Abstract
Bunyaviruses replicate in the cytoplasm of infected cells. New viral particles are formed by budding of nucleocapsids into the Golgi apparatus. We have previously shown that the IFN-induced human MxA protein inhibits bunyavirus replication by an unknown mechanism. Here we demonstrate that MxA binds to the nucleocapsid protein of La Crosse virus (LACV) and colocalizes with the viral protein in cytoplasmic complexes. Electron microscopy revealed that these complexes accumulated in the perinuclear area and consisted of highly ordered fibrillary structures. A similar MxA-mediated redistribution of viral nucleocapsid proteins was detected with other bunyaviruses, such as Bunyamwera virus and Rift Valley fever virus. MxA(E645R), a carboxy-terminal mutant of MxA without antiviral activity against LACV, did not lead to complex formation. Wild-type MxA, but not MxA(E645R), was able to bind to LACV nucleocapsid protein in coimmunoprecipitation assays, demonstrating the importance of the carboxy-terminal effector domain of MxA. These results illustrate an efficient mechanism of IFN action whereby an essential virus component is trapped in cytoplasmic inclusions and becomes unavailable for the generation of new virus particles.
La Crosse virus (LACV), a member of the Bunyaviridae family, is the most important arboviral cause of pediatric encephalitis in North America. As many as 300,000 infections have been estimated to occur annually in the midwestern United States alone (1). Infections in older children and adults are generally mild and often go unrecognized suggesting that humans have an efficient defense mechanism against LACV. In fact, humans seem to be dead-end hosts. The virus has never been isolated from human blood and has rarely been found in the cerebrospinal fluid (1).
Human MxA is an IFN-induced cytoplasmic protein (2) with antiviral activity against a number of RNA viruses including bunya- and orthomyxoviruses (3). It belongs to a family of conserved large GTPases (4) and to the superfamily of dynamin-like GTPases, which are involved in a wide range of intracellular transport processes (5, 6). They represent force-generating enzymes that use energy from GTP hydrolysis to induce a conformational change required for their mechanochemical function.
Human MxA was shown to inhibit LACV growth in cell culture and in transgenic mice (7, 8). It acts independently from other IFN-induced proteins as demonstrated by its protective effect against LACV in IFN-nonresponsive animals (8). Also, MxA protein is able to mediate resistance against LACV in transfected mosquito cells (9). These results suggest that MxA is a powerful antiviral agent on its own and may be important in protecting humans from the potentially harmful outcome of infection with LACV.
We were interested to define the mechanism by which MxA inhibits LACV. Similar to other members of the Bunyaviridae family, LACV has a segmented single-strand RNA genome in negative-sense orientation (i.e., opposite to the mRNA sense). The three RNA segments encode three structural proteins: the RNA polymerase, a glycoprotein precursor (which is processed into the envelope glycoproteins G1 and G2), the nucleocapsid (N) protein, and two nonstructural proteins (NSm and NSs). LACV transcribes and replicates its genome exclusively in the cytoplasm. The genomic RNA (vRNA) of the incoming virus is first transcribed into mRNA by the viral RNA polymerase in a process called primary transcription. After mRNA translation, replication of the genome occurs via synthesis of full-length plus-strand complementary RNA. Transcription and replication are both catalyzed by the viral RNA polymerase. However, newly synthesized N protein is required for the RNA polymerase to shift from the transcription to the replication mode (10). Thus, limitation of unassembled N protein reduces LACV genome replication (11). Presumably, unassembled N associates with the viral polymerase to form the replication complex. In addition, the N protein tightly associates with the vRNA and, together with L, forms the viral nucleocapsids. These are targeted to the Golgi apparatus where the viral envelope glycoproteins accumulate and budding of virus particles into the Golgi cisternae leads to the release of new virions (10). Previous experiments had shown that human MxA inhibits replication rather than transcription of LACV (7). Thus, primary transcription of the S segment was not affected and N protein synthesis was almost normal. Nevertheless, amplification of the viral genome was severely suppressed (7).
Using LACV, we now describe the antiviral action of MxA against bunyaviruses. We demonstrate that MxA specifically recognizes N of LACV in infected cells. We further show that the two proteins form an assembly of elongated tubular structures resulting in cytoplasmic inclusions. This is to our knowledge the first account of a physiological interaction between an IFN-induced antiviral protein and its viral target, leading to relocalization of both components in infected cells. We propose that the MxA-mediated sequestration of N depletes the infected cell from unassembled N and that this depletion accounts for the observed block in viral genome replication.
Materials and Methods
Cells and Viruses.
Cells were maintained in DMEM supplemented with 10% of FCS and antibiotics (1.5 mg of G418/ml). We used stably transfected African green monkey kidney (Vero) cells constitutively expressing MxA (clone VA9) or the variant MxA(E645R) [clone VA(E645R)] and control cells without MxA (VN36) (7).
The original strain of LACV was kindly provided by Ramasamy Raju, Meharry Medical College, Nashville, TN (12). The attenuated MP12 strain of Rift Valley fever virus (RVFV) was kindly provided by Michele Bouloy, Institut Pasteur, Paris, France (13). BUNV was kindly provided from Richard M. Elliott, Institute of Virology, University of Glasgow, U.K. (14).
Virus plaque assays were performed as described in ref. 7. Vero cell monolayers in six-well plates were infected with 200 plaque-forming units (pfu) of LACV. After 5 days the agar overlay was removed and the cells were stained with crystal violet.
Expression Vectors and Transfection.
pHMG expression vectors were used for production of recombinant wild-type MxA (15), MxA(T103A) (16), MxA(L612K) (17), MxA(E645R), and TMxA (18). The ORF of LACV N that also encodes the NSs ORF was amplified from plasmid pGEM3-LACV (provided by Ramasamy Raju) (19). The PCR product was cloned into pSC (20), and the overlapping ORF of NSs was disrupted by site-directed mutagenesis leading to pSC-N.
For transient transfection, Vero cells were seeded onto glass coverslips and transfected with 2 μg of plasmids by using LipofectAMINE (GIBCO/BRL). After 24 h the cells were infected with LACV for 20 h and than analyzed by immunofluorescence.
Abs and Immunoanalysis.
mAb M143, mAb 2C12 (21), a polyclonal rabbit antiserum (16) and a polyclonal guinea pig antiserum (provided by Ilkka Julkunen, National Public Health Institute, Helsinki) were used to detect MxA. Polyclonal rabbit antiserum directed against N of LACV was provided by Ramasamy Raju. The mAb directed against the G1 glycoprotein of LACV was obtained from Francisco Gonzalez-Scarano, University of Pennsylvania, Philadelphia (22); the mAb recognizing N of RVFV was provided by Michele Bouloy, and a polyclonal rabbit antiserum directed against BUNV N was a gift from Richard M. Elliott.
Immunoprecipitation was performed as described (23). Cell lysates were mixed with 2 μl of anti-LACV N antiserum and protein A-Sepharose in the presence of 200 μM guanosine 5′-O-(thiotriphosphate) (GTP-γS) for 2 h at 4°C. After washing of the beads, bound proteins were analyzed by Western blotting by using mAb M143.
For immunofluorescence analysis, cells were seeded onto glass coverslips and infected with viruses for 20 h. After fixation with 3% paraformaldehyde, cells were stained for MxA and viral antigens by using specific Abs. Antigen-bound primary Abs were detected with fluorophore (Cy2, Cy3, Cy5)-conjugated goat Abs. Cells were analyzed with a Leica (Deerfield, IL) TCSSP2 confocal laser scanning microscope.
Electron Microscopy (EM).
The cells were trypsinized, collected by centrifugation, resuspended in DMEM, and drawn into cellulose capillary tubes (24). Cells were fixed in 1.75% glutaraldehyde, postfixed with 1% OsO4, and followed by staining in 1% uranyl acetate. For ultrathin sectioning, the capillary tubes were dehydrated in graded series of ethanol and embedded in ERL (Spurr's) resin.
For immuno-EM, cells were fixed in 2.5% paraformaldehyde, postfixed, and stained in 1% uranyl acetate. After fixation, tubes were dehydrated in ethanol by progressive lowering of temperature method as described (25) in a Leica EM AFS freeze substitution unit. After dehydration, the tubes were embedded in Lowicryl HM20 (Agar Scientific, Essex, U.K.) and finally polymerized at −35°C by UV irradiation. For postembedding labeling of the ultrathin Lowicryl sections of the cells, the mAb M143 (dilution of 1:1,000) and the affinity-purified anti-LACV N Ab (dilution of 1:200) were incubated overnight at 4°C. The immune complexes were detected with protein A conjugated to 10-nm gold particles. Ultrathin sections were counterstained with 2% uranyl acetate and lead citrate. All electron micrographs were taken in a Philips CM 120 transmission electron microscope at 80 kV.
Results
MxA Sequesters the N Protein of LACV into Perinuclear Complexes.
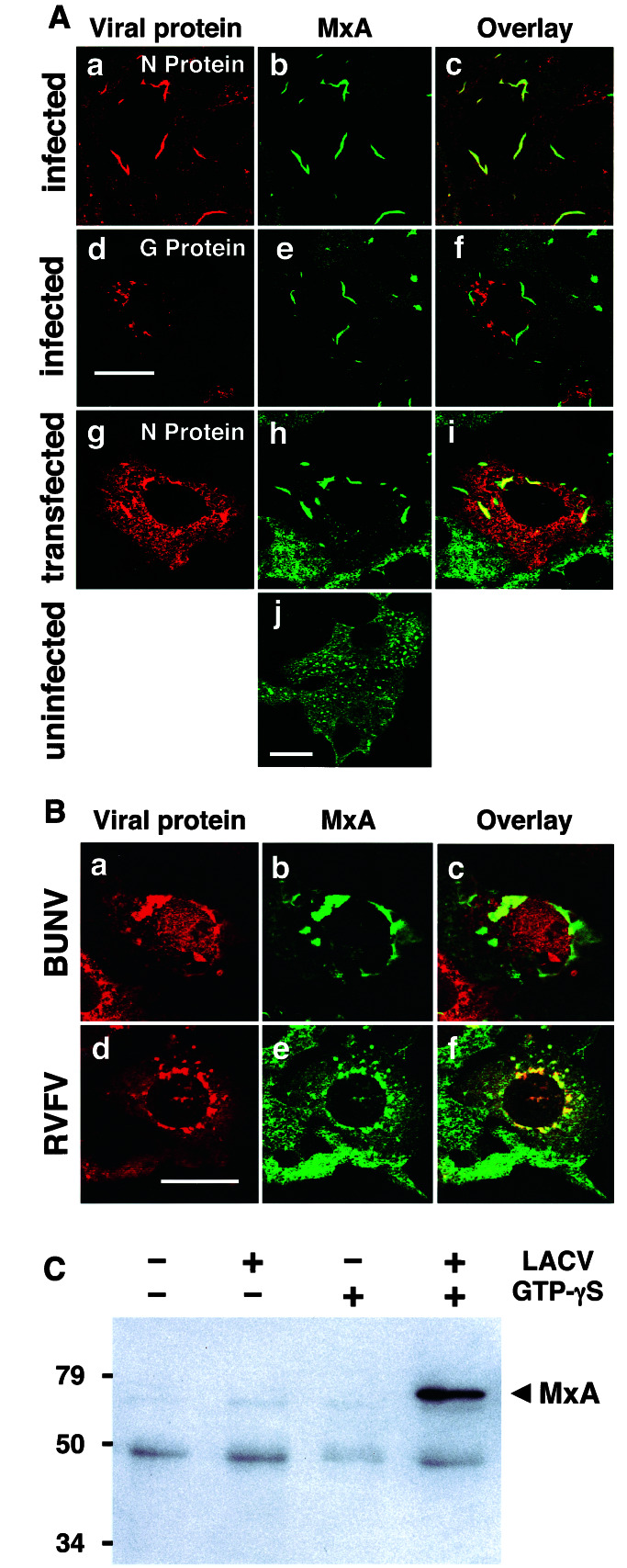
To investigate the effect of human MxA on LACV multiplication, we infected MxA-expressing Vero cells with a high input multiplicity of LACV. The interaction of MxA with the newly synthesized viral proteins was investigated by double-immunofluorescence by using confocal microscopy. Vero cells lacking MxA were used as a control. In these control cells, viral N showed normal diffuse distribution in the cytoplasm and accumulated in the Golgi compartment (data not shown). Surprisingly, in MxA-expressing cells, large complexes of N were formed near the nucleus (Fig. 1Aa). These N complexes colocalized perfectly with MxA (Fig. 1 Ab and Ac). Staining for the viral G protein showed that the glycoproteins accumulated in the Golgi area and did not overlap with the MxA/N complexes (Fig. 1A d–f). The MxA/N colocalization was accompanied by a loss of the typical granular distribution of MxA, indicating that MxA had completely relocated from the characteristic cytoplasmic dots into the newly formed perinuclear complexes. In contrast, uninfected MxA-expressing cells exhibited the classical dot-like MxA granula that are distributed regularly throughout the cytoplasm (Fig. 1Aj). Thus, N of LACV and MxA form perinuclear complexes that are spatially separated from the Golgi apparatus where N normally accumulates in the course of virus infection, as shown in Fig. 4 a–c.
Figure 1.
MxA binds to the N protein of bunyaviruses and colocalizes with N in large perinuclear complexes. (A) MxA-expressing Vero cells were either infected with 10 pfu of LACV per cell (a–f) or were transfected with plasmid pSC-N coding for the N protein of LACV (g–i, note that the transfected cell is surrounded by nontransfected cells expressing only MxA) or were left untreated (j). Cells were fixed 24 h later and analyzed by immunofluorescence for the presence of viral N or G protein (red) and MxA (green), using specific Abs. (B) MxA-expressing Vero cells were infected with 20 pfu per cell of either BUNV or RVFV for 20 h, fixed, and analyzed for MxA and N expression. The cells were stained with Abs directed against the N proteins of either BUNV (red; a) or RVFV (d) together with an MxA-specific Ab (green; b and e). Panels at the right show the superimposition of the two images. Bars, 20 μm. (C) MxA-expressing Vero cells were infected with 10 pfu of LACV per cell for 20 h or were left untreated. Cell lysates were used for immunoprecipitation in the presence or absence of GTPγS by using the N-specific antiserum. The coprecipitated proteins were detected by Western-blotting with the MxA-specific mAb M143. Positions of Mr markers (in kDa) are indicated at the left.
Figure 4.
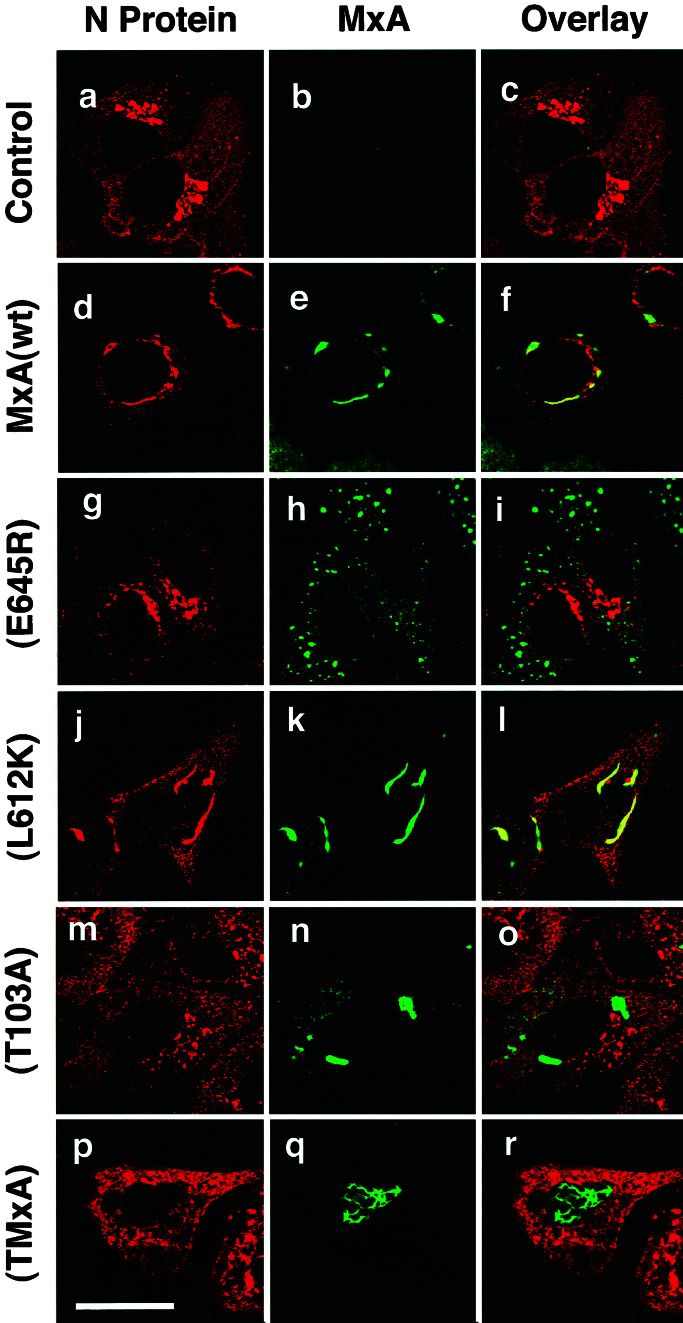
The antiviral activity of MxA correlates with the formation of MxA/N complexes. Control cells (a–c) or Vero cells constitutively expressing MxA (d–f) or MxA(E645R) (g–i) were used for infection. In parallel, Vero cells that transiently expressed MxA(L612K) (j–l), MxA(T103A) (m–o), or TMxA (p–r) were used. All cells were infected with 10 pfu/cell of LACV. Twenty hours later, the cells were fixed and analyzed for the viral N (red) and the various MxA proteins (green) by immunofluorescence. Panels at the right show the superimposition of the two images. Bar, 20 μm.
We further investigated whether LACV infection had a similar effect on the intracellular distribution of endogenous MxA in IFN-treated human hepatoma HuH-7 cells. As expected, MxA was quantitatively redistributed together with the viral N in LACV-infected cells (data not shown). We concluded that N of LACV and MxA formed these complexes as part of the IFN-induced antiviral state.
The possibility existed that the MxA/N complexes contained additional viral components, such as viral RNAs, and might in fact represent MxA/viral ribonucleoprotein complexes. We used fluorescence in situ hybridization to detect genomic or antigenomic viral RNAs in infected MxA-expressing cells. Fluorochrome-labeled plus- and minus-sense RNA probes were used to visualize the respective viral RNA species. The RNA probes specifically hybridized to viral RNAs in LACV-infected cells, but no obvious colocalization with the MxA/N complexes was detected (not shown). These findings suggested that the observed complex formation presumably did not require the presence of viral RNAs.
To further demonstrate that the interaction of MxA with N was independent of additional viral components, Vero cells were transfected with a cDNA expression construct (pSC-N), which encoded the viral N. In the transfected cells, N was overexpressed in the absence of viral RNAs. Immunofluorescence analysis revealed large perinuclear complexes of recombinant N in the transfected MxA-expressing cells (Fig. 1Ag) but not in transfected control cells (not shown). MxA had lost its granular appearance in transfected cells and was almost quantitatively relocated into the perinuclear complexes together with N protein, which was synthesized in great excess (Fig. 1A h and i). In contrast, MxA was distributed throughout the cytoplasm in neighboring cells that were not transfected and did not express the N protein (Fig. 1A h and i). Thus, expression of N from a plasmid had the same effect as expression of N during viral infection, indicating that the relocation of MxA was a direct effect of N expression and was independent of other viral structures.
MxA is known to be inhibitory to most members of the Bunyaviridae family (7). Therefore, we analyzed the ability of MxA to colocalize with the N proteins of two additional bunyaviruses, namely Bunyamwera virus (BUNV, representing a second member of the genus Bunyavirus) and RVFV, belonging to the genus Phlebovirus). Similar to the situation with LACV, infection of MxA-expressing Vero cells with either BUNV or RVFV lead to the formation of large perinuclear MxA/N complexes, as demonstrated by double-immunofluorescence analysis (Fig. 1B).
To investigate whether MxA binds directly to N of LACV, we performed an immunoprecipitation analysis. Cell lysates were prepared from either infected or uninfected MxA cells and subjected to immunoprecipitation, using an N-specific Ab. We have previously noticed that GTP-binding is critical for the interaction of MxA with viral target structures (23). Therefore, the immunoprecipitation procedure was performed in the presence of the nonhydrolyzable GTP analog GTPγS. By Western blotting using an MxA-specific Ab it was evident, that MxA precipitated together with N of LACV (Fig. 1C). This interaction only happened in the presence of GTPγS. No coimmunoprecipitation occurred in the absence of GTPγS or in the absence of viral infection (Fig. 1C). Thus, MxA physically interacts with the viral N protein in infected cells, and this interaction is stabilized by the nonhydrolyzable GTP analogue.
Ultrastructure of MxA/N Complexes.
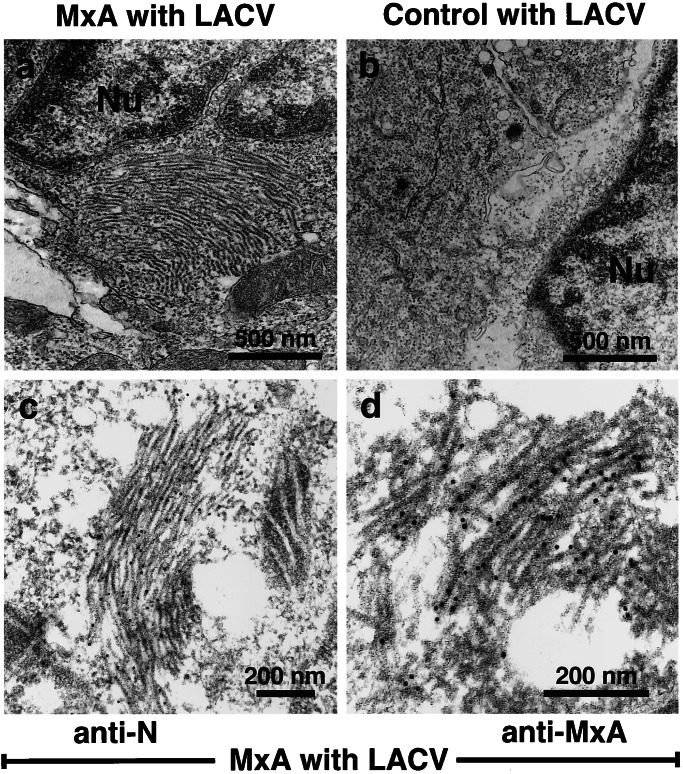
To study the structure of the MxA/N complexes, we performed EM of infected MxA cells, using the cellulose capillary tube technique (24). After virus infection, stacks of filamentous bundles were detected in MxA-expressing cells (Fig. 2a). These structures consisted of individual filaments with a diameter of 15–20 nm, were highly ordered, and located in the perinuclear area of the cells. The filaments were only visible in MxA-expressing cells infected with LACV, whereas no such structures could be detected in sections of LACV-infected control cells (Fig. 2b).
Figure 2.
Ultrastructure of MxA/N complexes. MxA-expressing Vero cells (a) or control cells (b) were infected with 10 pfu of LACV per cell for 20 h. Cells were prepared for transmission EM by cellulose capillary technique. The nucleus is marked by Nu. For immuno-EM, MxA-expressing cells infected with LACV were prepared by using the progressive lowering of temperature method. Thin sections of the cells were labeled with the N-specific Ab (c) or with mAb M143 directed against MxA (d). Primary Abs were detected with gold-labeled protein A. The magnification is indicated by the bars. The diameter of the gold particles is 10 nm.
To confirm that these structures were composed of MxA and LACV N, sections of infected cells were incubated with Abs directed either against N or MxA. The bound primary Abs were visualized by using immunogold-labeled Protein A. Both Abs specifically stained the filamentous structures in LACV-infected MxA cells indicating that the filaments were composed of both viral N and MxA (Fig. 2 c and d). In all other cell compartments, no such staining could be detected.
This ultrastructural analysis revealed no obvious connection of the MxA/N complexes to other subcellular structures. A possible association with intracellular membranes was excluded by immunofluorescence analysis, using structure specific markers for the endoplasmic reticulum or the pre-, cis-, and transGolgi compartments (data not shown). We conclude that the MxA/N filamentous complexes developed independently from other cellular structures and localized preferentially to the perinuclear area of the cytoplasm.
Formation of MxA/N Complexes Correlates with the Antiviral Activity of MxA.
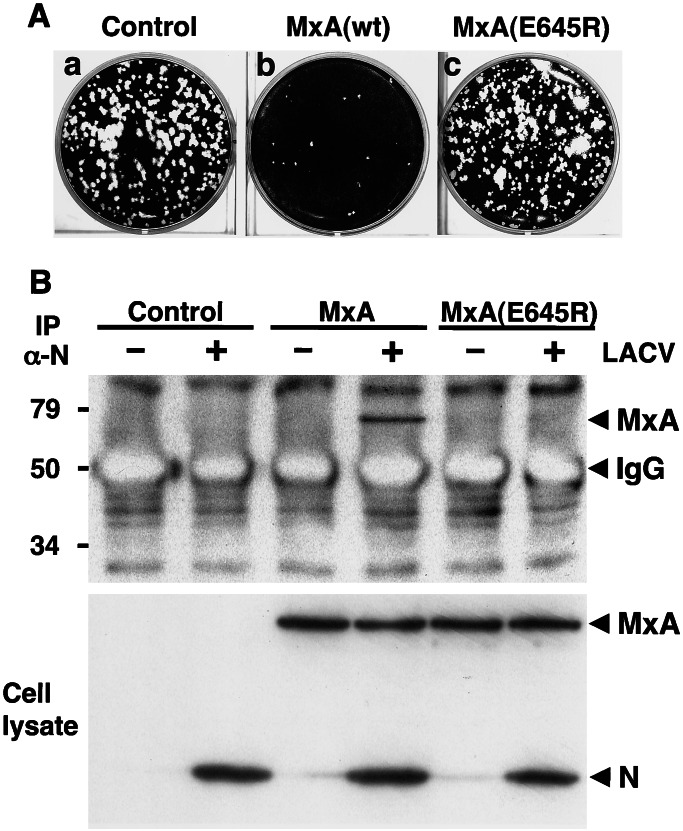
A mutant form of MxA, MxA(E645R), has a glutamic acid to arginine substitution at position 645. This single amino acid exchange in the C terminus influences the antiviral specificity of the molecule (18) without affecting its GTPase activity (26). MxA(E645R) has specifically lost its antiviral activity against vesicular stomatitis virus (VSV) but not against influenza A virus (FLUAV) and Thogoto virus (THOV) (18, 27). We first tested the ability of MxA(E645R) to interfere with the multiplication of LACV. Vero cells that constitutively expressed wild-type MxA or MxA(E645R) were infected with LACV, and virus growth was analyzed by plaque formation. As expected, wild-type MxA strongly inhibited the appearance of plaques (Fig. 3Ab). In contrast, the mutant MxA had no effect on the number and size of the plaques, which were comparable to those of control cells (Fig. 3A a and c). This demonstrated that MxA(E645R)-expressing cells were fully permissive for LACV. Next, we investigated whether MxA(E645R) had also lost the ability to interact with the N protein of LACV. To address this point, the interaction of MxA(E645R) with N was assessed in coimmunoprecipitation assays. Vero cells expressing MxA or MxA(E645R) and control cells were infected with LACV or were left untreated. Proteins in the cell lysates were immunoprecipitated with the N-specific antiserum in the presence of GTPγS and analyzed by SDS/PAGE and immunoblot assay. In addition, the expression levels of MxA, MxA(E645R), and N were confirmed by Western blot analysis of the crude cell lysates (Fig. 3B Lower). The immunoprecipitation analysis showed that the mutant form of MxA did not coprecipitate with N, in contrast to wild-type MxA (Fig. 3B Upper), indicating that MxA(E645R) was unable to recognize this viral component.
Figure 3.
MxA(E645R) lacks antiviral activity against LACV and does not interact with the viral N protein. (A) Plaque formation of LACV is not inhibited by MxA(E645R). Vero cells expressing wild-type MxA, MxA(E645R), or control cells were infected with 200 pfu of LACV and stained with crystal violet 5 days later. (B) MxA(E645R) does not coimmunoprecipitate with N of LACV. Vero cells expressing MxA or MxA(E645R) and control cells were infected with 10 pfu per cell of LACV or were left untreated. Cell lysates were prepared 20 h later and immunoprecipitated with the N-specific antiserum in the presence of GTPγS. The immunoprecipitate was analyzed for the presence of MxA with mAb M143 by immunoblot (Upper). The coprecipitated background bands visible in all lanes are of unknown origin. The expression levels of MxA, MxA(E645R), and N were assessed by immunoblot analysis of the crude cell lysates, using the same Abs (Lower).
We then determined the intracellular distribution of MxA(E645R) in LACV-infected cells by immunofluorescence analysis. In uninfected cells, mutant MxA(E645R) exhibited the characteristic granular immunofluorescence pattern, similar to wild-type MxA (not shown). Interestingly, LACV infection did not change this pattern, and the mutant protein remained in small dots distributed all over the cytoplasm (Fig. 4h). Also, viral N accumulated in the Golgi compartment of these cells, similar to the situation in infected control cells (Fig. 4 a–c and g–i). In contrast, wild-type MxA dislocated from the punctate pattern to large perinuclear complexes, together with N (Fig. 4 d–f). These findings are consistent with the lack of antiviral activity of MxA(E645R) against LACV.
We tried to further correlate the antiviral activity of MxA with the mislocalization of N, using additional MxA mutants. MxA(L612K) has a single amino acid substitution from leucine to lysine at position 612. As a consequence, it has no GTPase activity and is defective in self-assembly (17, 28, 29). This mutant, however, is antivirally active against FLUAV, THOV, VSV, and LACV, like wild-type MxA (17, 29, 30). MxA(T103A) differs from wild-type MxA by a threonine to alanine substitution within the N-terminal GTP-binding domain. This mutant lacks GTPase activity, is antivirally inactive, and tends to form large homooligomers when expressed in transfected cells (16). TMxA carries at its N terminus a foreign nuclear translocation signal derived from the simian virus 40 large T-antigen. This modified wild-type MxA accumulates in the nucleus and is antivirally active against FLUAV and THOV but, in contrast to cytoplasmic MxA, not against VSV or bunyaviruses, which replicate in the cytoplasm of infected cells (18, 27; M. Sandrock, O.H., and G.K., unpublished observations). These mutant MxA proteins were transiently expressed in Vero cells to investigate colocalization with LACV N. Expression of MxA(L612K) lead to the formation of large complexes together with N (Fig. 4 j–l). In contrast, the distribution of MxA(T103A) and TMxA was not affected by LACV (Fig. 4 m–r). The GTPase-inactive MxA(T103A) did not colocalize with N which partially accumulated in the Golgi compartment. Likewise, the nuclear TMxA had no influence on the normal localization of N. These results support our hypothesis that the intracellular sequestration of N into the large perinuclear complexes leads to the inhibition of virus replication in MxA-expressing cells.
Discussion
Here we demonstrate that the human MxA protein inhibits replication of LACV by targeting the viral N protein to perinuclear complexes. The N protein, which is the major component of the viral nucleocapsid, has a central role not only in virus assembly but also in genome amplification. It is essential in the process of vRNA synthesis because it helps the viral polymerase to switch from the transcription into the replication mode. We now show that MxA interferes with this process by sequestering newly synthesized viral N into large perinuclear complexes. This leads to a depletion of freely available unassembled N and a block in LACV genome amplification. As a consequence, the assembly of nucleocapsids and the formation of new viral particles are severely hampered. We further show that this antiviral mechanism is of general significance because it works in the same way against other bunyaviruses, such as BUNV and RVFV.
We have demonstrated that MxA and N form tightly packed fibrillary bundles in the cytoplasm of infected cells. The same structures are formed when N is expressed from a plasmid, provided MxA is present. This result indicates that additional viral components are not required for the observed MxA/N complex formation. This is remarkable because, in the case of THOV, we have previously found the MxA recognizes nucleocapsids rather than free nucleoprotein (23, 31, 37). Given the tendency of bunyavirus N proteins to associate into multimers (32, 33), it is conceivable that N of LACV spontaneously forms nucleocapsid-like structures that are preferentially sequestered by MxA into the perinuclear complexes described here. In the absence of MxA, comparable structures are not detectable, indicating that MxA and N have to cooperate to form these highly ordered structures. Immunogold labeling revealed that the filaments are indeed composed of both the viral N and MxA. The exact stoichiometry and morphology of these filaments is presently unknown and awaits further structural analysis at higher resolution.
Using mutant forms of MxA, we show that the antiviral activity and N recognition by MxA are most likely connected. MxA is organized in two functional domains, an N-terminal GTP-binding domain and a C-terminal effector domain containing two highly conserved leucine-zipper motifs (4). It has previously been shown that mutations within the N-terminal GTP-binding domain result in a loss of antiviral activity (16, 34). Accordingly, when mutant MxA(T103A) is expressed in LACV-infected cells, this inactive MxA mutant failed to form complexes with N, and the viral protein was able to reach the Golgi compartment. The importance of GTP-binding also was demonstrated in coimmunoprecipitation assays. Coimmunoprecipitation of wild-type MxA with N was strongly dependent on the presence of GTPγS, a nonhydrolyzable GTP analog. This is in agreement with previous findings showing that GTPγS stabilizes MxA in a conformation optimal for target binding (23). The C-terminal effector domain of MxA plays an important role in target recognition (18). A single amino acid exchange (E645R) within the distal leucine-zipper motif changed the antiviral specificity of MxA, such that the mutant protein loses its antiviral activity against VSV, but maintains wild-type activity against FLUAV and THOV (18, 27). The present results show that MxA(E645R) has also lost antiviral activity against LACV and, coincidentally, the capacity to recognize the viral N, as demonstrated in immunoprecipitation and immunofluorescence assays. These findings demonstrate that the formation of MxA/N complexes relies on a highly specific protein-protein recognition that is governed by the C terminus of MxA. They also support the hypothesis that MxA/N complex formation and antiviral activity are causally linked.
In MxA-expressing cells, immunofluorescence analysis usually shows a granular appearance of MxA throughout the cytoplasm. In LACV-infected cells, these punctate granula gradually disappeared, and a complete redistribution of MxA into the perinuclear complexes was observed. We have previously proposed that the MxA granula found in uninfected cells represent a storage form of inactive MxA oligomers, from which active MxA monomers can be recruited (17). In infected cells, MxA monomers in the active conformation would bind to their targets and recruit more MxA monomers into these complexes. The present findings are compatible with this view. They also are supported by the finding that monomeric MxA(L612K) was equally efficient as wild-type MxA in sequestering LACV N. Obviously, MxA(L612K) has lost its ability to form large homooligomers (28, 29) but is still capable of forming assemblies with the viral target structures resulting in perinuclear complexes. In summary, these data suggest that MxA can form two types of oligomers, namely homooligomeric storage complexes distributed throughout the cytoplasm and perinuclear copolymers with the N protein.
The ultimate fate of the MxA/N complexes is presently not known. They appear to be stable, localize predominantly in the perinuclear area, and have no obvious connection to defined subcellular compartments, indicating that they represent autonomous structures. It has previously been described that misfolded or defective cellular proteins are sequestered to metabolically stable inclusion bodies located close to the nucleus by a dynein-dependent retrograde transport on microtubules. These cytoplasmic deposits of aggregated proteins were called aggresomes and were proposed to help to protect cells from potentially toxic protein aggregates (35). The MxA/N complexes described here resemble aggresome-like structures, and the involvement of a microtubule-dependent transport mechanism in their formation has to be determined.
MxA has antiviral activity against a variety of RNA viruses (for a review see ref. 3). In most cases, MxA seems to inhibit an early step in the virus multiplication cycle. It inhibits primary transcription of VSV, measles virus, and THOV (15, 31, 36), most likely by interfering with the transcriptional activity of the infecting nucleocapsids, which represent the transcription and replication units of negative strand RNA viruses. In the case of THOV, MxA prevents incoming viral nucleocapsids from being transported into the nucleus in which THOV primary transcription occurs (31, 37). In the case of LACV and other bunyaviruses, as shown here, MxA recognizes newly synthesized viral N protein and forms large MxA/N copolymers that accumulate in the cytoplasm of infected cells. As a consequence, the drop in the amount of free N below a critical concentration may block genome amplification, without affecting viral transcription. Likewise, FLUAV and parainfluenzavirus type 3 seem to be blocked by MxA at the level of genome amplification (38, 39), and it is conceivable that a similar antiviral mechanism is at work. Elucidating the mechanism of MxA action should help to better understand innate immunity against RNA viruses and provide new means to control these human pathogens.
Acknowledgments
We thank Michele Bouloy, Richard M. Elliott, Francisco Gonzalez-Scarano, Illka Julkunen, and Ramasamy Raju for providing reagents; Simone Gruber and Carola Huckhagel for excellent technical assistance; and Peter Staeheli, Kevin Tan, and Friedemann Weber for suggestions and critical comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Ko 1579/1-4).
Abbreviations
- BUNV
Bunyamwera virus
- FLUAV
influenza A virus
- LACV
La Crosse virus
- N
nucleocapsid protein
- pfu
plaque-forming units
- RVFV
Rift Valley fever virus
- THOV
Thogoto virus
- VSV
vesicular stomatitis virus
- GTPγS
guanosine 5′-O-(thiotriphosphate)
- EM
electron microscopy
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Calisher C H. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi M, Fäh J, Hurt N, Samuel C E, Thomis D, Bazzigher L, Pavlovic J, Haller O, Staeheli P. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haller O, Frese M, Kochs G. Rev Sci Tech OIE. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 4.Staeheli P, Pitossi F, Pavlovic J. Trends Cell Biol. 1993;3:268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 5.Sever S, Damke H, Schmid S L. Traffic. 2000;1:385–392. doi: 10.1034/j.1600-0854.2000.010503.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Bliek A M. Trends Cell Biol. 1999;9:96–102. doi: 10.1016/s0962-8924(98)01490-1. [DOI] [PubMed] [Google Scholar]
- 7.Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. J Virol. 1996;70:915–923. doi: 10.1128/jvi.70.2.915-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefti H P, Frese M, Landis H, DiPaolo C, Aguzzi A, Haller O, Pavlovic J. J Virol. 1999;73:6984–6991. doi: 10.1128/jvi.73.8.6984-6991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura T A, Carlson J O, Beaty B J, Bowen R A, Olson K E. J Virol. 2001;75:3001–3003. doi: 10.1128/JVI.75.6.3001-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmaljohn C S. In: Virology. Fields B N, editor. New York: Raven; 1996. pp. 1447–1471. [Google Scholar]
- 11.Hacker D, Raju R, Kolakofsky D. J Virol. 1989;63:5166–5174. doi: 10.1128/jvi.63.12.5166-5174.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson W, Kalfayan B, Anslow R. Am J Epidemiol. 1965;81:245–253. doi: 10.1093/oxfordjournals.aje.a120512. [DOI] [PubMed] [Google Scholar]
- 13.Caplen H, Peters C J, Bishop D H L. J Gen Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 14.Watret G E, Pringle C R, Elliott R M. J Gen Virol. 1985;66:473–482. doi: 10.1099/0022-1317-66-3-473. [DOI] [PubMed] [Google Scholar]
- 15.Pavlovic J, Zürcher T, Haller O, Staeheli P. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponten A, Sick C, Weeber M, Haller O, Kochs G. J Virol. 1997;71:2591–2599. doi: 10.1128/jvi.71.4.2591-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janzen C, Kochs G, Haller O. J Virol. 2000;74:8202–8206. doi: 10.1128/jvi.74.17.8202-8206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zürcher T, Pavlovic J, Staeheli P. EMBO J. 1992;11:1657–1661. doi: 10.1002/j.1460-2075.1992.tb05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akashi H, Bishop D H. J Virol. 1983;45:1155–1158. doi: 10.1128/jvi.45.3.1155-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgiev O, Bourquin J P, Gstaiger M, Knoepfel L, Schaffner W, Hovens C. Gene. 1996;168:165–167. doi: 10.1016/0378-1119(95)00764-4. [DOI] [PubMed] [Google Scholar]
- 21.Flohr F, Schneider-Schaulies S, Haller O, Kochs G. FEBS Lett. 1999;463:24–28. doi: 10.1016/s0014-5793(99)01598-7. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Scarano F, Shope R E, Calisher C E, Nathanson N. Virology. 1982;120:42–53. doi: 10.1016/0042-6822(82)90005-8. [DOI] [PubMed] [Google Scholar]
- 23.Kochs G, Haller O. J Biol Chem. 1999;274:4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- 24.Hohenberg H, Mannweiler K, Muller M. J Microsc (Oxford) 1994;175:34–43. doi: 10.1111/j.1365-2818.1994.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 25.Carlemalm E, Garavito R M, Villiger W. J Microsc (Oxford) 1982;126:123–143. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwemmle M, Weining K C, Richter M F, Schumacher B, Staeheli P. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 27.Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. J Virol. 1995;69:3904–3909. doi: 10.1128/jvi.69.6.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher B, Staeheli P. J Biol Chem. 1998;273:28365–28370. doi: 10.1074/jbc.273.43.28365. [DOI] [PubMed] [Google Scholar]
- 29.DiPaolo C, Hefti H P, Meli M, Landis H, Pavlovic J. J Biol Chem. 1999;274:32071–32078. doi: 10.1074/jbc.274.45.32071. [DOI] [PubMed] [Google Scholar]
- 30.Janzen J. Ph.D. thesis. Germany: University of Freiburg; 2000. [Google Scholar]
- 31.Kochs G, Haller O. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alfadhli A, Love Z, Arvidson B, Seeds J, Willey J, Barklis E. J Virol. 2001;75:2019–2023. doi: 10.1128/JVI.75.4.2019-2023.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaukinen P, Koistinen V, Vapalahti O, Vaheri A, Plyusnin A. J Gen Virol. 2001;82:1845–1853. doi: 10.1099/0022-1317-82-8-1845. [DOI] [PubMed] [Google Scholar]
- 34.Pitossi F, Blank A, Schröder A, Schwarz A, Hüssi P, Schwemmle M, Pavlovic J, Staeheli P. J Virol. 1993;67:6726–6732. doi: 10.1128/jvi.67.11.6726-6732.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopito R R. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 36.Schneider-Schaulies S, Schneider-Schaulies J, Schuster A, Bayer M, Pavlovic J, ter Meulen V. J Virol. 1994;68:6910–6917. doi: 10.1128/jvi.68.11.6910-6917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber F, Haller O, Kochs G. J Virol. 2000;74:560–563. doi: 10.1128/jvi.74.1.560-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlovic J, Haller O, Staeheli P. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, De B P, Das T, Banerjee A K. Virology. 1996;220:330–338. doi: 10.1006/viro.1996.0321. [DOI] [PubMed] [Google Scholar]