Abstract
Cryptococcus neoformans (CN), an encapsulated, ubiquitous environmental yeast, is pathogenic for humans, primarily those with compromised immune function. CN is believed to be a facultative intracellular pathogen. Time-lapsed video microscopy revealed that yeast began to replicate and divide 2 hours after ingestion by J774.16 macrophage cells, with the average cell hosting 10–40 organisms of varying morphologies before ultimately lysing and releasing organisms, either singly or in clumps. Intracellular growth was accompanied by the accumulation of polysaccharide-filled vesicles in the macrophage. Studies with fluorescently labeled dextran revealed that the phagolysosomal compartment became leaky during the course of intracellular infection. Consistent with this observation, phagosomes containing CN had an increased pH relative to similar phagosomes containing inert magnetic beads, as indicated by a colorimetric change in the pH-sensitive Lysosensor dye. Immunocytochemistry revealed differences in the reactivity of polysaccharide elaborated by CN inside macrophages relative to that expressed in vitro. Taken together these results are suggestive of a novel mechanism of intracellular survival by an encapsulated organism, whereby ingestion is followed by damage to the phagosomal membrane resulting in continuity with the cytoplasm, accumulation of polysaccharide-containing vesicles, and possibly, production of a structurally different polysaccharide.
Cryptococcus neoformans (CN) is the causative agent of cryptococcosis, a disease that predominantly afflicts immunocompromised hosts but that can occur in healthy individuals as well (reviewed in ref. 1). This yeast is ubiquitous in the environment and is acquired through inhalation of desiccated particles. Infection may be accompanied by dissemination and clinical disease that usually manifests itself as meningitis. The available antifungal therapy is often inadequate for eradicating the infection in individuals with impaired immune function. With the AIDS epidemic and advances in organ transplant technology, there has been an ever increasing population of individuals with compromised immunity, resulting in a large pool of susceptible individuals.
CN is unique among the pathogenic fungi in that it has a polysaccharide capsule that is required for virulence. The capsule is antiphagocytic under in vitro conditions without opsonins and immunosuppressive in vivo. The role of the capsule in promoting virulence is complex and includes immunosuppressive effects resulting from shed polysaccharides (reviewed in ref. 1). After ingestion, CN elaborates its capsule, resulting in larger phagosomes. This action potentially serves to dilute lysosomal contents and it provides a physical separation between the surfaces of the yeast cell and the phagosomal membrane where microbicidal compounds are released. However, electron microscopic studies of infected tissue suggest that the capsule may actively function as an intracellular aggressin. CN sheds its polysaccharide in the intracellular confines of the macrophage and proceeds to fill lysosomes that have fused to the CN-containing compartment as they empty their lysosomal hydrolases. This process continues until the host cell is full of polysaccharide, which when cross-sectioned for microscopic study lends the host cell the appearance of having many holes. Thus, these cells were referred to as Hueco cells, where “hueco” translates from “hole” in Spanish (2). One of the hallmarks of the disease is the accumulation of the polysaccharide in the extracellular spaces (reviewed in ref. 1).
These observations have refocused attention on the contribution of intracellular parasitism to the pathogenesis of infection (3). Comparison of the intracellular survival strategy of C. neoformans to that of other intracellular pathogens suggest that it subverts macrophage function through a unique mechanism that has no parallel among other organisms that have been studied. Unfortunately, in vivo studies are necessarily limited to static observations at discrete intervals and are not amenable to dissecting the mechanism(s) by which CN survives in macrophages. Hence, it is important to develop in vitro systems that reproduce the features of in vivo infection to study the pathogenic process. In the present study we provide unequivocal evidence that CN can reproduce inside macrophages in a process that is associated with formation of a leaky phagosome and accumulation of cytoplasmic vesicles filled with capsular polysaccharide. The results indicate that CN subverts macrophage fungicidal mechanisms through a unique mechanism for intracellular pathogenesis.
Materials and Methods
Yeast Strains and Growth Conditions.
CN strains 24067, serotype D (American Type Culture Collection), and H99, serotype A (courtesy of Gary Cox, Duke University), were grown in Sabouraud dextrose broth (Difco) at 30°C with moderate shaking to stationary phase (about 30–48 h.).
Cell Lines and Culture Media.
J774.16 (ATCC) is a murine (BALB/c, haplotype H-2d) macrophage-like cell line derived from a reticulum sarcoma (4). Cells were maintained at 37°C in 10% CO2 in DMEM (Life Technologies, Rockville, MD) that was supplemented with 10% heat-killed FCS (Harlan Bioproducts, Indianapolis), Cellgro 1× nonessential amino acids (Mediatech, Herndon, VA), Cellgro 100 μg/ml penicillin/streptomycin, and 10% NCTC-109 medium (Life Technologies). The cell line was used between 4 and 15 passages.
Isolation of Murine Alveolar Macrophages.
Male BALB/c mice (The Jackson Laboratory) were killed, and a 20-gauge Angiocath (Becton Dickinson) was inserted and sewn into the trachea as described (5). Lungs were lavaged 5–10 times with an infusion of 0.8 ml of cold, Ca+2, and Mg+2-free Hanks' balanced salt solution (HBSS). Washed cells were suspended in 1 ml of 0.17 M ammonium chloride (pH 7.2) and incubated at room temperature for 10 min to lyse red blood cells. Cells were then washed twice with 50 ml of HBSS as above and suspended in 1 ml of HBSS per mouse used. Viable cells were counted as those that excluded the dye Trypan blue. Cells were maintained in culture and used within 24 h of harvest.
Macrophage Phagocytosis Assay.
Approximately 18–24 h before the assay, macrophages were plated at a density of 1–3 × 105 cells per ml in culture media as above. Stationary CN cells were washed 3 times in PBS and counted in a hemacytometer. Yeast were added to cultured cells at a multiplicity of infection of 1:1 or 2:1 in the presence of 10 μg/ml of mAb 18B7, which binds to the capsular polysaccharide and is opsonic (6), or 10% human serum. mIFNγ (Genzyme or Roche Molecular Biochemicals) and lipopolysaccharide (LPS) (Sigma) were added at 50 units per ml and 0.3 μg/ml, respectively. Phagocytosis was allowed to proceed at 37°C for 30 min or 1 h in 10% CO2. For live studies, extracellular yeast cells were removed by three successive washes with regular culture feeding medium without LPS or mIFNγ. The monolayer was then maintained in feeding medium for various intervals before microscopy. For immunofluorescence/histochemistry studies, the infected monolayer was washed free of extracellular yeast with PBS and fixed with formaldehyde (see below). Magnetic beads conjugated with 18B7 were added similarly. mAb 18B7 was linked to M-280 tosyl-activated Dynabeads (Dynal, Great Neck, NY) as per the manufacturer's instructions.
Immunofluorescence Staining of Cultured Cells on Coverslips.
Infected or treated cells [where infections or treatments were performed on glass coverslips in MaTek (Ashland, MA)-brand dishes] were washed with PBS to remove trace serum/medium. Monolayers were fixed with 3.7% formaldehyde in 1× Fix Buffer pH 7.2 (2×: 10 mM KCl/274 mM NaCl/8 mM NaHCO3/0.8 mM KH2PO4/2.2 mM Na2HPO4/4 mM MgCl2/10 mM Pipes, pH 7.2/4 mM EGTA/11 mM glucose) for 5 min at 37°C. Monolayers were then permeabilized for 20 min with 0.5% Triton X-100 at 25°C. To quench autofluorescence, cells were incubated in 0.1 M glycine for 10 min. Conjugation of Alexa 488 dye to mAb 18B7 was as per the manufacturer's instructions (Molecular Probes). Ab was added at 1:500 dilution for 1 h at 25°C, followed by 3 washes with PBS.
Immunochemistry.
Infected J774 cells were fixed as above and incubated with 5% goat serum in PBS for 1 h. After 2 washes in PBS, developing anti-glucuronoxylomunnan (GXM) Ab 3E5 (IgGγ3κ) was added at 2.6 μg/ml and incubated overnight. Monolayers were washed twice with PBS, and horseradish peroxidase-conjugated goat anti-mouse IgG3 was added at 1/250 dilution for 1 h at room temperature. After two PBS washes, the monolayer was developed with diaminobenzidine.
Microscopic Imaging.
Live imaging was performed with a Photometrics SenSys cooled charge-coupled device (CCD) camera (Roper Scientific, Munich) on an Olympus (New Hyde Park, NY) IX70 microscope. The temperature was kept constant at 37°C by a heated stage and heated air unit supplied by Olympus. The Plexiglas environmental surrounding the microscope was kept under positive pressure with 5% CO2/95% air. Imaging software was ip lab spectrum (Scanalytics, Fairfax, VA) running on a Macintosh G3. Magnification was ×60-×90. Images were stacked with Scion (Frederick, MD) image software. Confocal images were collected with a Bio-Rad Radiance 2000 laser scanning confocal microscope and manipulated in Scion image software to generate three-dimensional projections. Samples for transmission electron microscopy were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through immersion in a graded series of ethanol, and embedded in LX112 resin (Ladd Research Industries, Burlington, VT). Ultrathin sections were cut on a Reichert Ultracut E, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 1200EX transmission electron microscope at 80 kV.
Microinjection of J774.16.
Gauge no. 5 needles were pulled from Sutter Instruments (Novato, CA) glass (BF100-78-10). The transjector (model 5246) and micromanipulator (model 5171) were from Eppendorf (Brinkmann). The injection pressure was 100 hPa, and the injection time was 0.25 sec.
Pinocytic Loading of Dyes and Tracers into J774 Cells.
Lysosensor Yellow/Blue DND-160 (Molecular Probes) was added at a 1:300 dilution of 1 mM stock (MW, 366.42) to cell monolayers (7 × 105 cells per dish) grown in standard tissue culture media lacking the pH indicator phenol red (Life Technologies) and allowed to incubate overnight. Monolayers were washed with media before performing the phagocytosis assay. Lysosensor Yellow/Blue is excited in the UV range, and the resultant emissions can be captured through a long pass filter that includes blue wavelengths from 420 to 450 nm. Color-sensitive film (Elite Chrome ISO400) from Kodak substituted for the lack of such a filter block, and slides were scanned to Adobe Systems (Mountain View, CA) photoshop. Dextran 3000, Texas red (Molecular Probes) was added similarly.
Results
Ingestion of CN by Either J774.16 Cells or Alveolar Macrophages Results in Intracellular Replication and the Appearance of Polysaccharide-Containing Vesicles in Host Cell Cytoplasm.
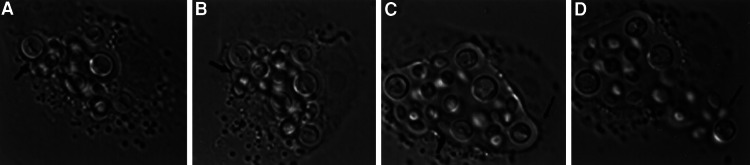
Adding CN opsonized with 18B7 to J774 cells at a ratio that limited the number of CN to approximately one per host cell allowed us to study the fate of the internalized yeast over the course of a day by obtaining successive images at 30-sec or 1-min intervals. Compiling these images with ip lab or Scion image software created animated movies. Several observations were made about the phagocytic process and the outcome of the infection. Phagocytosis of Ab-opsonized CN proceeded rapidly such that yeast cells were internalized by macrophages in ≈1.5 min. Phagocytosis was accompanied by a rapid migration of host cell vesicles to the macrophage surface in contact with the yeast cell. In many instances, the host cell seemed to release its focal contacts from the underlying dish to engulf the fungal cell. Thereafter, the macrophages flattened back onto the dish but remained mobile. Frustrated phagocytosis was also observed, as the fungal cells seemed to adhere to the macrophage and then to glide back and forth along the perimeter of the host cell before floating away into the surrounding medium. Approximately 2 h after ingestion, there was an onset of intracellular cryptococcal budding (Fig. 1 and Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org), which occurred at approximately the same time as those CN that had remained extracellular. The rate of the actual budding event varied but was also rapid. Budding was accompanied by a morphogenic switch from symmetrical to elongated bud growth, such that two patterns of bud formation and bud growth were observed within the same phagosome; unipolar budding with elongated bud growth, and bipolar budding with round “all-over-the-bud” growth (7, 8).
Figure 1.
Intracellular budding of C. neoformans strain 24067 in J774 cell. This figure represents individual frames from a composite movie (Movie 1). Arrows indicate CN budding site.
J774 cells were mixed with CN opsonized with either human serum or IgG (18B7-mouse mAb specific to the capsular polysaccharide) and then examined by direct immunofluorescence and histochemistry for the appearance of vesicles containing polysaccharide (Fig. 2 and figure 3 in ref. 3). Vesicles of uniform shape that were positive for CN polysaccharide were present at 18 and 28 h after infection and were dispersed throughout the host cell cytoplasm as well as around the CN-containing phagosome. The vesicles were found at higher density near the CN-containing phagosome.
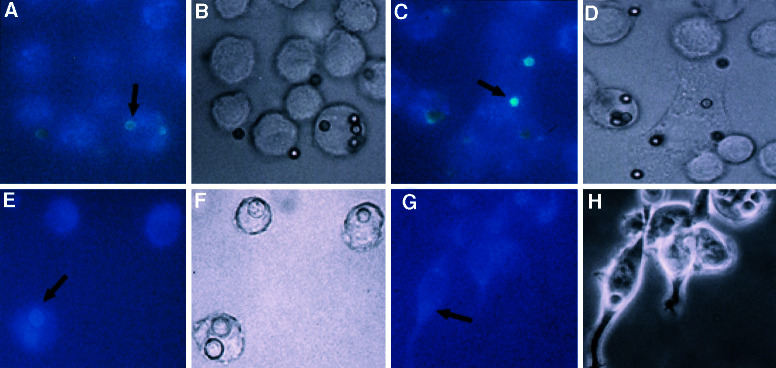
Figure 2.
Intracellular shedding and accumulation of C. neoformans polysaccharide 18 h after phagocytosis. (A) Phase image of infected cell. (B) Corresponding fluorescent image. Immunodetection of CN polysaccharide was with fluorescently labeled primary Ab to GXM. CN was ingested by means of opsonization with human complement. (C) Uninfected control monolayer of J774 cells. (D) Horseradish peroxidase staining of cells infected with CN strain 24067. CN was ingested by means of opsonization with GXM-specific Ab (IgG1) followed by detection with GXM-specific Ab of a different isotype (IgG3). Arrows in B and D point to released GXM. Original magnification is ×90.
CN Resides in a Phagosome That Becomes Permeable to Cytoplasmic Contents.
Electron microscopy (EM) of infected mouse lung (2) and J774 cells revealed discontinuities in the phagosomal membrane surrounding engulfed CN in cells where other organelle membranes were intact (Fig. 3). Although strongly suggestive of membrane disruption, this observation was not conclusive because the phagosomal membrane may have been more vulnerable to disruption by EM preparation. To investigate the integrity of the phagolysosomal membrane in living cells, we used a strategy similar to that used to study the permeability of the Mycobacterial phagosome that relies on the addition of fluorescent tracers (9). By using live imaging techniques, vesicles preloaded with fluorescently tagged dextran (MW, 3,000) were followed as they fused and merged their contents with the CN-containing phagosome shortly after phagocytosis. Fusion was apparent by the bright ring of fluorescence that encircled the fungal cell with time (Fig. 4A). The fluorescent signal of selected fields had a tendency to photobleach within 5 min of observation. However, observations were made of multiple fields from duplicate dishes. Serial images revealed a qualitative change in the location and intensity of the fluorescent signal. Initially the signal was localized primarily to the CN-containing phagosome. However, with time a fluorescent haze developed in the vicinity of the phagosome that diffused throughout the cell cytoplasm and was visible at multiple focal planes throughout the cell. Furthermore, dimming of the CN-containing phagosome and vesicles associated with the phagosome occurred at a rate that differed from collective photobleaching, indicating that the attenuation of signal in the phagosome was a different phenomenon. Additionally, CN-containing phagosomes in dextran-microinjected host cells were compared with the actual phagosome as seen in phase contrast to determine the area of fluorescence exclusion. Measurements indicate that there was an influx of labeled dextran into the phagosome because the diameter of the phagosome in phase contrast is larger than the apparent diameter in the same phagosome observed under fluorescence and measured in the same plane (Fig. 4 C and D). The efflux of CN-derived polysaccharide was also observed to occur as seen by a time course evaluation with three-dimensional reconstruction of serial confocal images (Fig. 4 E and F; supporting Movies 2 and 3).
Figure 3.
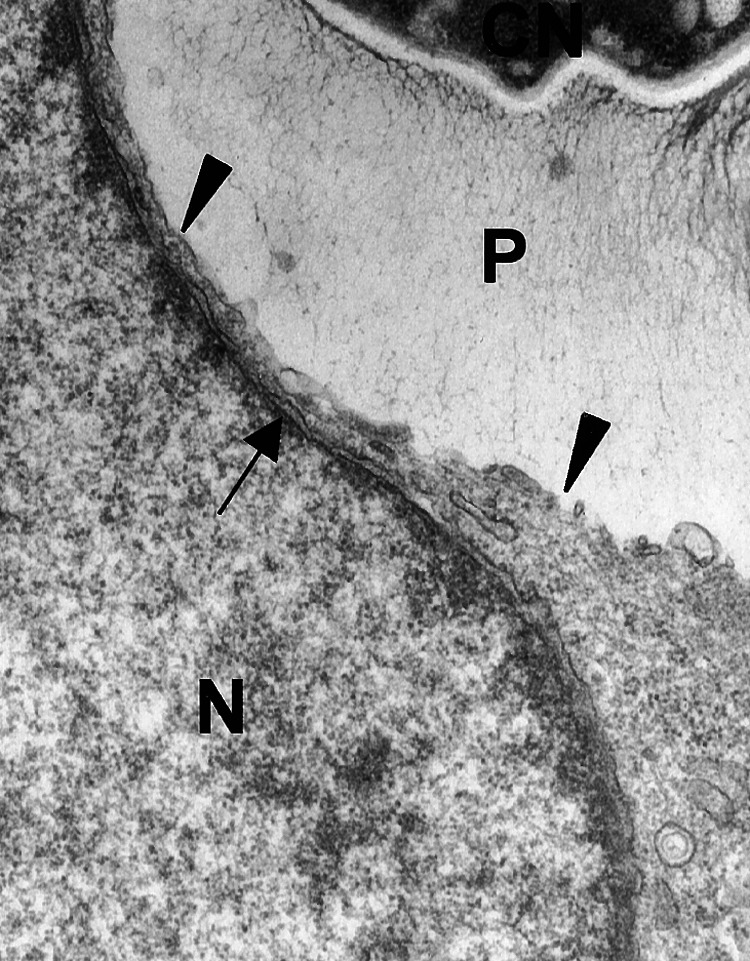
Dissolution of J774 phagosomal membrane by intracellular C. neoformans opsonized with complement (20 K magnification). Left arrowhead indicates an area of in tact phagosomal membrane. Right arrowhead points to dissolved phagosomal membrane. Arrow points to nuclear membrane that is intact. P, polysaccharide; N, host cell nucleus.
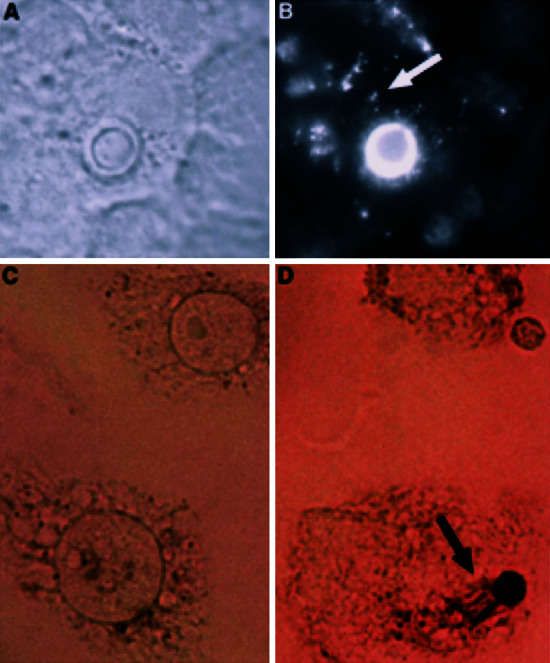
Figure 4.
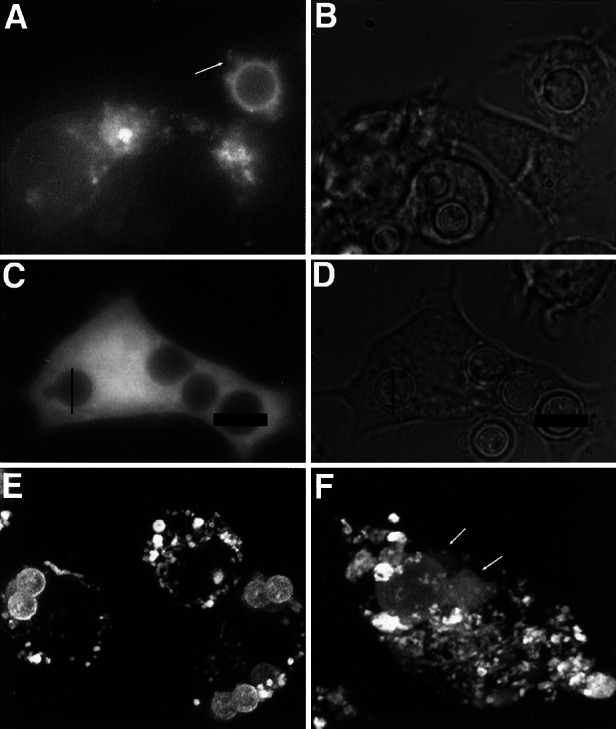
Phagosomes containing C. neoformans become permeable to macromolecules. (A) Fluorescence image of J774 cells that have taken up fluorescent dextran by means of pinocytosis. Arrow points to dextran leaking from CN-containing phagosome. (B) Phase image of A. (C) Fluorescence image of infected J774 cell microinjected with fluorescent dextran. (D) Phase image of C. The diameter of two CN-containing phagosomes has been traced with bars and layered onto the fluorescence image of the same plane of focus for comparison. (E) Stacked z-series of confocal fluorescence images of inert beads within intact phagosome. (F) Similar confocal series of CN-containing phagosome where tongue-like projections of leaking polysaccharide are indicated by arrows. (Movies 2 and 3 correspond to E and F, respectively.)
A secondary method to evaluate the integrity of the phagosomal membrane was used. The pH of the phagosome was examined, with the premise that the acidic milieu in this compartment could not be maintained if the membrane was damaged. J774 cells in culture were supplemented with a pH-sensitive dye that is commercially available. Inert beads were chosen as a control based on the work of Nyberg et al. (10). At early time intervals both intracellular beads and CN fluoresced yellow, indicating that they were in the acidic environment of the phagolysosome (Fig. 5 A and E). As expected, compartments containing inert beads remained yellow with time (Fig. 5 A and C). However, the color of the CN-containing phagosomes changed to the blue hue of the more neutral cytoplasmic compartment (Fig. 5 E and G). The inability of CN-containing phagosomes to maintain an acidic pH, together with the dextran migration studies, indicates continuity between the cytoplasm and the phagosome that implies a leaky phagolysosomal membrane.
Figure 5.
C. neoformans phagosome becomes less acidic with time as determined by changes in pH indicator color. J774 cells with ingested magnetic beads at 30 min and 3 h (A and C, respectively); corresponding phase images (B and D). Arrows in A and C point to beads. J774 cells with intracellular CN at 30 min and 3 h (E and G, respectively); corresponding phase images (F and H). Arrows in E and G point to CN. Yellow indicates acidic pH.
Intracellular Replication Leads to Host Cell Lysis and Production of Qualitatively Different Polysaccharide.
After 6–18 h of infection, depending on the field observed, the host cells lysed releasing fungal cells into the media (Fig. 6; supporting Movie 4). The lysis event occurs suddenly in cells that are alive and functional and seems to be the result of catastrophic stretching of the phagolysosomal membrane by proliferating yeast cells in close proximity to the cell membrane. Cells containing CN-filled phagosomes continued to function with regard to phagocytic capacity and motility. Two strains (H99 and 24067) representing serotypes A and D, respectively, were observed to replicate intracellularly and caused macrophage lysis in this study. Interestingly, 24067 cells that had replicated intracellularly were released from the host cell as a clumped mass that either remained attached to the lysed host cell or drifted away as a clump. However, H99 released from lysing macrophages floated in all directions as single cells (data not shown and ref. 11).
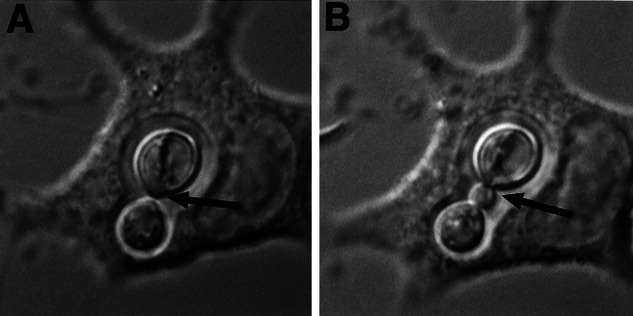
Figure 6.
Intracellular growth of CN strain 24067 in mouse primary alveolar macrophage leads to host cell lysis. A–D represent single frames from Movie 4. Arrows indicate morphological switch to oval budding pattern (A–C) and rupture of host cell in D (Movie 4).
While examining J774 cells for Hueco formation (see above), it was noted that the staining pattern of the specific Ab directed to the capsular polysaccharide of CN was more diffuse and punctate than the smooth annular pattern seen on the organism before it enters the macrophage. Sloughing of polysaccharide within the phagosome was also apparent (data not shown).
Progression of CN Intracellular Pathogenesis Events.
On the basis of these in vitro results and several prior observations we propose a model by which CN survives inside macrophages after ingestion, lysosomal fusion, and phagolysosomal maturation (Fig. 7). Our results indicate and are consistent with a process whereby ingestion follows residence in a phagosome that acidifies. After several hours the phagosome becomes leaky with an increase in pH, polysaccharide-filled vesicles accumulate in the cytoplasm, and internalized CN replicate. Continued CN intracellular growth results in distended phagosomes filled with yeast cells and polysaccharides that rupture killing the macrophage. The time interval between ingestion and cell rupture requires 8–18 h.
Figure 7.
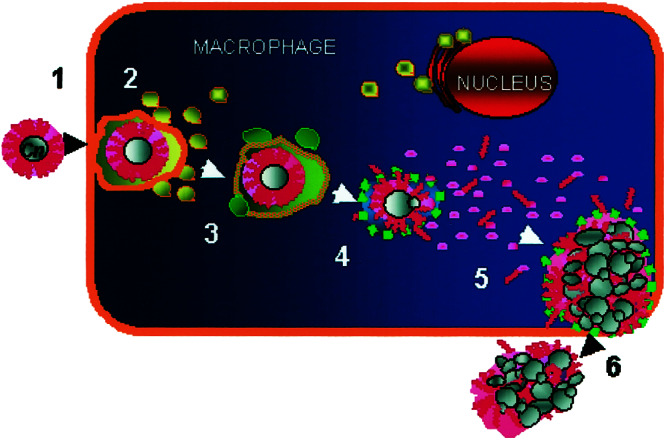
Proposed model of events leading to C. neoformans intracellular survival within macrophages. After phagocytic uptake (1), phagolysosomal fusion occurs (2). Late-stage trafficking markers, such as LAMP-1, are represented in green (3). CN appears to dissolve the phagosomal compartment and intracellular replication ensues (4), while CN-derived polysaccharide builds up within the host cell (5). Ultimately, the host cell ruptures, releasing the newly replicated CN (6).
Discussion
Although CN has been suspected of being an intracellular pathogen for a long time, sufficient evidence has only recently accumulated to indicate that this is true (1, 3, 12). Intracellular parasitism may be particularly important in the pathogenesis of infection because CN is found almost exclusively in macrophages of hosts with chronic or latent infection. Analysis of murine tissues revealed a higher budding index for intracellular organisms and the accumulation of polysaccharide-filled vesicles in the cytoplasm of infected cells (2). To date, all in vitro observations have been limited to the analysis of discrete time intervals that could not address the possibility that accumulation of intracellular yeast reflected continuing phagocytosis or preferential uptake of budding yeast. Such measurements are currently impossible in vivo, although intravital imaging may allow this in the future (13). In this study, we applied time-lapse microscopy to demonstrate unequivocally that CN replicates inside macrophages cultured in vitro with a generation time that is similar to that observed for extracellular yeast cells. Furthermore, this technique allowed us to define the process of intracellular pathogenesis in detail and validate the concept that C. neoformans has a unique strategy for intracellular survival (3).
Time-lapse microscopy revealed that the ingestion process is very rapid, being accomplished in less than 2 min despite the relatively large size of the yeast cell. Prior studies have shown that ingestion is followed by formation of a mature phagosome that expresses LAMP-1 and acidifies and those findings were confirmed here, indicating that CN does not interfere with phagosomal maturation but survives in a mature phagolysosomal vesicle (14). However, dextran diffusion studies and pH measurements revealed that the phagolysosomal membrane becomes leaky subsequent to ingestion of CN by macrophages. This result confirms the validity of the discontinuities observed in the cell membrane of the phagolysosomal vesicle by electron microscopy. In contrast, inert particles were contained in a well-defined phagosome without leakage of labeled dextran into the cytoplasm. By preloading the host cells with a soluble fluorochrome, we were able to examine the influence of CN and inert particles on the pH of the phagolysosomal compartments, thus alleviating the need to conjugate a fluorochrome to the surface of the yeast cell and potentially affecting normal yeast cell function. Three hours after ingestion, the CN-containing phagosomes appeared blue, indicating an increase in pH, whereas those phagosomes containing magnetic beads remained yellow, corresponding to an acidified milieu. Because the vesicles containing inert particles remained intact with regard to pH, it is likely that phagosomal membrane permeabilization reflects microbe-mediated damage. In this regard, CN is known to elaborate enzymes that could damage cell membranes including phospholipases and proteases (15–17).
Our results were similar to several other comparative studies of fungi such as Aspergillus fumigatus, Saccharomyces cerevisiae, and Candida albicans with inert particles (10, 18). The intracellular pH of these fungi-containing phagosomes also increases by 3 h. However, except for S. cerevisiae, the pH reacidifies by 24 h. This decrease in pH at later time points probably reflects intracellular growth of the pathogenic fungi and therefore accumulation of metabolic waste. However, because CN is able to breach the phagosome, accumulation of metabolic waste capable of changing the vesicular pH does not occur.
Electron microscopic studies have established that intracellular residence by C. neoformans is accompanied by the accumulation of polysaccharide-containing vesicles. The origin of these vesicles was uncertain because they could have arisen from the phagosome, pinocytosis of extracellular polysaccharide, or both. In this study we noted that polysaccharide-containing vesicles originate from the phagosome. This phenomenon was evident regardless of whether the opsonin that contributed to phagocytosis was serum or mAb to the polysaccharide. The outcome of intracellular survival and growth was likewise unaffected by the choice of opsonins, as CN was observed to replicate within the host cells regardless of which receptor pathway was stimulated. This observation indicates that the organism may respond to additional signals that are independent of those proinflammatory signals that accompany ligation of Fc gamma receptors (FcγRs) or complement receptor (CR3) C3bi-dependent uptake (19).
Most of the infected macrophages in our study underwent lysis at 24 h after considerable intracellular replication that produced at least 40 yeast per macrophage cell. Permeabilization of the phagosomal cell membrane suggests an explanation for the observation that ingested CN can replicate as rapidly as extracellular yeast cells in vitro, because a leaky membrane would provide access to nutrients in the cytoplasm. Within the timeframe of our studies, the breach was not large enough to liberate replicated yeast cells into the nutrient-rich cytoplasm. However, fluorescently labeled dextran molecules with a molecular weight of 3,000 freely crossed the phagosomal membrane in a bidirectional manner, indicating a wide berth for any number of macromolecules. It is improbable that the yeast internalized enough nutrients from the culture medium to sustain 4–5 rounds of replication during the original phagocytosis event, as they were internalized in 1.5 min via a zippering mechanism as opposed to coiling phagocytosis (20–22).
Intracellular replication was accompanied by the generation of oval-shaped yeast that were morphologically different from those added to the monolayer and ingested in the initial bout of phagocytosis. Initiation of a stress response may have been responsible for the appearance of oval-shaped yeast cells. However, it is unclear whether the morphological differences for cells replicated within the phagosomal compartment, i.e., round bipolar budding pattern vs. the elongated pattern, were a reflection of ploidy or stress response (8, 23, 24). The dimorphic fungus Histoplasma capsulatum is also known to undergo phenotypic variation once inside host cells, where the variant oblong forms are able to persist without killing the resident macrophage (25). CN may respond similarly to the intracellular environment leading to its persistence in vivo. However, in vitro CN continues to replicate until the macrophage is lysed and oblong forms have been noted in host cells that have undergone disruption in vivo (2), indicating that although the yeast can be induced to switch morphology, the outcome is organism-specific. We noted that polysaccharide synthesized inside cells did not stain well with mAb to GXM. This finding suggests that a switch in the type or organization of polysaccharide made accompanies intracellular residence (26). Hence the morphologic changes observed in the phagosome may be associated with the elaboration of a different GXM. The contribution of this morphologic switch to survival in macrophages and host cell lysis is unknown.
By using J774.16 cells we were able to recapitulate in vitro the in vivo observation of polysaccharide-filled vesicles. However, preliminary data from our lab (data not shown) indicates that the organism responds to macrophage extracts by eliciting a large capsule. Therefore, the Hueco phenomenon is likely to be an active process established by the organism in response to a host cell factor(s) that remains to be identified. Given that the CN phagolysosome becomes compromised with time, the polysaccharide may then be free to affect host cell function by altering osmotic conditions within the cell or by directly interacting with host cell products. In this regard, soluble polysaccharide has been shown to affect the water metabolism of glial cells (27, 28) and may have similar effects on J774 cells.
Microbes and parasites alike exercise a variety of strategies to establish a protective intracellular niche (representative literature in refs. 29–32). Most of these strategies, which involve phenotypic modulation of the organism (33) as well as the host (34), evolved to customize the phagosome to the advantage of the microbe. Some like Legionella pneumophila forego fusion with lysosomes in an effort to prevent acidification of their vacuole, whereas others like Listeria monocytogenes use vacuole acidification to activate enzymes that serve to lyse the vacuolar compartment to gain access to the cytoplasm for nutrients (35). Our results indicate that CN uses a qualitatively different strategy than that described for other intracellular pathogens to survive phagocytosis that involves permeabilization of the phagolysosomal membrane coupled with intracellular secretion of capsular polysaccharide into vesicles.
Supplementary Material
Acknowledgments
We thank Michael Cammer and the AECOM Analytical Imaging Facility for assistance. A.C. is supported by National Institutes of Health Awards AI33774, AI3342, and HL-59842-01 and by a Burroughs Wellcome Development Therapeutics Scholar award. S.C.T. is supported in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases Training Grant 5T32AI07506.
Abbreviations
- CN
Cryptococcus neoformans
- GXM
glucuronoxylomannan
References
- 1.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, DC: Am. Soc. Microbiol Press; 1998. [Google Scholar]
- 2.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmesser M, Tucker S, Casadevall A. Trends Microbiol. 2001;9:273–278. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee S, Feldmesser M, Casadevall A. J Infect Dis. 1996;173:1222–1231. doi: 10.1093/infdis/173.5.1222. [DOI] [PubMed] [Google Scholar]
- 5.Feldmesser M, Casadevall A. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 6.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L A, Rivera J, et al. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lew D J, Reed S I. Curr Opin Gen Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 8.Blacketer M J, Madaule P, Myers A M. Genetics. 1995;140:1259–1275. doi: 10.1093/genetics/140.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teitelbaum R, Cammer M, Maitland L, Freitag N E, Condeelis J, Bloom B R. Proc Natl Acad Sci USA. 1999;96:15190–15195. doi: 10.1073/pnas.96.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyberg K, Nessa K, Johansson A, Jarstrand C, Camner P. J Med Vet Mycol. 1996;34:11–17. doi: 10.1080/02681219680000031. [DOI] [PubMed] [Google Scholar]
- 11.Luberto C, Toffaletti D L, Wills E A, Tucker S C, Casadevall A, Perfect J R, Hannun Y A, Del Poeta M M. Genes Dev. 2001;15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond R D, Bennett J E. Infect Immun. 1973;7:231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farina K L, Wyckoff J B, Rivera J, Lee H, Segall J E, Condeelis J S, Jones J G. Cancer Res. 1998;58:2528–2532. [PubMed] [Google Scholar]
- 14.Levitz S M, Nong S H, Seetoo K F, Harrison T S, Speizer R A, Simons E R. Infect Immun. 1999;67:885–890. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer D B, Peberdy J F. Crit Rev Biotechnol. 1997;17:273–306. doi: 10.3109/07388559709146616. [DOI] [PubMed] [Google Scholar]
- 16.Cox G M, McDade H C, Chen S C, Tucker S C, Gottfredsson M, Wright L C, Sorrell T C, Leidich S D, Casadevall A, Ghannoum M A, Perfect J R. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 17.Vidotto V, Sinicco A, Di Fraia D, Cardaropoli S, Aoki S, Ito-Kuwa S. Mycopathologia. 1996;136:119–123. doi: 10.1007/BF00438916. [DOI] [PubMed] [Google Scholar]
- 18.Nessa K, Palmberg L, U J, Malmberg P, Jarstrand C, Camner P. Environ Res. 1997;75:141–148. doi: 10.1006/enrs.1997.3788. [DOI] [PubMed] [Google Scholar]
- 19.Caron E, Hall A. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 20.Aderem A, Underhill D M. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 21.Rittig M G, Wilske B, Krause A. Microbes Infect. 1999;1:727–735. doi: 10.1016/s1286-4579(99)80074-4. [DOI] [PubMed] [Google Scholar]
- 22.Rittig M G, Schroppel K, Seack K-H, Sander U, N′Diaye E-N, Maridonneau-Parini I, Solbach W, Bogdan C. Infect Immun. 1998;66:4331–4339. doi: 10.1128/iai.66.9.4331-4339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alspaugh J A, Davidson R C, Heitman J. Contrib Microbiol. 2000;5:217–238. doi: 10.1159/000060352. [DOI] [PubMed] [Google Scholar]
- 24.San-Blas G, Travassos L R, Fries B C, Goldman D L, Casadevall A, Carmona A K, Barros T F, Puccia R, Hostetter M K, Shanks S G, et al. Med Mycol. 2000;38:79–86. [PubMed] [Google Scholar]
- 25.Groppe Eissenberg L, Poirier S, Goldman W E. Infect Immun. 1996;64:5310–5314. doi: 10.1128/iai.64.12.5310-5314.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fries B C, Goldman D L, Cherniak R, Ju R, Casadevall A. Infect Immun. 1999;67:6076–6083. doi: 10.1128/iai.67.11.6076-6083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano A, Zimmerman H M, Levine S. J Pathol Bacteriol. 1966;91:149–155. doi: 10.1002/path.1700910119. [DOI] [PubMed] [Google Scholar]
- 28.Levine S, Hirano A, Zimmerman H M. Res Publ Assoc Res Nerv Ment Dis. 1968;44:393–423. [PubMed] [Google Scholar]
- 29.Sinai A P, Joiner K A. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 30.Moulder J W. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackstadt T. Traffic. 2000;1:93–99. doi: 10.1034/j.1600-0854.2000.010201.x. [DOI] [PubMed] [Google Scholar]
- 32.Sibley L D, Andrews N W. Traffic. 2000;1:100–106. doi: 10.1034/j.1600-0854.2000.010202.x. [DOI] [PubMed] [Google Scholar]
- 33.Abu Kwaik Y, Harb O S. Electrophoresis. 1999;20:2248–2258. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2248::AID-ELPS2248>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Bliska J B, Galán J E, Falkow S. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 35.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J P. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.