Abstract
Genetically accessible host models are useful for studying microbial pathogenesis because they offer the means to identify novel strategies that pathogens use to evade immune mechanisms, cause cellular injury, and induce disease. We have developed conditions under which the human pathogen Pseudomonas aeruginosa infects Dictyostelium discoideum, a genetically tractable eukaryotic organism. When D. discoideum is plated on nutrient agar plates with different P. aeruginosa strains, the bacteria form lawns on these plates with amoebae embedded in them. Virulent P. aeruginosa strains kill these amoebae and leave an intact bacterial lawn. A number of P. aeruginosa mutants have been identified that are avirulent in this assay. Amoebae feed on these bacteria and form plaques in their bacterial lawns. One avirulent mutant strain carries an insertional mutation in the lasR gene. LasR is a transcription factor that controls a number of virulence genes in a density-dependent fashion. Another class of avirulent P. aeruginosa mutants is defective in type III secretion. One mutant lacks the PscJ protein, a structural component of the secretion apparatus, suggesting that cytotoxins are injected into the D. discoideum cell. One of these cytotoxins is ExoU, and exoU mutants are avirulent toward D. discoideum. Complementation of the lasR and exoU mutations restores virulence. Therefore, P. aeruginosa uses conserved virulence pathways to kill D. discoideum.
Keywords: slime mold‖type III secretion‖host–pathogen interactions‖LasR‖ExoU
Pseudomonas aeruginosa is an opportunistic pathogen that causes life-threatening infections in individuals with compromised immune systems, such as cancer patients undergoing chemotherapy or patients with cystic fibrosis. Immunocompromised individuals are at high risk of becoming infected in a hospital setting where P. aeruginosa causes a variety of nosocomial infections, including pneumonia, urinary tract infections, surgical wound infections, and blood stream infections (for review, see ref. 1).
Cystic fibrosis patients, who carry mutations in both alleles of the cystic fibrosis transmembrane conductance regulator, are at high risk of developing chronic lung infections at some stage in their lives. Initially, P. aeruginosa colonizes the airways with other pathogens, like Haemophilis influenzae and Staphylococcus aureus. After a variable period, chronic lung disease develops in which the bacterial population consists almost exclusively of mucoid P. aeruginosa in the form of biofilms (2). Biofilms are structured communities of bacteria embedded in a polysaccharide matrix, and growth in this form renders P. aeruginosa innately resistant to antimicrobial treatment (3).
The fact that P. aeruginosa is resistant to treatment with antibiotics demands alternative strategies to treat infections with this pathogen. One such approach is to interfere with the interaction between P. aeruginosa and its host. Several experimental systems involving the plant Arabidopsis thaliana, the nematode Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and mice have been used to study such host–pathogen interactions (for review, see ref. 4). To study the interaction of P. aeruginosa and a host cell, we suggest the genetically tractable organism Dictyostelium discoideum. D. discoideum is a complex eukaryote that lives part of its life cycle as a unicellular amoeba. Free-living amoebae feed on bacteria by a phagocytic mechanism that resembles that of mammalian macrophages (for review, see ref. 5). Using Dictyostelium as a genetic model host takes advantage of the organism's ability to integrate complicated activities in a single cell, which includes responses to pathogens. It is this unicellular lifestyle that enables us to study the interaction between a host and a pathogen directly with only these two organisms present. D. discoideum is well suited for studying the host biology of pathogens, because it offers a variety of different genetic manipulations. Because D. discoideum is haploid, mutations are not masked by additional alleles, so that mutations, including recessive ones, are readily identified. The small size of the genome (≈34 Mb) and the small amount of noncoding sequence allow us to readily saturate the genome with mutations. The entire genomic sequence will be known soon, and this will accelerate the identification of genes that emerge from any genetic screen for resistance to P. aeruginosa. D. discoideum has already been shown to be useful in studying the virulence mechanisms of the intracellular pathogen Legionella pneumophilia (6).
A simple plating assay has been developed to study the interaction between P. aeruginosa and D. discoideum. P. aeruginosa formed lawns on these plates with amoebae embedded in them. Virulent P. aeruginosa strains kill these amoebae. If a mutation causes a particular strain to be avirulent toward D. discoideum, the amoebae feed on these bacteria and form plaques in bacterial lawns after a few days. We have identified a number of P. aeruginosa mutants that are avirulent in this assay. Analysis of these avirulent mutants suggests that P. aeruginosa utilizes at least two major virulence pathways, namely quorum-sensing mediated virulence and type III secretion of virulence factors, to infect amoebae. We propose that D. discoideum is a genetically tractable system that can be used to study the host biology of P. aeruginosa.
Materials and Methods
Strains and Culture Conditions.
Dictyostelium strain AX3 was used in all experiments (7). AX3 was grown in liquid HL/5 cultures or in lawns of Klebsiella aerogenes on SM/5 plates, as described by Sussman (8). In some of the experiments, low-nutrient plates were used to limit bacterial growth. SM/25, SM/50, and SM/100-plates contained 1/25, 1/50, or 1/100 of a SM-stock solution [1% glucose/1% Bacto peptone (Difco)/0.1% Bacto yeast extract (Difco)/4.2 mM MgSO4,], respectively. Solutions were buffered to pH 6.5 by the addition of 13.2 mM KH2PO4 and 6.9 mM K2HPO4.
The lasR-mutant 12A1, the phenA/phenB-mutant ΔphenA/phenB, the TnphoA-mutant 8C12, as well as their parent strain UCB-PP PA14, were kind gifts from Fred Ausubel (Massachusetts General Hospital, Boston) (9–11). Strains SUP16 and SUP17 were generated by introducing plasmid pUCP18 (12) and plasmid pLasR-PUH (see below) into lasR-mutant 12A1. A rhlA mutant of PA14 was constructed with knockout-plasmid pEX100T-rhlA∷Gm by using the site-specific insertional mutagenesis strategy of Schweizer and Hoang (13, 14).
The exoU-mutant PA103∷exoU (mut 8) (15), the pscJ-mutant PA103∷pscJ (mut N) (15), and their isogenic parent PA103 (16) were gifts from Joanne Engel (University of California, San Francisco). The exoU mutation was complemented by introducing plasmid pAH807 (pLAFRSK1 with a 3.8-kb genomic fragment containing exoU) (17); the resulting strain was also a kind gift from Joanne Engel.
Creation of pLasR-PUH.
To complement the lasR mutation in P. aeruginosa strain 12A1, we designed a plasmid that provides high expression levels of lasR. The lasR gene was recovered from plasmid pKDT17 (18) as a 0.8-kb EcoRI/HindIII fragment and subcloned in front of the lac promoter of EcoRI/HindIII-digested pUCP18 (12).
Plate Killing Assay.
Bacteria were grown in Luria broth for 16 h, pelleted by centrifugation (1,600 × g; 15 min), washed once, and resuspended in SorC (16.7 mM Na2H/KH2PO4/50 μM CaCl2, pH 6.0) at a final optical density of 5.5 at 600 nm. D. discoideum cells from midlogarithmic cultures were collected by centrifugation (1,000 × g; 4 min), washed once with SorC (8), and added to the bacterial suspensions at a final concentration of 5 × 102 cells/ml suspension; 0.2 ml of this mixture was plated out on SM/5 plates and allowed to dry under a sterile flow of air. Plates were incubated for 3–5 days and examined for plaques formed by Dictyostelium amoebae.
Elastase and Pyocyanin Assays.
Elastolytic activity was determined as described by Gambello and Iglewski (19). Pyocyanin levels in bacterial cultures were determined as described by Essar et al. (20).
Gentamicin Protection Assay.
To test whether P. aeruginosa is internalized and digested by D. discoideum, a modification of the gentamicin protection assay was used (21). D. discoideum cells from midlogarithmic cultures were collected by centrifugation (1,000 × g; 4 min), washed once with fresh HL/5 (8), and resuspended in HL/5 at a concentration of 106 cells/ml. Aliquots of 1.0 ml were added to 24-well tissue culture dishes (Falcon) and incubated for 60 min at 22°C to allow cells to adhere to the plastic surface. Bacteria were grown in Luria broth for 16 h, pelleted by centrifugation (1,600 × g; 15 min), and resuspended in HL/5. Bacteria were added to the tissue culture wells at a final optical density of 0.1 at 600 nm (multiplicity of infection = 100). Infection was initiated by centrifugation (750 × g; 10 min). Thirty minutes after the initiation of infection, cells were washed twice and resuspended in SorC (16.7 mM Na2H/KH2PO4/50 μM CaCl2, pH 6.0) containing 400 μg/ml of gentamicin to kill all bacteria that were not ingested by the amoebae. To determine the number of viable bacteria inside the amoebae at various time points, cells were washed twice with SorC and lysed in SorC containing 0.05% Triton X-100. The lysates were diluted and plated on Luria broth agar plates. After an incubation at 37°C for 16 h, the colony-forming units were counted.
Results
D. discoideum Is Susceptible to Infection with P. aeruginosa.
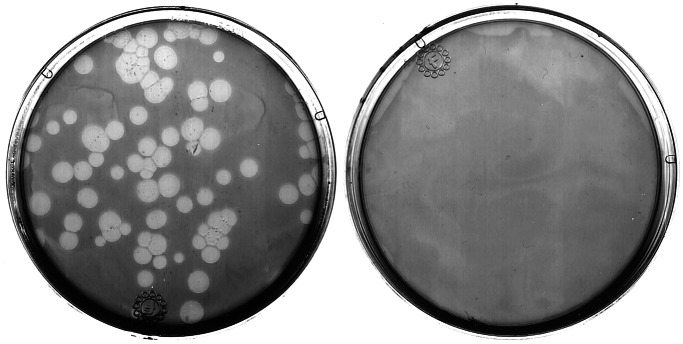
To test whether P. aeruginosa could infect D. discoideum, the pathogenicity of P. aeruginosa toward D. discoideum cells was determined by using a simple plating assay. D. discoideum cells were plated on nutrient agar plates with nonpathogenic Klebsiella aerogenes or virulent P. aeruginosa strain PA14. Over the course of a few days, the bacteria formed lawns on these plates with amoebae embedded in them. As shown in Fig. 1, D. discoideum feeds on K. aerogenes and forms plaques that become readily apparent after a few days. D. discoideum does not form any plaques on the lawns of P. aeruginosa strain PA14. This experiment does not distinguish between killing and growth inhibition, but we have shown that the bacteria kill the amoebae (see below). P. aeruginosa therefore has the capacity to kill D. discoideum under these conditions.
Figure 1.
D. discoideum cells do not form plaques in lawns of P. aeruginosa strain PA14. D. discoideum cells (AX3) were plated on SM/5 with K. aerogenes (Left) and P. aeruginosa strains PA14 (Right) at a density of ≈100 D. discoideum cells/plate. After 3 days, D. discoideum plaques appeared on plates with K. aerogenes. No plaques were formed on plates with the virulent P. aeruginosa strain.
Virulence Factors Controlled by Quorum Sensing Kill D. discoideum.
A number of virulence factors of P. aeruginosa are produced only when bacteria are present at high titers. This “quorum-sensing mechanism” is thought to be an important regulator of pathogenesis, because it guarantees that certain virulence factors are made only when P. aeruginosa has reached a crucial density and can launch a successful infection (22).
We chose to investigate the role of the quorum-sensing mechanism by testing the virulence of quorum-sensing mutants of P. aeruginosa strain PA14 (11). PA14 is a multihost pathogen that has been used widely for studying the molecular basis of biofilm formation (9–11, 23). As shown in Fig. 2 Top, D. discoideum is unable to form plaques in lawns of parental strain PA14 but forms plaques in lawns of the isogenic lasR-mutant 12A1. Mutant strain12A1 carries a TnphoA transposon in the ORF of lasR (9). lasR encodes the main transcriptional regulator of the las quorum-sensing pathway, and the lasR-mutant 12A1 has been shown to be avirulent toward C. elegans (9).
Figure 2.
Complementation of the lasR mutation restores LasR function and virulence but does not affect synthesis of the pigment pyocyanin. (Top) D. discoideum cells (AX3) were plated on nutrient agar (SM/5) with P. aeruginosa strain PA14, the isogenic lasR-mutant 12A1, complemented strains SUP16 (ΔlasR with pUCP18) and SUP17 (ΔlasR with plasR-PUH), and the pyocyanin-deficient strain ΔphenA/ΔphenB. Plates were incubated at 22°C for 5 days, and virulence was assessed by determining the number of Dictyostelium plaques. (Middle) Elastase activity of supernatants from overnight cultures were assayed by using elastin Congo red (ECR) as the substrate. Data represent the means of duplicate elastase assays, and activity is expressed as the activity relative to wild-type PA14 (=100%) +/−SDn−1. (Bottom) The ability of each strain to secrete the pigment pyocyanin was determined by assaying the amount of pyocyanin present in overnight cultures of each strain. Pyocyanin was extracted from supernatants and assayed as described in (20). Data represent the means of duplicate assays, and amounts of secreted pyocyanin are expressed as micrograms/milliliter culture medium +/− SDn−1.
To test whether the ability of Dictyostelium to form plaques on strain 12A1 is due to the removal of LasR, we carried out a plasmid complementation experiment. We constructed a plasmid, pLasR-PUH, which carries lasR fused to the lacZ promoter. Plasmid pLasR-PUH and vector control pUCP18 were introduced into lasR-mutant 12A1, resulting in strains SUP17 and SUP16, respectively. To ensure that the introduction of pLasR-PUH restores LasR function, we determined the activity of the LasR-controlled lasB gene product, elastase. As shown in Fig. 2 Middle, lasR-mutant 12A1 and vector control strain SUP16 show low elastase activity, compared with wild-type PA14. The complemented lasR strain SUP17 shows elastase levels comparable to PA14, indicating that the function of LasR is restored in this strain. We then determined the virulence of these strains in our plaque assay. Dictyostelium formed plaques in lawns of SUP16, but only a few petite plaques could be identified in lawns of SUP17 (see Fig. 2 Top). Complementation of lasR therefore restores virulence that is comparable to wild-type strain PA14. For various reasons, elastase is unlikely to be a virulence factor in this host–pathogen system (see Discussion).
Work with C. elegans as a model host showed that P. aeruginosa secretes a pigment, pyocyanin, that exerts its toxic effect on eukaryotic cells through reactive oxygen species (10). Because pyocyanin synthesis is controlled by quorum sensing, we investigated whether P. aeruginosa-induced killing of Dictyostelium was mediated by pyocyanin. First, we determined the amount of pyocyanin that was secreted by PA14 and its mutants during stationary phase. Pyocyanin (≈6–7 μg/ml) were found in cultures of wild-type PA14 (see Fig. 2 Bottom). The isogenic lasR-mutant 12A1 produced about one-third of this amount of pyocyanin. Reduced pyocyanin production is expected in a lasR mutant because LasR directly controls the expression of RhlR, a transcription factor that is involved in pyocyanin production (24). Overexpression of lasR from plasmid pLasR-PUH did not restore pyocyanin production in strain SUP17. We speculate that this is because high levels of intracellular LasR inhibit the activity of the transcription factor RhlR at a posttranslational level (25). These results showed that virulent strain SUP17 and avirulent strains 12A1 and SUP16 release similar amounts of pyocyanin into the culture medium. Furthermore, a PA14 mutant that is unable to secrete pyocyanin because of a deletion in the phenA and phenB genes is still virulent in our plaque assay (see Fig. 2 Top) (10). The addition of purified pyocyanin to axenic cultures of D. discoideum at a concentration of 7 μg/ml, the highest amount of pyocyanin we ever found in P. aeruginosa supernatants did not affect the viability of growing amoebae (data not shown). Pyocyanin is, therefore, unlikely to be responsible for killing D. discoideum amoebae.
Another class of virulence factors that is controlled by the lasR quorum-sensing mechanism are the rhamnolipids. Rhamnolipids are biosurfactants that lyse host cells by disrupting their cell membranes (26). We investigated whether the avirulent phenotype of the lasR-mutant 12A1 was due to a reduction in rhamnolipid secretion. We measured the amounts of extracellular rhamnolipids from P. aeruginosa cultures in stationary phase and found no significant differences among the lasR-mutant 12A1, the plasmid-complemented strain SUP17, and wild-type PA14 (data not shown). To determine the role of rhamnolipids in the plaque assay, we tested the virulence of a PA14 mutant defective in the rhlA gene. RhlA encodes a subunit of rhamnosyltransferase 1, an essential enzyme in the synthesis of rhamnolipids, and mutations in rhlA result in the inability to secrete any rhamnolipids (13). We constructed a rhlA mutant of P. aeruginosa PA14 by using the site-specific insertional mutagenesis strategy of Schweizer and Hoang (14). As expected, the resulting mutant, PA14ΔrhlA, showed a defect in rhamnolipid synthesis (data not shown). We then tested the virulence of this strain in our plaque assay. D. discoideum amoebae were plated on nutrient agar plates in combination with K. aerogenes or P. aeruginosa strains PA14 and PA14ΔrhlA. D. discoideum formed plaques in lawns of K. aerogenes but was unable to do so in lawns of PA14 and PA14ΔrhlA. Therefore, rhamnolipids do not play a role in the virulence of P. aeruginosa on D. discoideum (data not shown).
Plaque Formation on Strain 12A1 Is Not Due to Increased Phagocytosis.
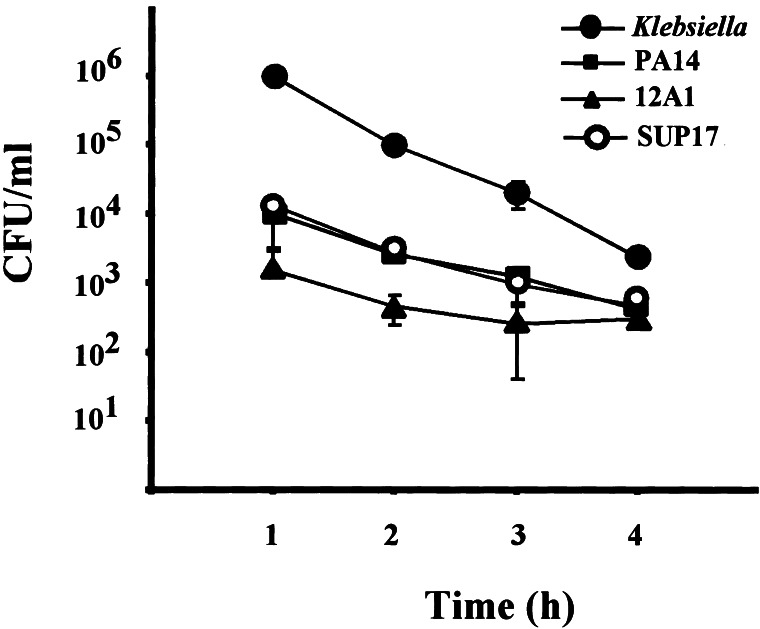
D. discoideum may form plaques in lawns of the lasR-mutant 12A1 but not in lawns of its isogenic parent PA14, because amoebae phagocytose strain 12A1 more efficiently. For example, strain 12A1 might be taken up at an increased rate if the loss of LasR had affected a virulence mechanism that inhibits phagocytosis by the host cell. To determine whether this is the case, we applied a modified gentamicin protection protocol to measure the uptake rates of various P. aeruginosa strains, including strains 12A1 and PA14 (21). D. discoideum and P. aeruginosa were coincubated for 30 min, and any bacteria that were not taken up by the amoebae were killed by the addition of gentamicin. The fate of intracellular bacteria was monitored over a period of 4 h. As shown in Fig. 3, avirulent K. aerogenes is readily phagocytosed by the amoebae. Once internalized, K. aerogenes is digested by the endosomal pathway (27). The virulent P. aeroginosa strain PA14 is phagocytosed at a much lower rate (≈100-fold less compared with K. aerogenes), but once internalized, PA14 is also rapidly digested. The same is true for another virulent strain, SUP17, which carries the lasR gene in trans on a high-copy plasmid. Uptake and digestion rates are comparable to those of PA14. The avirulent lasR-mutant 12A1, although ingested at an even lower rate, is degraded at rates comparable to those of PA14 and SUP17. Therefore, the ability of D. discoideum to form plaques in lawns of strain 12A1 is not because of increased phagocytosis of this particular mutant. The low phagocytosis rate of strain 12A1 is reflected in reduced plaque size. D. discoideum forms plaques in lawns of 12A1 that are approximately one-tenth the size of those formed in lawns of K. aerogenes (data not shown). The gentamicin protection experiment demonstrates that D. discoideum has the capacity to phagocytose all P. aeruginosa strains tested. Therefore, the ability of D. discoideum to form plaques in lawns of 12A1 is because of the loss of a virulence mechanism that does not involve the inhibition of phagocytosis.
Figure 3.
Uptake of P. aeruginosa by D. discoideum. D. discoideum cells were placed in tissue culture wells at 106 cells/ml. Cells were infected with bacteria at a multiplicity of infection of approximately 100:1. Cultures were incubated at 22°C for 30 min, at which time gentamicin was added to kill all extracellular bacteria. Amoebae were collected at indicated time points, lysed, and plated on nutrient agar plates to determine the colony-forming units (cfu) in these lysates. Data represent the means of duplicate experiments, and the number of internalized bacteria per well is expressed as cfu/ml +/− SDn−1.
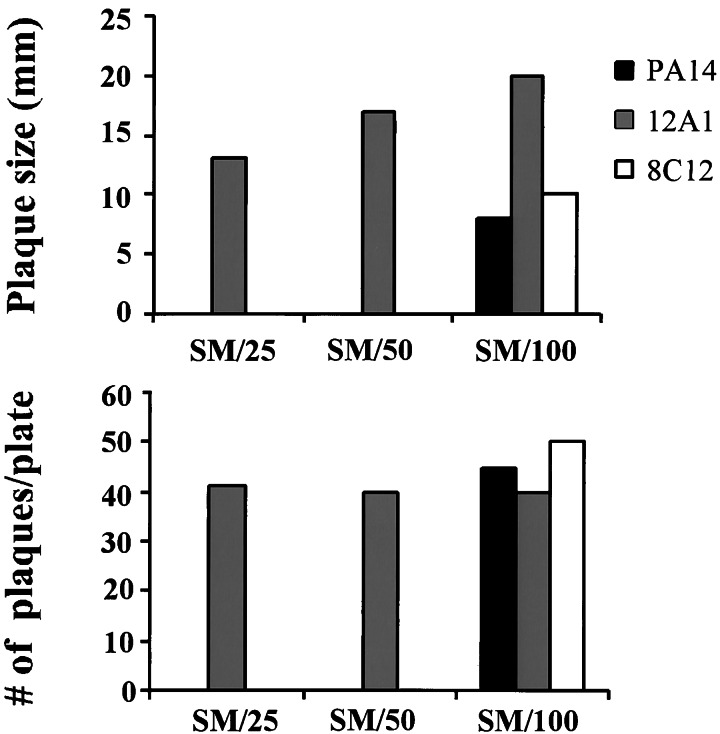
When exposed to low titers of bacterial cells in a liquid medium, D. discoideum amoebae ingested all P. aeruginosa strains by phagocytosis in our gentamicin-protection experiment. We asked whether the same is true on plates when bacteria were plated at low titers. Bacteria and amoebae were plated on agar plates with decreasing nutrient content to control the thickness of the bacterial layer, which is proportional to the nutrient concentration in the agar. D. discoideum plaques were scored after 4 days (see Fig. 4). On SM/25 and SM/50 plates, which contain one-fifth and one-tenth of the nutrient concentration of SM/5, respectively, plaques emerged in lawns of the lasR-mutant 12A1. No plaques could be seen in lawns of wild-type PA14 and the isogenic mutant 8C12 on SM/25 and SM/50 plates. Strain 8C12 is identical to 12A1, except that the TnphoA transposon is in another gene, which does not affect virulence toward amoebae (10). When nutrient content was reduced 20-fold compared with SM/5, plaques emerged in all lawns, including PA14. Therefore, D. discoideum amoebae are able to feed on virulent P. aeruginosa when bacteria are present at low abundance. The plaques formed in lawns of PA14 and 8C12 are smaller in size compared with those in lawns of 12A1. Reduced plaque size might be a result of residual virulence that PA14 and 8C12 exhibit toward D. discoideum on low-nutrient plates (see Fig. 4).
Figure 4.
D. discoideum forms plaques in thin lawns of P. aeruginosa PA14. D. discoideum cells (AX3) were plated with P. aeruginosa strains PA14 (wild type) and PA14 mutants 8C12 and 12A1 (ΔlasR) on nutrient agar plates with decreasing nutrient content. Agar plates contained 1/25 (SM/25), 1/50 (SM/50), and 1/100 (SM/100) of the nutrient content in SM plates. After 4 days at 22°C, D. discoideum formed plaques in lawns of 12A1 on SM/25 and plates with lower nutrient content, 8C12 and PA14 allowed plaque formation only on SM/100 plates.
The Acute Cytotoxin ExoU Kills Dictyostelium Amoebae.
P. aeruginosa utilizes virulence factors that are injected into the host cell through a “type III secretion apparatus” (for review, see ref. 28). This apparatus is a multiprotein complex that is embedded in the outer membrane of the bacterium and that inserts a pilus-like structure into the host cell. This structure allows cytotoxins to be transferred from the bacterium to the host cell. Four toxins that are injected in this way, ExoS, ExoT, ExoU, and ExoY, have been identified (15, 29, 30).
We examined the role of type III secretion of toxins in the observed virulence of P. aeruginosa toward D. discoideum by testing the virulence of the clinical P. aeruginosa isolate, PA103. Previous work demonstrated that PA103 uses type III secretion to kill macrophages and epithelial cells in vitro (31). When amoebae were plated on nutrient agar plates with lawns of PA103, no Dictyostelium plaques emerged (see Figs. 5 and 6A). To address the possibility that the amoebae simply cannot eat PA103 and therefore cannot grow but remain alive, we monitored the fate of amoebae in lawns of virulent PA103 by recovering amoebae from these lawns after an incubation period of 24 h. Recovered amoebae were plated with K. aerogenes on nutrient agar plates supplemented with 200 μg/ml of kanamycin that kill P. aeruginosa but not K. aerogenes. Only about 2.5% of the amoebae that were plated with PA103 could be recovered from these lawns (data not shown), whereas more than 95% of amoebae that were plated on agar plates without a bacterial associate survived such starvation treatment. Thus, the loss of amoebae in lawns of PA103 is because of killing rather than starvation. The same is true for PA14 (data not shown).
Figure 5.
Complementation of the exoU mutation restores virulence. D. discoideum cells (AX3) were plated with different bacterial associates on nutrient agar plates (SM/5) at a density of approximately 40 amoebae/plate. Emerging D. discoideum plaques on each plate were scored after 5 days. Virulence is expressed as the average number of emerging plaques per plate, and error bars represent the standard deviation of five replicates. The virulence of PA103, as well as isogenic mutants PA103∷pscJ (ΔpscJ), PA103∷exoU (ΔexoU), PA103∷exoU+pLAFRSKI (ΔexoU with plasmid control pLAFRSKI), and PA103∷exoU+pAH807 [ΔexoU with plasmid pAH807 (exoU+/spcU+)] was tested in this assay. As a reference for plating efficiency, AX3 was also cultured with K. aerogenes.
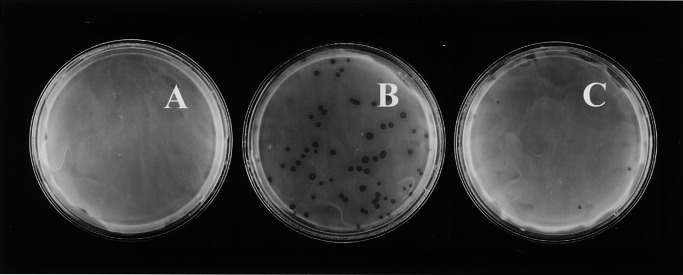
Figure 6.
Expression of ExoU in P. aeruginosa PA103 prohibits plaque formation by D. discoideum. D. discoideum cells (AX3) were plated on SM/5 with P. aeruginosa strains PA103 (A), PA103∷exoU (ΔexoU) (B), and PA103∷exoU + pAH807 (ΔexoU with plasmid pAH807 [exoU+/spcU+]) (C) at a density of ≈100 D. discoideum cells/plate. Plaques were scored after 4 days of incubation at 22°C.
To test whether killing is because of the translocation of toxins, we examined the virulence of a mutant of PA103 that carries a mutation in the pscJ gene and is therefore defective in type III secretion (15). pscJ exhibits 83% similarity to the type III secretion apparatus component YscJ from Yersinia enterocolitica and is essential for the export of toxins (32). The mutation in pscJ prohibits export of the four known cytotoxins by P. aeruginosa strain PA103 (15, 33). Dictyostelium amoebae formed plaques in lawns of the pscJ mutant, suggesting that during infections with wild-type strain PA103, cytotoxins are injected into the D. discoideum host cells (see Fig. 5).
Knowing that a type III secretion apparatus was involved, we asked which virulence factors were responsible for the killing of Dictyostelium amoebae. Because strain PA103 does not produce cytotoxins ExoY and ExoS, we determined the virulence of a PA103 mutant that carries a Tn5-transposon insertion in the exoU gene (15, 30, 34). This exoU mutant is less virulent toward D. discoideum than PA103, allowing D. discoideum to form plaques (see Figs. 5 and 6B).
The exoU operon contains the exoU and spcU genes. SpcU (for specific Pseudomonas chaperone for ExoU) encodes a chaperone that is necessary for proper translocation of ExoU (35). The transposon insertion at the 5′ end of the gene, 125 bp downstream from the translational start site, results in a polar mutation that disrupts both exoU and spcU in strain PA103ΔexoU. To confirm that the loss of virulence is exclusively because of the inability of this strain to export active ExoU, we complemented the exoU mutation by introduction of plasmid pAH807, which carries both the exoU and spcU genes (17). If ExoU is the cause of lethality, then complementation of the mutation should prevent growth of D. discoideum on lawns of the complemented mutant. D. discoideum amoebae could not form plaques in the complemented exoU mutant (see Figs. 5 and 6C) but were able to form plaques in lawns of the exoU mutant that carries the empty plasmid control (see Fig. 5). Thus, ExoU is responsible for killing D. discoideum.
D. discoideum is able to form plaques in thin lawns of virulent P. aeruginosa strains. Any genetic manipulation of P. aeruginosa that results in a growth defect could lead to the formation of thin bacterial lawns on rich-medium plates, allowing D. discoideum to form plaques. To determine whether the ability of D. discoideum to feed on particular mutants of P. aeruginosa strain PA103 was because of reduced growth rates of these strains, we examined the growth properties of the mutant bacteria. Liquid SM/5 medium, the same medium that was used to prepare nutrient agar plates for the plaque assay, was inoculated with various PA103 mutants, and their growth at 22°C was followed over 30 h. The growth properties of strains defective in the exoU or pscJ genes did not differ from their isogenic parent PA103. Introduction of plasmids pAH807 (ExoU+/SpcU+) or pLAFRSKI (plasmid control) into the exoU mutant also did not affect growth (data not shown). We can therefore exclude the possibility that the exoU and pscJ mutants are avirulent, because they do not reach high enough titers on nutrient agar plates to kill D. discoideum. Virulence of the complemented exoU strain is not because of an increased growth rate.
Discussion
The results presented here establish a host–pathogen interaction between the human pathogen P. aeruginosa and the social amoeba D. discoideum. When the two organisms are coincubated on nutrient agar plates, P. aeruginosa is capable of killing D. discoideum. Defined mutants of P. aeruginosa, however, allow D. discoideum to feed on these bacteria and to form plaques in the bacterial lawns. These mutants are impaired in two conserved virulence pathways: quorum-sensing-mediated virulence and type III secretion of cytotoxins.
Quorum sensing is thought to be an important determinant of P. aeruginosa virulence, because it guarantees that the organism expresses virulence factors only when it is present at high numbers. A mutant in the lasR gene, which codes for the transcriptional activator of the lasR quorum-sensing mechanism, is less virulent toward D. discoideum and allows plaque formation. Complementation of the lasR mutation restores virulence, as D. discoideum amoebae are not able to form plaques in lawns of this complemented strain. Thus, P. aeruginosa predation of D. discoideum depends on LasR, which likely regulates expression of a downstream effector gene through the quorum-sensing pathway. This LasR-controlled virulence factor remains to be identified, but we considered rhamnolipids, phenazines, and elastase as possible candidates. We directly examined the contribution of rhamnolipids and phenazines to D. discoideum killing but found that these factors were not involved. Furthermore, addition of purified pyocyanin did not affect the viability of axenically growing amoebae. The fact that D. discoideum is resistant to pyocyanin at concentrations that kill mammalian cells is not surprising. Pyocyanin is a phenazine that applies oxidative stress to eukaryotic cells. D. discoideum is a soil organism that normally encounters a variety of different oxidative radicals and has developed a wide range of mechanisms to deactivate such radicals (5). We are currently examining the role of elastase in the P. aeruginosa–D. discoideum interaction.
Besides quorum-sensing mediated virulence, P. aeruginosa utilizes a type III secretion mechanism to kill D. discoideum. Cytotoxin ExoU contributes to the pathogenicity of P. aeruginosa PA103 toward D. discoideum, because PA103 mutants that are defective in type III secretion, or production of cytotoxin ExoU are avirulent toward D. discoideum. ExoU is a 71-kDa protein that, once translocated into a host cell, causes lysis of the host cell within a few hours (17, 31, 36, 37). Isogenic mutants that do not produce or secrete ExoU are defective in virulence in a mouse model of pneumonia (17, 36). The mechanism by which ExoU induces rapid lysis is not yet known. The use of D. discoideum as a host model may lead to the identification of cellular host components that are targeted by ExoU.
Most P. aeruginosa isolates from cystic fibrosis patients lack the exoU gene or are unable to secrete the toxin, whereas a higher proportion of strains isolated from corneal infections are reported to contain ExoU (38–40). This finding suggests that P. aeruginosa strains that use ExoU as a virulence factor are readily cleared from the lungs of cystic fibrosis patients. The ExoU cytotoxin may induce an unusually strong host response that results in rapid clearing from the lungs. Identification of the host targets of ExoU may reveal a signaling pathway that can be stimulated with existing or novel drugs. Increasing the host response in such a manner may help cystic fibrosis patients to clear infections with P. aeruginosa that lack ExoU.
The work presented here suggests that D. discoideum represents a permissive host for P. aeruginosa infections. Using D. discoideum as a genetically tractable model host takes advantage of the organism's ability to integrate complicated activities in a single cell, which includes responses to pathogens. A number of different genetic manipulations, including insertional mutagenesis, chemical mutagenesis, complementation, and cloning, are available to identify mutations that confer resistance to Pseudomonas infections (for a review of available methods, see ref. 5). For example, insertional mutagenesis by restriction-enzyme-mediated integration (REMI) may introduce null mutations that result in the genetic removal of proteins from cells (41). If such a protein is a host component that is targeted by a particular virulence factor, its removal may confer resistance to the pathogen. Identification of the affected gene is straightforward because the gene can be readily cloned because of the insertion of a selectable plasmid during the REMI procedure. With such a genetic approach, two major virulence pathways, quorum-sensing-mediated virulence and type III secretion of cytotoxins, can be studied in more detail from the host's perspective.
Acknowledgments
We are grateful to Herbert Ennis, Jakob Franke, Felipe B. Manalo, Grant P. Otto, Mary Wu, and members of the Mekalanos laboratory for helpful discussions, and to Su Chiang for critically reading the manuscript. We thank Lynne Garrity-Ryan (University of California, San Francisco) and Joanne Engel for P. aeruginosa strain PA103 and its isogenic mutants, as well as Fred Ausubel for P. aeruginosa strain PA14 and its isogenic mutants. We thank Bradley Britigan (University of Iowa) and Barry Schniep (Jeneil Biosurfactant Co., Saukville, WI) for their kind gifts of purified pyocyanin and rhamnolipids, respectively. We also thank Urs Ochsner (University of Colorado Health Sciences Center) for knockout plasmid pEX100T-rhlA∷Gm. This work was supported by National Institutes of Health Grant GM33136–20 (to R.H.K.) and Grant AI26289 (to J.J.M.), as well as by a grant from the Cystic Fibrosis Foundation (to S.U.P.).
References
- 1.Deretic V. In: Persistent Bacterial Infections. Nataro J P, Blaser M J, Cunningham-Rundles S, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 305–326. [Google Scholar]
- 2.Govan J R, Deretic V. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Nature (London) 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan-Miklos S, Rahme L G, Ausubel F M. Mol Microbiol. 2000;37:981–988. doi: 10.1046/j.1365-2958.2000.02056.x. [DOI] [PubMed] [Google Scholar]
- 5.Kessin R H. Dictyostelium—Evolution, Cell Biology, and the Development of Multicellularity. Cambridge, U.K.: Cambridge Univ. Press; 2001. [Google Scholar]
- 6.Solomon J M, Rupper A, Cardelli J A, Isberg R R. Infect Immun. 2000;68:2939–2947. doi: 10.1128/iai.68.5.2939-2947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomis W F. Exp Cell Res. 1971;64:484–486. doi: 10.1016/0014-4827(71)90107-8. [DOI] [PubMed] [Google Scholar]
- 8.Sussman M. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- 9.Tan M W, Rahme L G, Sternberg J A, Tompkins R G, Ausubel F M. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan-Miklos S, Tan M W, Rahme L G, Ausubel F M. Cell. 1999;96:47–56. doi: 10.1016/s0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 11.Rahme L G, Stevens E J, Wolfort S F, Shao J, Tompkins R G, Ausubel F M. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 12.Schweizer H P. Gene. 1991;97:109–121. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 13.Rahim R, Ochsner U A, Olvera C, Graninger M, Messner P, Lam J S, Soberon-Chavez G. Mol Microbiol. 2001;40:708–718. doi: 10.1046/j.1365-2958.2001.02420.x. [DOI] [PubMed] [Google Scholar]
- 14.Schweizer H P, Hoang T T. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 15.Kang P J, Hauser A R, Apodaca G, Fleiszig S M, Wiener-Kronish J, Mostov K, Engel J N. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 16.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K E, Wiener-Kronish J. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser A R, Kang P J, Engel J N. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambello M J, Iglewski B H. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essar D W, Eberly L, Hadero A, Crawford I P. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuqua C, Winans S C, Greenberg E P. Annu Rev Microbiol. 1996;50:727–551. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 23.O'Toole G A, Kolter R. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.Ochsner U A, Koch A K, Fiechter A, Reiser J. J Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pesci E C, Pearson J P, Seed P C, Iglewski B H. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Delden C, Iglewski B H. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornillon S, Pech E, Benghezal M, Ravanel K, Gaynor E, Letourneur F, Bruckert F, Cosson P. J Biol Chem. 2000;275:34287–34292. doi: 10.1074/jbc.M006725200. [DOI] [PubMed] [Google Scholar]
- 28.Galan J E, Collmer A. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 29.McGuffie E M, Frank D W, Vincent T S, Olson J C. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahr T L, Vallis A J, Hancock M K, Barbieri J T, Frank D W. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauser A R, Engel J N. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michiels T, Vanooteghem J C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G R. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauser A R, Fleiszig S, Kang P J, Mostov K, Engel J N. Infect Immun. 1998;66:1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahr T L, Mende-Mueller L M, Friese M B, Frank D W. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finck-Barbancon V, Yahr T L, Frank D W. J Bacteriol. 1998;180:6224–6231. doi: 10.1128/jb.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 37.Evans D J, Frank D W, Finck-Barbancon V, Wu C, Fleiszig S M. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dacheux D, Toussaint B, Richard M, Brochier G, Croize J, Attree I. Infect Immun. 2000;68:2916–2924. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser A R. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 40.Lomholt J A, Poulsen K, Kilian M. Infect Immun. 2001;69:6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuspa A, Loomis W F. Proc Natl Acad Sci USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]