Abstract
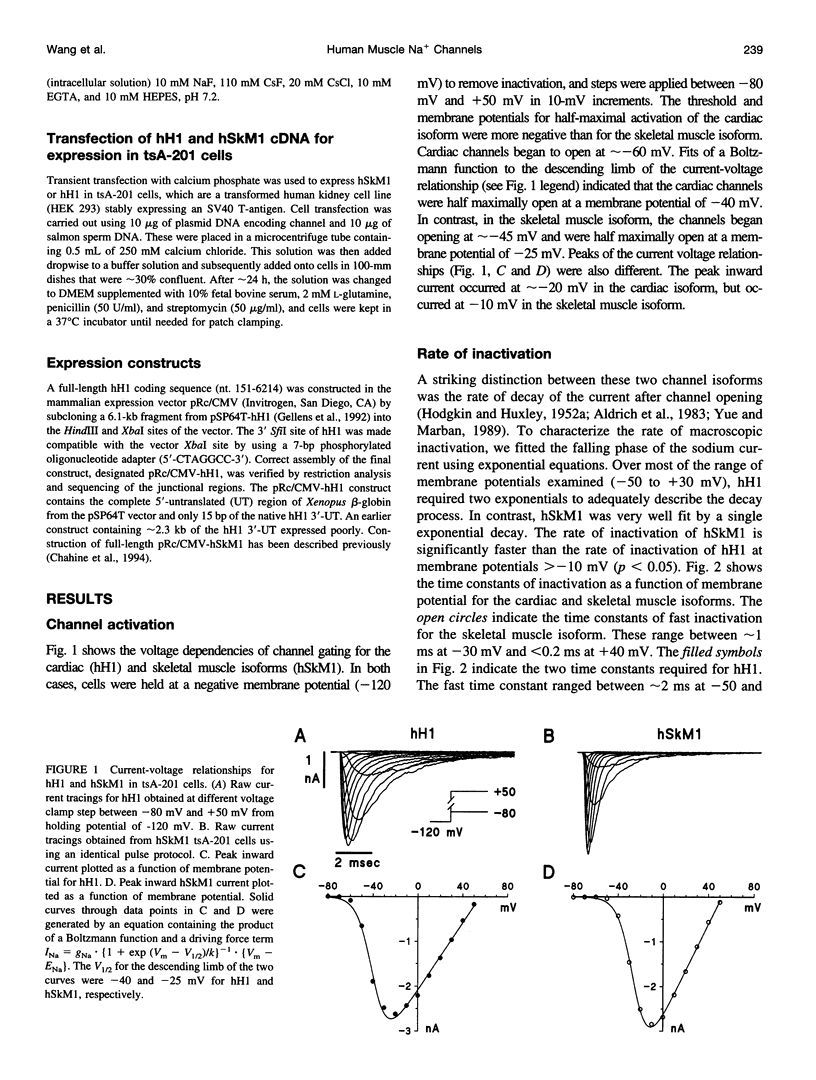
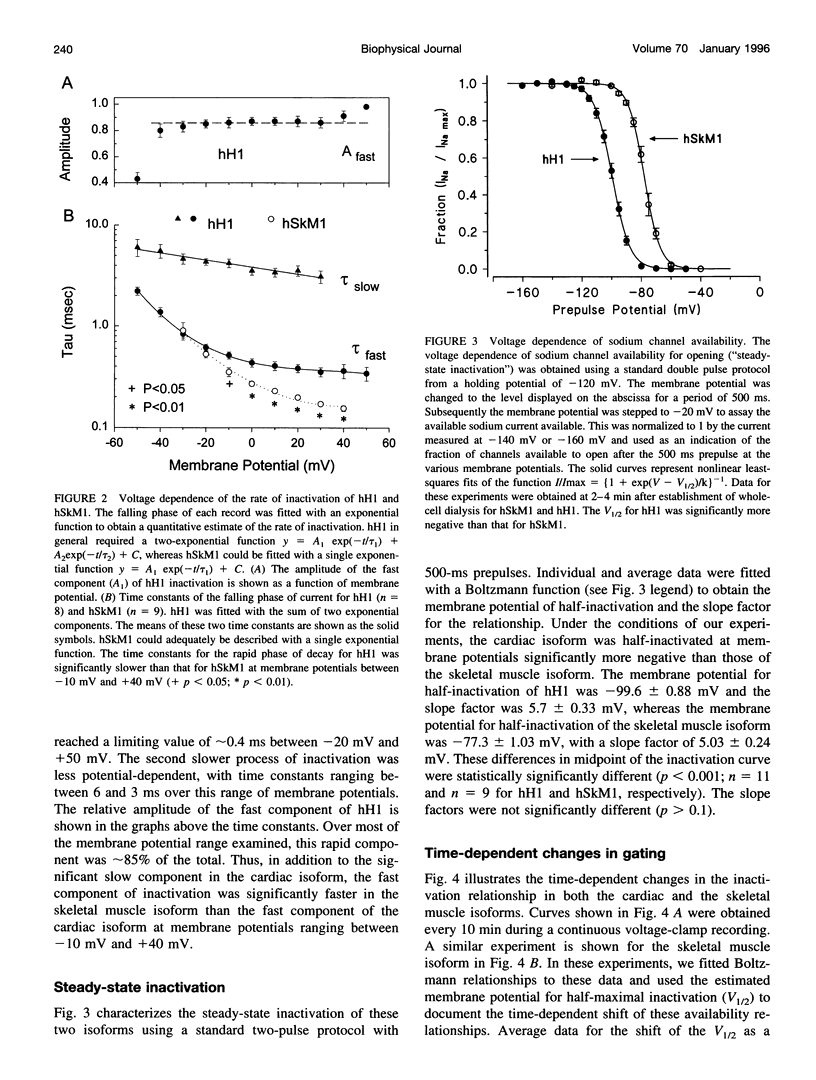
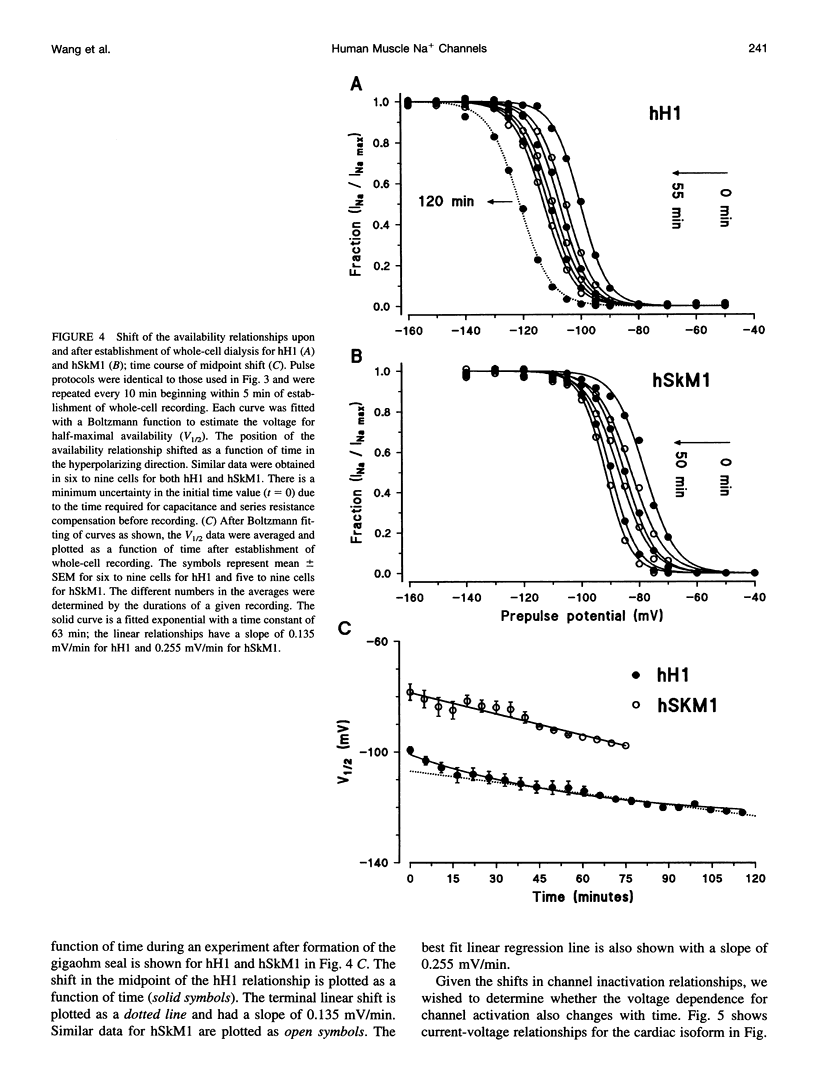
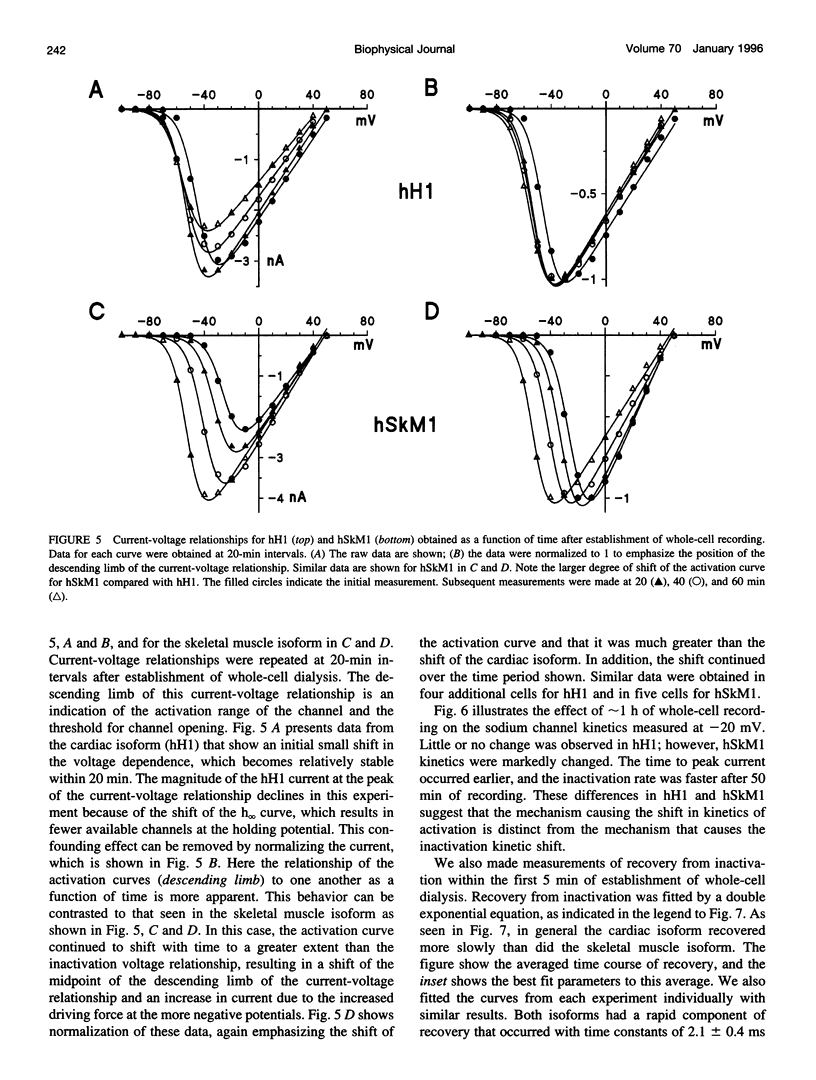
In this study we have expressed and characterized recombinant cardiac and skeletal muscle sodium channel alpha subunits in tsA-201 cells under identical experimental conditions. Unlike the Xenopus oocyte expression system, in tsA-201 cells (transformed human embryonic kidney) both channels seem to gate rapidly, as in native tissue. In general, hSkM1 gating seemed faster than hH1 both in terms of rate of inactivation and rate of recovery from inactivation as well as time to peak current. The midpoint of the steady-state inactivation curve was approximately 25 mV more negative for hH1 compared with hSkM1. In both isoforms, the steady-state channel availability relationships ("inactivation curves") shifted toward more negative membrane potentials with time. The cardiac isoform showed a minimal shift in the activation curve as a function of time after whole-cell dialysis, whereas hSkM1 showed a continued and marked negative shift in the activation voltage dependence of channel gating. This observation suggests that the mechanism underlying the shift in inactivation voltage dependence may be similar to the one that is causing the shift in the activation voltage dependence in hSkM1 but that this is uncoupled in the cardiac isoform. These results demonstrate the utility and limitations of measuring cardiac and skeletal muscle recombinant Na+ channels in tsA-201 cells. This baseline characterization will be useful for future investigations on channel mutants and pharmacology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Bennett P. B., Jr, Makita N., George A. L., Jr A molecular basis for gating mode transitions in human skeletal muscle Na+ channels. FEBS Lett. 1993 Jul 12;326(1-3):21–24. doi: 10.1016/0014-5793(93)81752-l. [DOI] [PubMed] [Google Scholar]
- Chahine M., Bennett P. B., George A. L., Jr, Horn R. Functional expression and properties of the human skeletal muscle sodium channel. Pflugers Arch. 1994 May;427(1-2):136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. L., Jr, Komisarof J., Kallen R. G., Barchi R. L. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann Neurol. 1992 Feb;31(2):131–137. doi: 10.1002/ana.410310203. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L. L., De Jongh K. S., Patton D. E., Reber B. F., Offord J., Charbonneau H., Walsh K., Goldin A. L., Catterall W. A. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992 May 8;256(5058):839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Krafte D. S., Goldin A. L., Auld V. J., Dunn R. J., Davidson N., Lester H. A. Inactivation of cloned Na channels expressed in Xenopus oocytes. J Gen Physiol. 1990 Oct;96(4):689–706. doi: 10.1085/jgp.96.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. 1986 Aug 28-Sep 3Nature. 322(6082):826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Proebstle T., Hombach V., Hannekum A., Rüdel R. Characterization of the sodium currents in isolated human cardiocytes. Pflugers Arch. 1994 Aug;428(1):84–90. doi: 10.1007/BF00374755. [DOI] [PubMed] [Google Scholar]
- Sheets M. F., Hanck D. A. Modification of sodium channel inactivation by alpha-chymotrypsin in canine cardiac Purkinje cells. J Cardiovasc Electrophysiol. 1993 Dec;4(6):686–694. doi: 10.1111/j.1540-8167.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Valenzuela C., Bennett P. B., Jr Gating of cardiac Na+ channels in excised membrane patches after modification by alpha-chymotrypsin. Biophys J. 1994 Jul;67(1):161–171. doi: 10.1016/S0006-3495(94)80465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]
- Zhou J. Y., Potts J. F., Trimmer J. S., Agnew W. S., Sigworth F. J. Multiple gating modes and the effect of modulating factors on the microI sodium channel. Neuron. 1991 Nov;7(5):775–785. doi: 10.1016/0896-6273(91)90280-d. [DOI] [PubMed] [Google Scholar]


