Abstract
Fluoxetine (Prozac) is the most widely prescribed medication for the treatment of depression. Nevertheless, little is known about the molecular basis of its clinical efficacy, apart from the fact that fluoxetine increases the synaptic availability of serotonin. Here we show that, in vivo, fluoxetine, given either acutely or chronically, regulates the phosphorylation state of dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32) at multiple sites in prefrontal cortex, hippocampus, and striatum. Acute administration of fluoxetine increases phosphorylation of DARPP-32 at the protein kinase A site, Thr-34, and at the casein kinase-1 site, Ser-137, and decreases phosphorylation at the cyclin-dependent kinase 5 site, Thr-75. Each of these changes contributes, through distinct signaling pathways, to increased inhibition of protein phosphatase-1, a major serine/threonine protein phosphatase in the brain. Fluoxetine also increases phosphorylation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR1 at Ser-831 and Ser-845. Both the fluoxetine-mediated increase in AMPA receptor phosphorylation at Ser-845–GluR1 and the beneficial responsiveness to fluoxetine in an animal test of antidepressant efficacy were strongly reduced in DARPP-32 knockout mice, indicating a critical role for this phosphoprotein in the antidepressant actions of fluoxetine. Mice chronically treated with fluoxetine had increased levels of DARPP-32 mRNA and protein and a decreased ability to increase phospho-Ser-137–DARPP-32 and phospho-Ser-831–GluR1. These chronic changes may be relevant to the delayed onset of therapeutic efficacy of fluoxetine.
Agents that enhance serotonergic neurotransmission, such as serotonin reuptake inhibitors (e.g., fluoxetine), and monoamine oxidase inhibitors (e.g., moclobemide), are effective as antidepressants. Although these agents immediately increase the synaptic availability of serotonin, there is a temporal delay in the onset of their beneficial actions. Recent studies in experimental animals have focused on understanding the effects of various antidepressant agents on signal transduction pathways in neurons located in brain regions thought to be implicated in depression (1, 2). In particular, it has been shown that treatment with various antidepressants, including fluoxetine, enhances the efficacy of the cAMP-dependent protein kinase A (PKA) pathway at several different levels in the frontal cortex and hippocampus (1). The possibility that the cAMP/PKA system is involved in the action(s) of antidepressants is strongly supported by evidence from basic and clinical studies showing that cAMP phosphodiesterase inhibitors, such as rolipram, have antidepressant efficacy (3, 4).
One major target protein for the cAMP/PKA pathway is dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32; ref. 5). DARPP-32 is enriched in striatum and the olfactory tubercle (6). Moderate to high levels of DARPP-32 also are found in several extrastriatal regions, such as cerebral cortex, hippocampus, amygdala, and the bed nucleus of stria terminalis. The function of DARPP-32 is determined by its phosphorylation state. It is well established that dopamine, by means of D1 receptor-mediated activation of PKA, phosphorylates DARPP-32 at Thr-34 and thereby converts DARPP-32 into a potent inhibitor of protein phosphatase-1 (PP-1; ref. 7). Phosphorylation of DARPP-32 at Ser-137 by casein kinase-1 prevents the dephosphorylation at Thr-34 by calcineurin and thereby potentiates the PKA/Thr-34–DARPP-32/PP-1 cascade (8). Phosphorylation at Thr-75 by cyclin-dependent kinase 5 (Cdk5) converts DARPP-32 into an inhibitor of PKA (9) and antagonizes the PKA/Thr-34–DARPP-32/PP-1 cascade. Consequently, DARPP-32 can act either as a phosphatase inhibitor or as a kinase inhibitor, depending on its relative state of phosphorylation at the PKA, casein kinase-1, and Cdk5 sites.
DARPP-32 is implicated in the regulation of the phosphorylation state and efficacy of several ion channels and ionotropic receptors (10, 11). For example, it has been shown that the PKA/Thr-34–DARPP-32/PP-1 signaling cascade plays an important role in regulating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation and currents (12, 13). Interestingly, recent work has shown that positive allosteric potentiators of AMPA receptors have antidepressant-like actions in rodents (14), which appear to be independent of an increase in the synaptic concentrations of monoamines (15).
In view of a large amount of evidence (10, 11) that DARPP-32 is critically implicated in regulating actions mediated via the cAMP/PKA pathway, we studied the effects of acute and chronic administration with fluoxetine on the level and phosphorylation state of DARPP-32 at multiple sites in prefrontal cortex, hippocampus, and striatum. By using DARPP-32 knockout (KO) mice, we examined the possible involvement of DARPP-32 in mediating the beneficial effects of fluoxetine in the tail-suspension test, a model used to predict antidepressant efficacy, and in regulating AMPA receptor phosphorylation.
Materials and Methods
Whole Animal Studies.
In experiments examining acute effects of fluoxetine, adult male C57/Bl6 mice were given an i.p. injection with saline or fluoxetine (5, 10, or 20 mg/kg) and killed 15-min postinjection by focused microwave irradiation (4.5–5 kW for 1.4 sec), by using a small animal microwave (Muromachi Kikai, Tokyo, Japan). In experiments examining chronic effects of fluoxetine, adult male C57/Bl6, wild-type (WT), or DARPP-32 KO mice (16) were injected i.p. with saline or fluoxetine (10 mg/kg) for 19 days and then challenged with saline or fluoxetine (5 or 10 mg/kg) and killed 15-min postinjection. In all experiments, hippocampi, prefrontal cortices, and striata were rapidly dissected out and stored at −80°C until assayed.
Preparation and Treatment of Slices from Hippocampus, Prefrontal Cortex, and Striatum.
Slices (300-μm) were prepared from adult male C57/Bl6, WT, or DARPP-32 KO mice as described (17). The slices were preincubated in Krebs buffer at 30°C under constant oxygenation (95% O2/5% CO2) for 60 min, with a change of buffer after 30 min. In all experiments with serotonin, the slices were pretreated with fluoxetine (10 μM) for 2 min, and then serotonin (100 μM) was added for an additional 2 min (for phospho-Thr-34– and phospho-Ser-137–DARPP-32) or 10 min (for phospho-Thr-75–DARPP-32 and phospho-Ser-831– and phospho-Ser-845–GluR1). Fluoxetine alone had no effect on DARPP-32 phosphorylation under any of the conditions tested (data not shown). After drug treatment, the buffer was removed and the slices were rapidly frozen on dry ice and stored at −80°C until immunoblotted.
Immunoblotting.
Frozen tissue samples from the in vitro and in vivo experiments were sonicated in 1% SDS and boiled for 10 min. Small aliquots of the homogenate were retained for protein determination by the bicinchoninic acid protein assay method (Pierce). Equal amounts of protein were processed by using 10% acrylamide gels as described (17). Immunoblotting was carried out with phosphorylation-state-specific antibodies against phospho-Thr-34–DARPP-32 (18), phospho-Thr-75–DARPP-32 (9), phospho-Ser-137–DARPP-32 (19), phospho-Ser-831–GluR1 (Upstate Biotechnology, Lake Placid, NY), phospho-Ser-845–GluR1 (Upstate Biotechnology), or antibodies that are not phosphorylation-state-specific against total DARPP-32 (20) and total GluR1 (Upstate Biotechnology). Antibody binding was detected by enhanced chemiluminescence (ECL; Amersham Pharmacia) and quantified by densitometry, using National Institutes of Health IMAGE 1.61 software. Data on protein phosphorylation are expressed as percentage of control.
In Situ Hybridization.
WT and DARPP-32 KO mice were injected i.p. with saline or fluoxetine (10 mg/kg) for 19 days and killed 20 min after the last injection by decapitation. Brains were rapidly dissected out and frozen at −80°C. Cryostat sections (12-μm) were prepared and hybridized with [α-35S]UTP-labeled riboprobes prepared by in vitro transcription from cDNA clones corresponding to full-length clones of DARPP-32 or inhibitor-1 as described (21). After hybridization, the sections were exposed to Biomax MR film (Kodak) for 2–14 days and analyzed with a Microcomputer Imaging Device system (M4, Imaging Research, St. Catherine's, ON, Canada).
Tail-Suspension Test.
Mice were injected i.p. with saline or fluoxetine (5 or 10 mg/kg) 30 min before the tail-suspension test trial. Mice were suspended by their tails 80 cm above the floor by having their tails secured to the edge of a platform with adhesive tape placed 1 cm from the tip of the tail. The trial was conducted for a period of 5 min, during which the duration of immobility was recorded with the program PORSOLT (Infallible Software, Rockville, MD). Mice were considered immobile when they hung passively and motionless (14).
Results
Regulation of DARPP-32 Phosphorylation in Vivo by Acute Treatment with Fluoxetine.
Mice were injected i.p. with saline or fluoxetine (5, 10, and 20 mg/kg) and killed 15 min later by focused microwave irradiation. The results shown in Fig. 1 demonstrate that fluoxetine caused an increased phosphorylation of DARPP-32 at Thr-34, the PKA site, and a decreased phosphorylation at Thr-75, the cyclin-dependent kinase 5 (Cdk5) site, in hippocampus, frontal cortex, and striatum (Fig. 1). Because of a low signal in extrastriatal areas, the level of phosphorylation of DARPP-32 at Ser-137, the casein kinase-1 site, could be accurately assayed only in striatum. In this region, fluoxetine increased phosphorylation at Ser-137.
Figure 1.
Regulation of DARPP-32 phosphorylation in vivo by acute treatment with fluoxetine. Data are shown for mice treated with saline or fluoxetine (5, 10, or 20 mg/kg) and killed 15-min postinjection. The amounts of (■) phospho-Thr-34–DARPP-32 and (▴) phospho-Thr-75–DARPP-32 were quantified in hippocampus, prefrontal cortex, and striatum, and the amounts of (○) phospho-Ser-137–DARPP-32 were quantified in striatum. Data represent means ± SE for six to ten mice per group. *, P < 0.05, **, P < 0.01 compared with saline-treated mice, one-way ANOVA followed by Dunnett's test.
Regulation of DARPP-32 Phosphorylation in Vivo by Chronic Treatment with Fluoxetine.
The antidepressant actions of fluoxetine characteristically require 2–3 weeks to be manifested. To examine the effects of chronic administration of fluoxetine on DARPP-32 phosphorylation, mice were treated with saline or fluoxetine (10 mg/kg, i.p.) once daily for 19 days and then challenged with a single i.p. injection of saline or fluoxetine at 5 or 10 mg/kg and killed 15-min postinjection. Mice chronically treated with saline and challenged with fluoxetine exhibited patterns of DARPP-32 phosphorylation (Fig. 2 Upper) similar to those obtained in the acute dose–response experiments (Fig. 1).
Figure 2.
Regulation of DARPP-32 phosphorylation in vivo by chronic treatment with fluoxetine. Data are shown for mice treated for 19 days with saline (Upper) or fluoxetine (10 mg/kg; Lower) and then challenged with fluoxetine (0, 5, or 10 mg/kg in saline) and killed 15-min postinjection. The amounts of (■, □) phospho-Thr-34–, (▴, ▵) phospho-Thr-75– and (●, ○) phospho-Ser-137–DARPP-32 were quantified and normalized to total DARPP-32 (see text). Data represent means ± SE for four to eight mice per group. *, P < 0.05, **, P < 0.01 compared with saline/saline-treated mice; +, P < 0.05, ++, P < 0.01 compared with fluoxetine/saline-treated mice; #, P < 0.05 compared with saline/fluoxetine-treated mice, one-way ANOVA followed by Dunnett's test.
In mice chronically treated with fluoxetine, there was an increase in total DARPP-32 protein in hippocampus and frontal cortex, but not in striatum (see Regulation of DARPP-32 mRNA and Protein Levels by Chronic Treatment with Fluoxetine). After normalization for these changes in the total levels of DARPP-32, there were no differences in the fraction of DARPP-32 phosphorylated at Thr-34, Thr-75, or Ser-137 between mice chronically treated with saline and challenged with saline and those chronically treated with fluoxetine and challenged with saline. A challenge with fluoxetine increased DARPP-32 phosphorylation at Thr-34 and decreased DARPP-32 phosphorylation at Thr-75 (Fig. 2 Lower) in mice chronically treated with fluoxetine to an extent similar to that seen in mice chronically treated with saline (Fig. 2 Upper) in all three regions examined. In contrast, the ability of a challenge with 10 mg/kg of fluoxetine to increase phosphorylation at Ser-137 in striatum (Fig. 2 Lower) was abolished in mice chronically treated with fluoxetine.
Regulation of DARPP-32 mRNA and Protein Levels by Chronic Treatment with Fluoxetine.
Mice treated chronically with fluoxetine (10 mg/kg, i.p.) exhibited increased levels of total DARPP-32 protein in prefrontal cortex (123 ± 3%) and hippocampus (121 ± 4%), but not striatum (102 ± 4%), when compared with mice treated chronically with saline. The changes in the total levels of DARPP-32 protein were not paralleled by changes in the total levels of inhibitor-1 protein (102 ± 2%, 98 ± 3%, and 97 ± 3%, for the three regions, respectively), a closely related homologue of DARPP-32. To determine whether effects of chronic fluoxetine on DARPP-32 protein levels in hippocampus and prefrontal cortex occurred at the transcriptional level, in situ hybridization experiments were carried out to study the effects of chronic administration (once daily for 19 days) of saline or fluoxetine (10 mg/kg, i.p.) on DARPP-32 mRNA levels. As illustrated in Fig. 3, chronic treatment with fluoxetine increased DARPP-32 mRNA expression in hippocampus and frontal cortex, but not in striatum (Fig. 3 Left). Chronic treatment with fluoxetine had no effects on inhibitor-1 mRNA expression in any of the examined regions (Fig. 3 Right).
Figure 3.
Levels of DARPP-32 mRNA and inhibitor-1 after chronic treatment for 19 days with saline or fluoxetine (10 mg/kg). The exposure time for the dark-field photomicrographs was 7 days, or, in the case of striatal DARPP-32 mRNA, 2 days. The amounts of DARPP-32 mRNA (Left) and inhibitor-1 mRNA (Right) were quantified by densitometry in hippocampus (Top), prefrontal cortex (Middle), and striatum (Bottom). Data represent means ± SE for four to six mice per group. *, P < 0.05 compared with saline-treated mice, Student's t test.
Regulation by Serotonin of DARPP-32 Phosphorylation in Slices.
To examine whether the effects of fluoxetine on DARPP-32 phosphorylation could be attributed to an action of serotonin on DARPP-32-containing neurons, slices were incubated with serotonin (100 μM). The results presented in Fig. 4 demonstrate that serotonin increased DARPP-32 phosphorylation at Thr-34 (Fig. 4 Upper) and decreased DARPP-32 phosphorylation at Thr-75 (Fig. 4 Lower), in slices prepared from hippocampus, prefrontal cortex, and striatum (Fig. 4). Serotonin also increased DARPP-32 phosphorylation at Ser-137 in striatum (22).
Figure 4.
Regulation of DARPP-32 phosphorylation by serotonin (100 μM) in vitro. The amounts of phospho-Thr-34–DARPP-32 (Upper) and phospho-Thr-75–DARPP-32 (Lower) in extracts of slices were quantified by densitometry. Data represent means ± SE for three to ten experiments. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared with control, Student's t test.
Regulation by Serotonin of AMPA Receptor Phosphorylation in Slices Prepared from WT and DARPP-32 KO Mice.
DARPP-32 regulates phosphorylation of AMPA receptors and increases AMPA currents in striatal neurons (12, 13). Moreover, positive allosteric modulators of AMPA receptors have beneficial effects in animal models of depression (14, 15). We therefore investigated, using WT and DARPP-32 KO mice, whether serotonin can regulate the phosphorylation state of the GluR1 subunit of the AMPA receptor at two different phosphorylation sites known to affect the functional properties of AMPA receptors, namely Ser-831–GluR1, a PKC/calmodulin-dependent kinase II (CamKII) site, and Ser-845–GluR1, a PKA site. The results shown in Fig. 5 demonstrate that serotonin (100 μM) increased the phosphorylation at Ser-831–GluR1 as well as at Ser-845–GluR1 in slices from hippocampus, frontal cortex, and striatum in WT mice. The increased phosphorylation at Ser-845–GluR1 in WT mice was significantly reduced in slices prepared from DARPP-32 KO mice (Fig. 5), showing that the DARPP-32/PP-1 signaling cascade is involved in the serotonin-mediated regulation of GluR1 phosphorylation at this site. The increased phosphorylation at Ser-831–GluR1 in WT mice was significantly reduced in striatal slices, but not in hippocampal or cortical slices, prepared from DARPP-32 KO mice (Fig. 5); the striatal data indicate that the DARPP-32/PP-1 signaling cascade may be involved in the serotonin-mediated regulation of phosphorylation at Ser-831–GluR1.
Figure 5.
Regulation of AMPA receptor phosphorylation by serotonin (100 μM) in vitro. The amounts of phospho-Ser-831–GluR1 (Left) and phospho-Ser-845–GluR1 (Right) in extracts of slices from WT (black bars) and DARPP-32 KO (white bars) mice were quantified by densitometry. Data represents means ± SE for three to ten experiments. *, P < 0.05, **, P < 0.01, ***, P < 0.001 compared with control; #, P < 0.05, ##, P < 0.01 compared with serotonin in WT, Student's t test.
Regulation of AMPA Receptor Phosphorylation by Chronic Treatment with Saline or Fluoxetine in WT and DARPP-32 KO Mice.
WT mice that had been treated chronically with saline and challenged with fluoxetine exhibited an increased phosphorylation state at Ser-831–GluR1 and Ser-845–GluR1 in all three brain regions examined (Table 1). WT mice chronically treated with fluoxetine and challenged with fluoxetine exhibited higher levels of phosphorylation at Ser-845–GluR1 in all three brain regions than did WT mice chronically treated with fluoxetine and challenged with saline. In contrast, the ability of a challenge with fluoxetine to increase phosphorylation at Ser-831–GluR1 was abolished in WT mice chronically treated with fluoxetine.
Table 1.
Regulation by chronic treatment with saline or fluoxetine (Fluo) of AMPA receptor phosphorylation in vivo
| Chronic treatment | Challenge | Ser-831–GluR1
|
Ser-845–GluR1
|
||
|---|---|---|---|---|---|
| WT | D32 KO | WT | D32 KO | ||
| Hippocampus | |||||
| Saline | Saline | 100 (2.0) | 97 (5.9) | 100 (0.9) | 104 (2.4) |
| Saline | Fluo 5 mg/kg | 111 (8.0) | 102 (6.7) | 133 (2.5)* | 115 (4.2)# |
| Saline | Fluo 10 mg/kg | 127 (6.1)* | 110 (4.8)# | 143 (7.8)** | 114 (3.9)# |
| Fluo | Saline | 96 (3.4) | 98 (3.7) | 96 (3.8) | 97 (5.3) |
| Fluo | Fluo 5 mg/kg | 98 (4.6) | 94 (3.3) | 136 (5.2)§ | 122 (4.4) |
| Fluo | Fluo 10 mg/kg | 107 (4.0)† | 105 (5.5) | 133 (5.9)§ | 110 (5.2)# |
| Prefrontal cortex | |||||
| Saline | Saline | 100 (5.2) | 88 (10.2) | 100 (2.2) | 91 (3.4) |
| Saline | Fluo 5 mg/kg | 94 (3.4) | 90 (5.0) | 136 (6.6)* | 118 (4.1)# |
| Saline | Fluo 10 mg/kg | 115 (2.8)* | 107 (4.3) | 133 (5.8)* | 110 (9.7)# |
| Fluo | Saline | 90 (10.0) | 88 (4.7) | 88 (8.8) | 84 (4.5) |
| Fluo | Fluo 5 mg/kg | 98 (6.7) | 88 (6.6) | 128 (8.6)§ | 107 (6.6)# |
| Fluo | Fluo 10 mg/kg | 99 (8.0)† | 94 (4.4) | 129 (8.2)§ | 109 (9.4)# |
| Striatum | |||||
| Saline | Saline | 100 (5.7) | 88 (8.0) | 100 (2.3) | 104 (8.0) |
| Saline | Fluo 5 mg/kg | 123 (1.1)* | 115 (5.0) | 141 (7.4)* | 116 (9.1)# |
| Saline | Fluo 10 mg/kg | 135 (4.9)* | 126 (10.2) | 159 (10.5)** | 128 (4.9)# |
| Fluo | Saline | 97 (8.2) | 86 (15.6) | 111 (8.1) | 109 (7.9) |
| Fluo | Fluo 5 mg/kg | 110 (2.8) | 112 (4.8) | 142 (7.9)§ | 123 (4.0)# |
| Fluo | Fluo 10 mg/kg | 111 (8.7)† | 109 (5.2) | 160 (7.4)§§ | 135 (6.6)# |
Data are shown for phospho-Ser-831–GluR1 and phospho-Ser-845–GluR1 in WT and DARPP-32 KO (D32 KO) mice treated for 19 days with saline or fluoxetine (10 mg/kg) and then challenged with saline or fluoxetine (5 or 10 mg/kg) and killed 15-min postinjection. Data represent means ± SEM for four to twelve mice per group.
, P < 0.05,
, P < 0.01 compared with saline/saline-treated mice;
, P < 0.05,
, P < 0.01 compared with fluoxetine/saline-treated WT mice;
, P < 0.05 compared with corresponding saline/fluoxetine-treated WT mice;
, P < 0.05 compared with corresponding WT mice, one-way ANOVA followed by Dunnett's test.
We also compared fluoxetine-induced phosphorylation of the AMPA receptor in WT and DARPP-32 KO mice to evaluate the possible involvement of DARPP-32 in mediating the effects of fluoxetine. Fluoxetine (5 and 10 mg/kg) was significantly less efficient in increasing phosphorylation at Ser-845–GluR1 in DARPP-32 KO mice than in WT mice (Table 1), regardless of whether the mice had received chronic treatment with saline or fluoxetine. The fluoxetine-mediated increase in phospho-Ser-831–GluR1 was significantly lower in hippocampus, but not in cortex or striatum, from DARPP-32 KO mice (Table 1, Hippocampus).
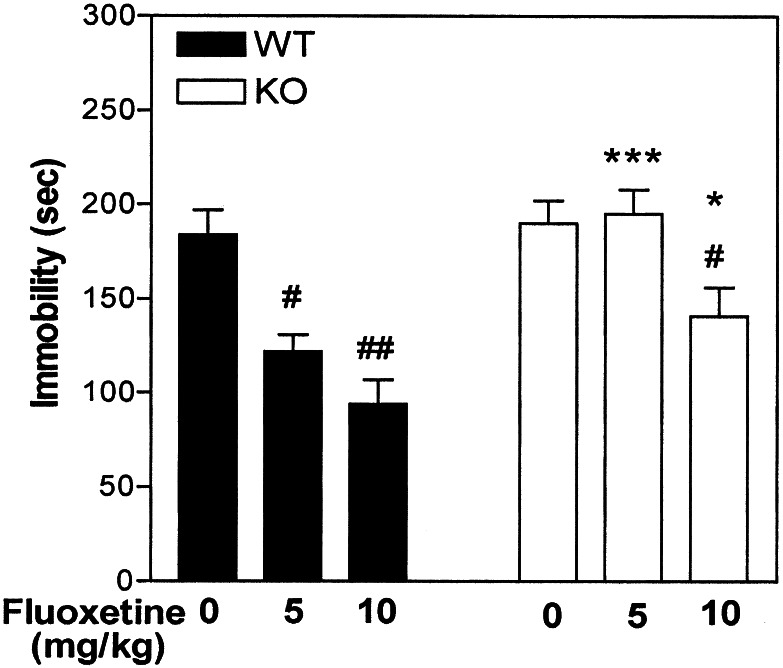
Involvement of DARPP-32 in Fluoxetine-Mediated Immobility in the Tail-Suspension Test for Antidepressant Efficacy.
Learned-helplessness models, in which experimental animals are exposed to inescapable aversive situations, e.g., the tail-suspension test, are of utility for predicting antidepressant efficacy. During these tests, mice show alternate periods of agitation and immobility (23). It is well established that acute treatment with various antidepressant drugs reduces immobility in these tests. In particular, agents that selectively inhibit serotonin reuptake, including fluoxetine, invariably appear to be efficacious in the tail-suspension test (24). To evaluate the possible involvement of DARPP-32 in fluoxetine-mediated antidepressant-like effects in the tail-suspension test, WT and DARPP-32 KO mice were injected with saline or fluoxetine (5 or 10 mg/kg) 30 min before the trial. Fluoxetine decreased immobility in WT animals (Fig. 6). This effect was strongly attenuated in DARPP-32 KO mice (Fig. 6). Nonspecific increases in activity could not account for the positive effect of fluoxetine in WT mice, because locomotion was not significantly affected by fluoxetine at the doses examined (data not shown).
Figure 6.
Involvement of DARPP-32 in fluoxetine-mediated immobility in the tail-suspension test for antidepressant efficacy. WT and DARPP-32 KO mice were injected with fluoxetine (5 or 10 mg/kg) 30 min before the trial. The trial was conducted for a period of 5 min, during which the duration of immobility was recorded. Data represents means ± SE for six to ten mice per experiment. #, P < 0.05, ##, P < 0.01 compared with saline; *, P < 0.05, ***, P < 0.001 compared with fluoxetine in DARPP-32 WT mice, two-way ANOVA followed by Duncan's test.
Discussion
The present studies have demonstrated that fluoxetine regulates phosphorylation of DARPP-32 at multiple sites and that DARPP-32 mediates biochemical and behavioral actions of fluoxetine.
Functional neuroimaging studies have shown that there is an altered activity in prefrontal cortex, hippocampus, amygdala, and striatum in depressed individuals that is, at least partly, reversible by antidepressant regimens (2). Moreover, adaptations in the cAMP/PKA pathway, together with morphological and electrophysiological changes, have been described in prefrontal cortex, hippocampus, amygdala, and striatum after administration of fluoxetine and other antidepressants in laboratory animals (1, 2). These regions receive a moderate to high serotonergic innervation (25) and contain DARPP-32 (6). In the present study, the ability of fluoxetine to regulate DARPP-32 phosphorylation was assessed in prefrontal cortex, hippocampus, and striatum. Because of its small size and complicated division into subnuclei, we were unable to study effects of fluoxetine on DARPP-32 phosphorylation in the amygdaloid complex.
Fourteen different serotonin receptors have been cloned and pharmacologically characterized (26). They are divided into seven different subclasses: 5HT1A-F, 5HT2A-C, 5HT3, 5-HT4, 5-HT5, 5HT6 and 5-HT7 receptors. All of them are metabotropic receptors, with the exception of 5-HT3 receptors, which are ionotropic. They act primarily by means of the following second messenger systems: 5-HT1- and 5-HT5-class receptors decrease cAMP formation; 5-HT2-class receptors increase inositol triphosphate and diacylglycerol formation; 5-HT3 receptors increase Na+ and Ca2+ influx; and 5-HT4, 5-HT6 and 5HT7 receptors increase cAMP formation. In the present studies, it was found that acute administration of fluoxetine to whole animals or application of serotonin to slices, increased phosphorylation of DARPP-32 at Thr-34, decreased phosphorylation at Thr-75, and increased phosphorylation at Ser-137. In the accompanying paper (22) we provide evidence that the following signaling cascades account for these three effects: (i) activation of 5-HT4 and 5-HT6 receptors/increased cAMP/activation of PKA/phosphorylation of Thr-34; (ii) activation of 5-HT4 and 5-HT6 receptors/increased cAMP/activation of PKA/activation of protein phosphatase-2A (PP-2A)/dephosphorylation of Thr-75; (iii) activation of 5-HT2 receptors/activation of PLC/increased calcium/activation of casein kinase-1/phosphorylation of Ser-137. As is also discussed in the accompanying paper (22), the changes at these three phosphorylation sites work synergistically, each contributing to the inhibition of PP-1.
Moreover, these three cascades also can account for the observations that the ability of fluoxetine to regulate DARPP-32 phosphorylation at Thr-34 and Thr-75, but not at Ser-137, is preserved in mice chronically treated with this compound. Thus, there is evidence that serotonin-mediated responses via the cAMP/PKA pathway persist (1, 27), but that responses via the PLC pathway are reduced (27–30), after prolonged fluoxetine treatment.
Chronic treatment with fluoxetine caused a significant increase in the total levels of DARPP-32 protein and mRNA in prefrontal cortex and hippocampus, but not striatum. These data extend previous observations that chronic treatment with the tricyclic antidepressant, imipramine, or the mood-stabilizer, lithium, increases the level of total DARPP-32 protein in the rat frontal cortex (31). The mechanisms underlying the regulation of DARPP-32 levels in frontal cortex and hippocampus remain to be determined. However, brain-derived neurotrophic factor (BDNF) may be involved, because it has been shown that chronic treatment with several different antidepressants, including fluoxetine, increases the levels of BDNF in frontal cortex and hippocampus in experimental animals (32), and that the levels of DARPP-32 mRNA and protein are significantly reduced in BDNF KO mice (33). Because central infusion of BDNF has been reported to have beneficial effects in animal tests of depression (34), it will be important in future work to clarify the relationship between BDNF and DARPP-32.
There is evidence that phospho-Thr-34–DARPP-32 increases the phosphorylation state and efficacy of several ion channels and ionotropic receptors, such as AMPA receptors (10, 11). Activation of PKA in hippocampal (35) or striatal (12) neurons increases AMPA receptor currents and AMPA receptor-mediated synaptic plasticity. The notion that these effects occur as the result of the phosphorylation of the receptor is supported by the observation that phosphorylation by PKA at Ser-845–GluR1 is required for PKA-mediated potentiation of peak current carried by homomeric GluR1 channels (36). AMPA receptors also can be regulated by phosphorylation of Ser-831–GluR1 after activation of calcium/calmodulin-dependent kinase II (CaMKII) or protein kinase C (PKC) leading to potentiation of AMPA currents in hippocampal neurons (37). Interestingly, it has recently been shown that LY392098 and LY404187, two allosteric potentiators of AMPA receptor function, have robust antidepressant-like actions in two tests of “learned helplessness,” the forced-swim test and the tail-suspension test (14, 15). Based on these findings, we examined the effects of fluoxetine on AMPA receptor phosphorylation. It was found that acute administration of fluoxetine increased the phosphorylation of both Ser-831–GluR1 and Ser-845–GluR1 in frontal cortex, hippocampus, and striatum. The fluoxetine-mediated regulation at Ser-845–GluR1 was strongly and consistently attenuated in DARPP-32 KO mice. After chronic administration of fluoxetine, the ability of a challenge with fluoxetine to induce phosphorylation at Ser-845–GluR1, in a DARPP-32-dependent manner, was preserved. In contrast, the fluoxetine-mediated phosphorylation at Ser-831–GluR1 was found only in mice chronically treated with saline and not in mice chronically treated with fluoxetine. As a consequence, the ratio of the phosphorylation state between Ser-845–GluR1 and Ser-831–GluR1 was increased. Such a change may be associated with altered synaptic plasticity (38) and may underlie an adaptive response relevant to the delayed onset of the antidepressant action of fluoxetine.
To evaluate the behavioral relevance of the biochemical actions of fluoxetine on DARPP-32 phosphorylation, the effect of fluoxetine in the tail-suspension test was evaluated in WT and DARPP-32 KO mice. This test is among the most commonly used procedures to detect clinically effective antidepressant agents because of its high degree of predictive validity (23, 24). Several selective serotonin reuptake inhibitors, including fluoxetine, reduce immobility in this test. Indeed, in the present study, fluoxetine decreased immobility in WT mice. Nonspecific activation could not account for this effect, because fluoxetine did not affect locomotion in WT mice. Interestingly, the effect of fluoxetine in the tail-suspension test was significantly attenuated in DARPP-32 KO mice. The suppression in DARPP-32 KO mice of the fluoxetine-induced reduction in immobility time in the tail-suspension test strongly suggests that DARPP-32 participates in the antidepressant actions of fluoxetine.
In conclusion, the data indicating that fluoxetine regulates the phosphorylation state of DARPP-32 and AMPA receptors, that DARPP-32 is involved in mediating the effect of fluoxetine on AMPA receptor phosphorylation and in the tail-suspension test, and that AMPA receptor potentiators mimic antidepressant agents in this behavioral test, suggest that a signaling pathway involving a DARPP-32-induced increase in AMPA receptor phosphorylation and conductance may play an important role in mediating the antidepressant actions of fluoxetine.
Acknowledgments
The exceptionally skilled technical assistance of Stacey Galdi is greatly acknowledged. We thank D. Nelson and A. Nishi for their critical reading of this manuscript. We also thank G. L. Snyder, J. A. Bibb, F. Liu, and H. C. Hemmings, Jr., for antibodies against phospho-Thr-34–DARPP-32, phospho-Thr-75–DARPP-32, phospho-Ser-137–DARPP-32, and total DARPP-32. This work was supported by National Institutes of Health Grants MH-40899 and DA10044 (to P. G.). P. S. is supported by a postdoctoral fellowship from Stiftelsen för Internationalisering av Högre Utbildning och Forskning (STINT).
Abbreviations
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of Mr 32,000
- PKA
protein kinase A
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PP-1
protein phosphatase-1
- KO
knockout
- WT
wild type
- BDNF
brain-derived neurotrophic factor
References
- 1.Duman R S, Heninger G R, Nestler E J. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 2.Manji H K, Drevets W C, Charney D S. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 3.Horowski R, Sastre-Y-Hernandez M. Curr Ther Res. 1985;38:23–29. [Google Scholar]
- 4.Fleischhacker W W, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wilf R, Gerlach W, Jaklitsch H, Sastre-Y-Hernandez M, et al. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- 5.Walaas S I, Aswad D W, Greengard P. Nature (London) 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 6.Ouimet C C, Miller P E, Hemmings H C, Jr, Walaas S I, Greengard P. J Neurosci. 1984;4:114–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmings H C, Jr, Greengard P, Tung H Y L, Cohen P. Nature (London) 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 8.Desdouits F, Siciliano J C, Greengard P, Girault J-A. Proc Natl Acad Sci USA. 1995;92:2682–2685. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibb J A, Snyder G L, Nishi A, Yan Z, Meijer L, Fienberg A A, Tsai L H, Kwon Y T, Girault J A, Czernik A J, et al. Nature (London) 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 10.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 11.Greengard P. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 12.Yan Z, Hsien-Wilson L, Feng J, Tomizawa K, Allen P B, Fienberg A A, Nairn A C, Greengard P. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 13.Snyder G L, Allen P B, Fienberg A A, Valle C G, Huganir R L, Nairn A C, Greengard P. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Tizzano J P, Griffey K, Clay M, Lindstrom T, Skolnick P. Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 15.Skolnick P, Legutko B, Li X, Bymaster F P. Pharmacol Res. 2001;43:411–422. doi: 10.1006/phrs.2000.0806. [DOI] [PubMed] [Google Scholar]
- 16.Fienberg A A, Hiroi N, Mermelstein P G, Song W J, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D B, Cole D G, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 17.Nishi A, Snyder G L, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder G L, Girault J A, Chen J Y C, Czernik A J, Kebabian J W, Nathanson J A, Greengard P. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Ma X H, Ule J, Bibb J A, Nishi A, DeMaggio A J, Yan Z, Nairn A C, Greengard P. Proc Natl Acad Sci USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmings H C, Jr, Greengard P. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeMoine C, Bloch B. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 22.Svenningsson P, Tzavara E T, Liu F, Witkin J M, Fienberg A A, Nomikos G G, Greengard P. Proc Natl Acad Sci USA. 2002;99:3188–3193. doi: 10.1073/pnas.052712699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steru L, Chermat R, Thierry B, Simon P. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt R D, Lenegre A. In: Experimental Approaches to Anxiety and Depression. Elliott J, Heal D J, Marsden C A, editors. London: Wiley; 1992. pp. pp.73–85. [Google Scholar]
- 25.Steinbusch H W. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 26.Barnes N M, Sharp T. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 27.Beasley C M, Masica D N, Potvin J H. Psychopharmacology. 1992;107:1–10. doi: 10.1007/BF02244958. [DOI] [PubMed] [Google Scholar]
- 28.Peroutka S L, Snyder S H. Science. 1980;21:88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- 29.Sanders-Bush E, Breeding M, Knoth K, Tsutsumi M. Psychopharmacology. 1989;99:64–69. doi: 10.1007/BF00634454. [DOI] [PubMed] [Google Scholar]
- 30.Bristow L J, O'Connor D, Watts R, Duxon M S, Hutson P H. Neuropharmacology. 2000;39:1222–1236. doi: 10.1016/s0028-3908(99)00191-4. [DOI] [PubMed] [Google Scholar]
- 31.Guitart X, Nestler E J. J Neurochem. 1992;59:1164–1167. doi: 10.1111/j.1471-4159.1992.tb08361.x. [DOI] [PubMed] [Google Scholar]
- 32.Nibuya M, Morinobu S, Duman R S. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivkovic S, Polonskaia O, Farinas I, Ehrlich M. Neuroscience. 1999;79:509–516. doi: 10.1016/s0306-4522(96)00684-7. [DOI] [PubMed] [Google Scholar]
- 34.Siucack J A, Lewis D R, Wiegand S J, Lindsay R M. Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 35.Greengard P, Jen J, Nairn A C, Stevens C F. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- 36.Roche K W, O'Brien R J, Mammen A L, Bernhardt J, Huganir R L. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 37.Barria A, Muller D, Derkach V, Griffith L C, Soderling T R. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 38.Lee H K, Barbarosie M, Kameyama K, Bear M F, Huganir R L. Nature (London) 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]