. 1978 Jun 19;32(2):213–224. doi: 10.1007/BF00239728. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975 Feb 21;85(1):103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977 Aug 19;132(1):121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Filtering of electromyographic signals. Am J Phys Med. 1970 Apr;49(2):142–146. [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Response to sudden torques about ankle in man. III. Suppression of stretch-evoked responses during phasic contraction. J Neurophysiol. 1980 Aug;44(2):233–246. doi: 10.1152/jn.1980.44.2.233. [DOI] [PubMed] [Google Scholar]
- Grillner S. Interaction between central and peripheral mechanisms in the control of locomotion. Prog Brain Res. 1979;50:227–235. doi: 10.1016/S0079-6123(08)60823-7. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975 Apr;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Grillner S. The role of muscle stiffness in meeting the changing postural and locomotor requirements for force development by the ankle extensors. Acta Physiol Scand. 1972 Sep;86(1):92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Andreassen S. Regulation of soleus muscle stiffness in premammillary cats: intrinsic and reflex components. J Neurophysiol. 1981 Feb;45(2):267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- Houk J. C. Regulation of stiffness by skeletomotor reflexes. Annu Rev Physiol. 1979;41:99–114. doi: 10.1146/annurev.ph.41.030179.000531. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Somatosensory unit input to the spinal cord during normal walking. Can J Physiol Pharmacol. 1981 Jul;59(7):627–635. doi: 10.1139/y81-097. [DOI] [PubMed] [Google Scholar]
- Nichols T. R., Houk J. C. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976 Jan;39(1):119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg E. D., Behrends H. B. The possibility of phase-dependent monosynaptic and polysynaptic is excitation to homonymous motoneurones during fictive locomotion. Brain Res. 1978 Mar 31;143(3):533–537. doi: 10.1016/0006-8993(78)90363-3. [DOI] [PubMed] [Google Scholar]
- Shik M. L., Orlovsky G. N. Neurophysiology of locomotor automatism. Physiol Rev. 1976 Jul;56(3):465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Steeves J. D., Jordan L. M., Lake N. The close proximity of catecholamine-containing cells to the 'mesencephalic locomotor region' (MLR). Brain Res. 1975 Dec 26;100(3):663–670. doi: 10.1016/0006-8993(75)90166-3. [DOI] [PubMed] [Google Scholar]
- Wetzel M. C., Stuart D. G. Ensemble characteristics of cat locomotion and its neural control. Prog Neurobiol. 1976;7(1):1–98. doi: 10.1016/0301-0082(76)90002-2. [DOI] [PubMed] [Google Scholar]


