Abstract
A theoretical model is presented that accounts for the facilitation of the pressure dissociation of R17 phage, and for the partial restoration of the concentration dependence of the dissociation, by the presence of subdenaturing concentrations of urea. As an indifferent osmolyte urea should promote the stability of the protein aggregates under pressure, and the decrease in pressure stability with urea concentration demonstrates that such indirect solvent effects are not significant for this case, and that the progressive destabilization is the result of direct protein-urea interactions. By acting as a "homogenizer" of the properties of the phage particles, urea addition converts the pressure-induced deterministic dissociation of the phage into a limited stochastic equilibrium. The model establishes the origin of the uniform progression from the stochastic equilibrium of dimers, to the temperature-dependent and partially concentration-dependent association of tetramers, to the fully deterministic equilibrium observed in many multimers and in the virus capsids.
Full text
PDF

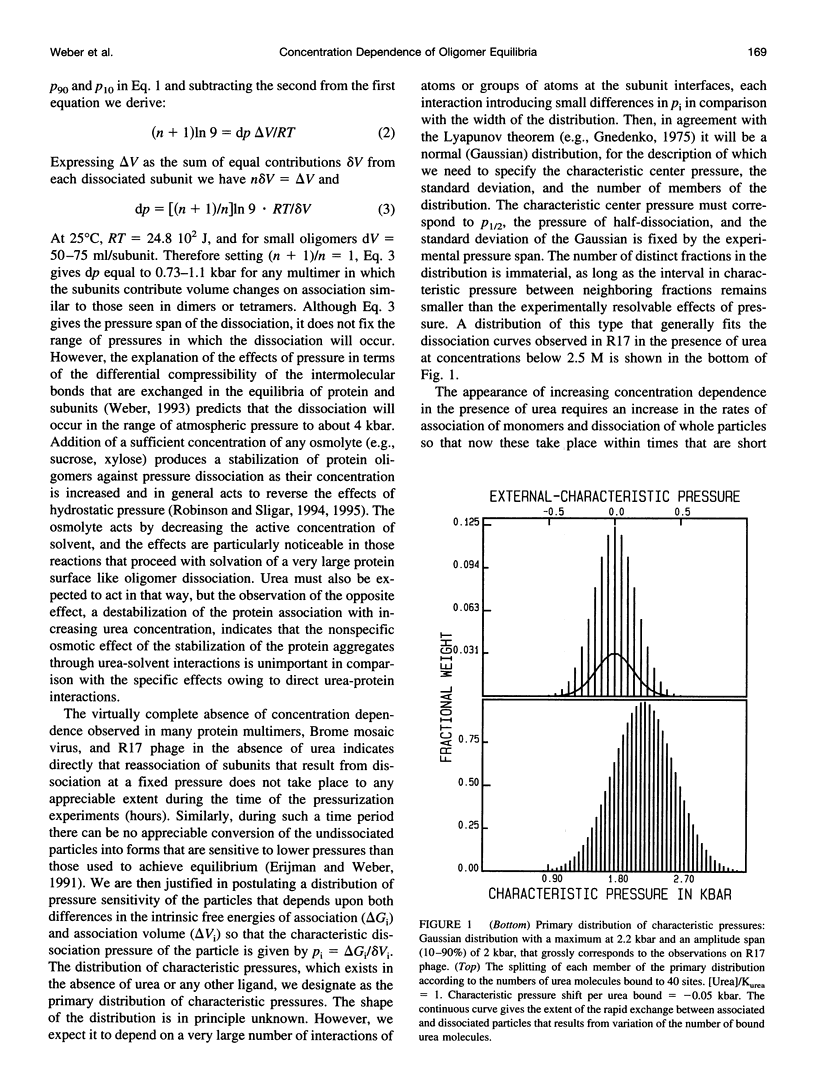
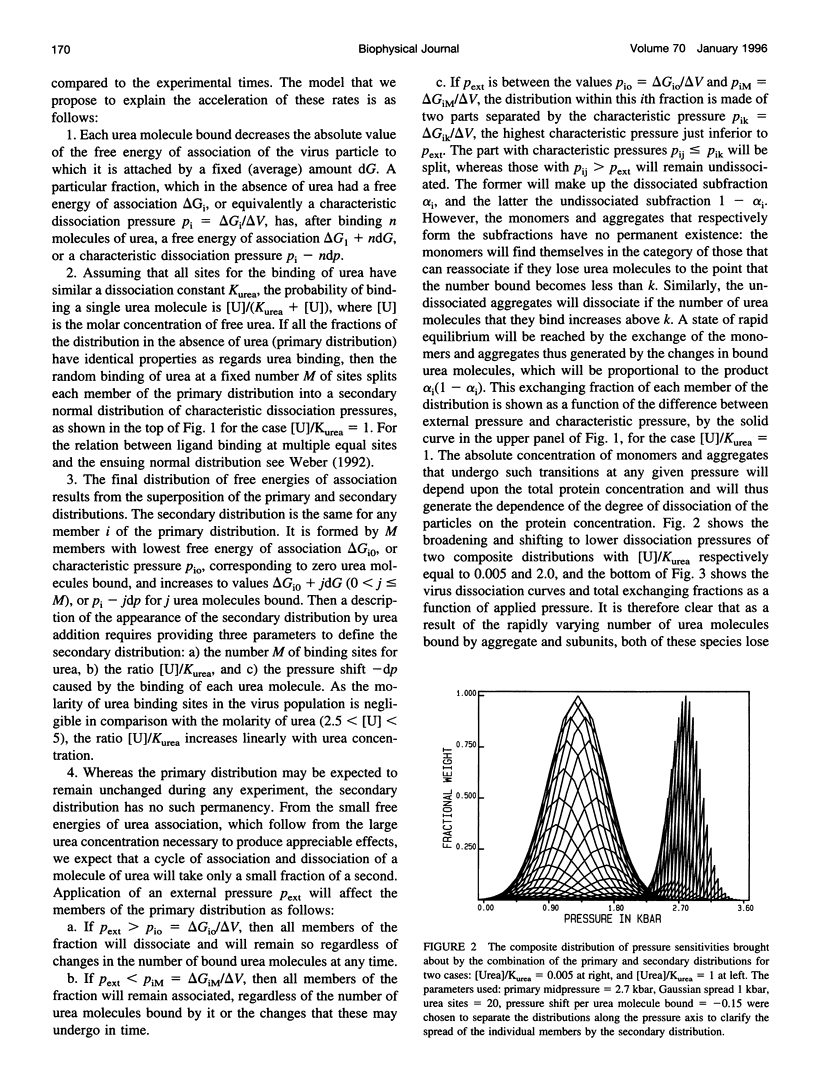
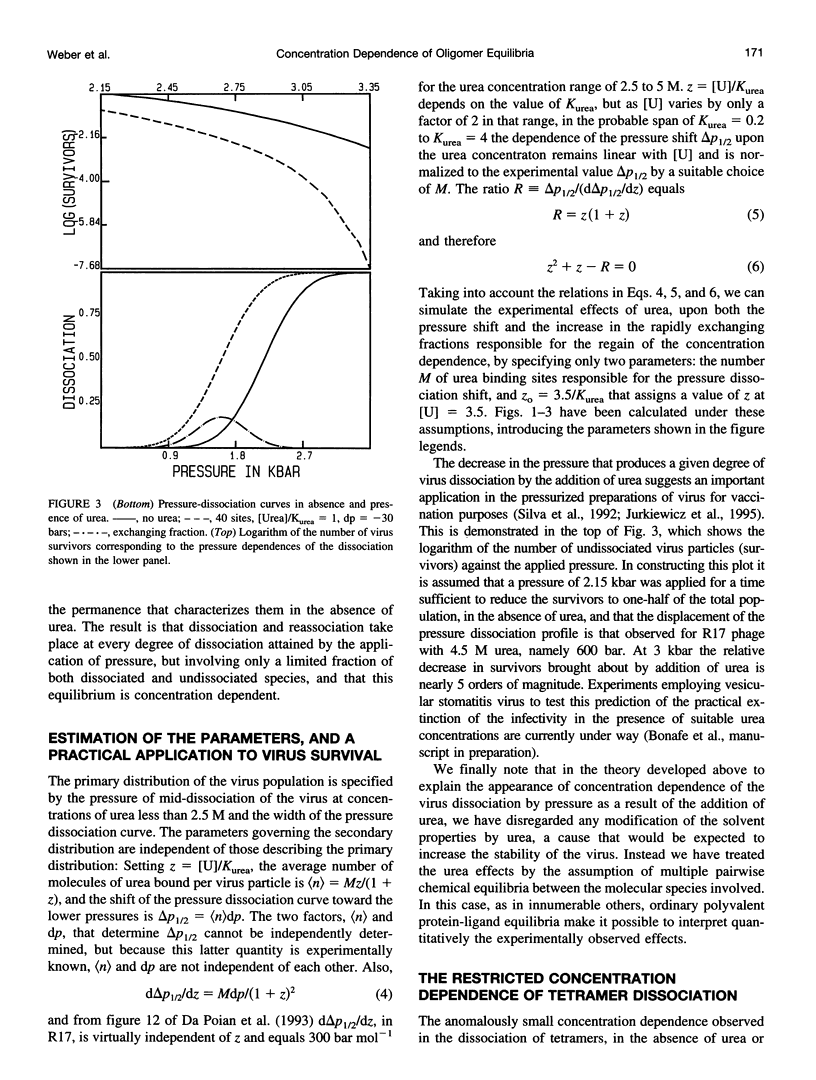


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonafe C. F., Araujo J. R., Silva J. L. Intermediate states of assembly in the dissociation of gastropod hemocyanin by hydrostatic pressure. Biochemistry. 1994 Mar 8;33(9):2651–2660. doi: 10.1021/bi00175a038. [DOI] [PubMed] [Google Scholar]
- Bonafe C. F., Villas-Boas M., Suarez M. C., Silva J. L. Reassembly of a large multisubunit protein promoted by nonprotein factors. Effects of calcium and glycerol on the association of extracellular hemoglobin. J Biol Chem. 1991 Jul 15;266(20):13210–13216. [PubMed] [Google Scholar]
- Da Poian A. T., Oliveira A. C., Gaspar L. P., Silva J. L., Weber G. Reversible pressure dissociation of R17 bacteriophage. The physical individuality of virus particles. J Mol Biol. 1993 Jun 20;231(4):999–1008. doi: 10.1006/jmbi.1993.1347. [DOI] [PubMed] [Google Scholar]
- Erijman L., Lorimer G. H., Weber G. Reversible dissociation and conformational stability of dimeric ribulose bisphosphate carboxylase. Biochemistry. 1993 May 18;32(19):5187–5195. doi: 10.1021/bi00070a030. [DOI] [PubMed] [Google Scholar]
- Erijman L., Weber G. Oligomeric protein associations: transition from stochastic to deterministic equilibrium. Biochemistry. 1991 Feb 12;30(6):1595–1599. doi: 10.1021/bi00220a022. [DOI] [PubMed] [Google Scholar]
- Erijman L., Weber G. Use of sensitized fluorescence for the study of the exchange of subunits in protein aggregates. Photochem Photobiol. 1993 Mar;57(3):411–415. doi: 10.1111/j.1751-1097.1993.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz E., Villas-Boas M., Silva J. L., Weber G., Hunsmann G., Clegg R. M. Inactivation of simian immunodeficiency virus by hydrostatic pressure. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6935–6937. doi: 10.1073/pnas.92.15.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L., Weber G. Conformational drift of dissociated lactate dehydrogenases. Biochemistry. 1986 Jun 17;25(12):3632–3637. doi: 10.1021/bi00360a023. [DOI] [PubMed] [Google Scholar]
- Robinson C. R., Sligar S. G. Hydrostatic pressure reverses osmotic pressure effects on the specificity of EcoRI-DNA interactions. Biochemistry. 1994 Apr 5;33(13):3787–3793. doi: 10.1021/bi00179a001. [DOI] [PubMed] [Google Scholar]
- Ruan K., Weber G. Hysteresis and conformational drift of pressure-dissociated glyceraldehydephosphate dehydrogenase. Biochemistry. 1989 Mar 7;28(5):2144–2153. doi: 10.1021/bi00431a028. [DOI] [PubMed] [Google Scholar]
- Ruan K., Weber G. Physical heterogeneity of muscle glycogen phosphorylase revealed by hydrostatic pressure dissociation. Biochemistry. 1993 Jun 22;32(24):6295–6301. doi: 10.1021/bi00075a025. [DOI] [PubMed] [Google Scholar]
- Silva J. L., Luan P., Glaser M., Voss E. W., Weber G. Effects of hydrostatic pressure on a membrane-enveloped virus: high immunogenicity of the pressure-inactivated virus. J Virol. 1992 Apr;66(4):2111–2117. doi: 10.1128/jvi.66.4.2111-2117.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. L., Miles E. W., Weber G. Pressure dissociation and conformational drift of the beta dimer of tryptophan synthase. Biochemistry. 1986 Sep 23;25(19):5780–5786. doi: 10.1021/bi00367a065. [DOI] [PubMed] [Google Scholar]
- Silva J. L., Villas-Boas M., Bonafe C. F., Meirelles N. C. Anomalous pressure dissociation of large protein aggregates. Lack of concentration dependence and irreversibility at extreme degrees of dissociation of extracellular hemoglobin. J Biol Chem. 1989 Sep 25;264(27):15863–15868. [PubMed] [Google Scholar]
- Silva J. L., Weber G. Pressure stability of proteins. Annu Rev Phys Chem. 1993;44:89–113. doi: 10.1146/annurev.pc.44.100193.000513. [DOI] [PubMed] [Google Scholar]
- Silva J. L., Weber G. Pressure-induced dissociation of brome mosaic virus. J Mol Biol. 1988 Jan 5;199(1):149–159. doi: 10.1016/0022-2836(88)90385-3. [DOI] [PubMed] [Google Scholar]
- Van Holde K. E., Brenowitz M. Subunit structure and physical properties of the hemocyanin of the giant isopod Bathynomus giganteus. Biochemistry. 1981 Sep 1;20(18):5232–5239. doi: 10.1021/bi00521a021. [DOI] [PubMed] [Google Scholar]
- Weber G. Phenomenological description of the association of protein subunits subjected to conformational drift. Effects of dilution and of hydrostatic pressure. Biochemistry. 1986 Jun 17;25(12):3626–3631. doi: 10.1021/bi00360a022. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Association-dissociation equilibria of Octopus hemocyanin. Biochemistry. 1985 Aug 13;24(17):4577–4582. doi: 10.1021/bi00338a014. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Haemocyanins. Q Rev Biophys. 1982 Feb;15(1):1–129. doi: 10.1017/s0033583500002705. [DOI] [PubMed] [Google Scholar]


