Abstract
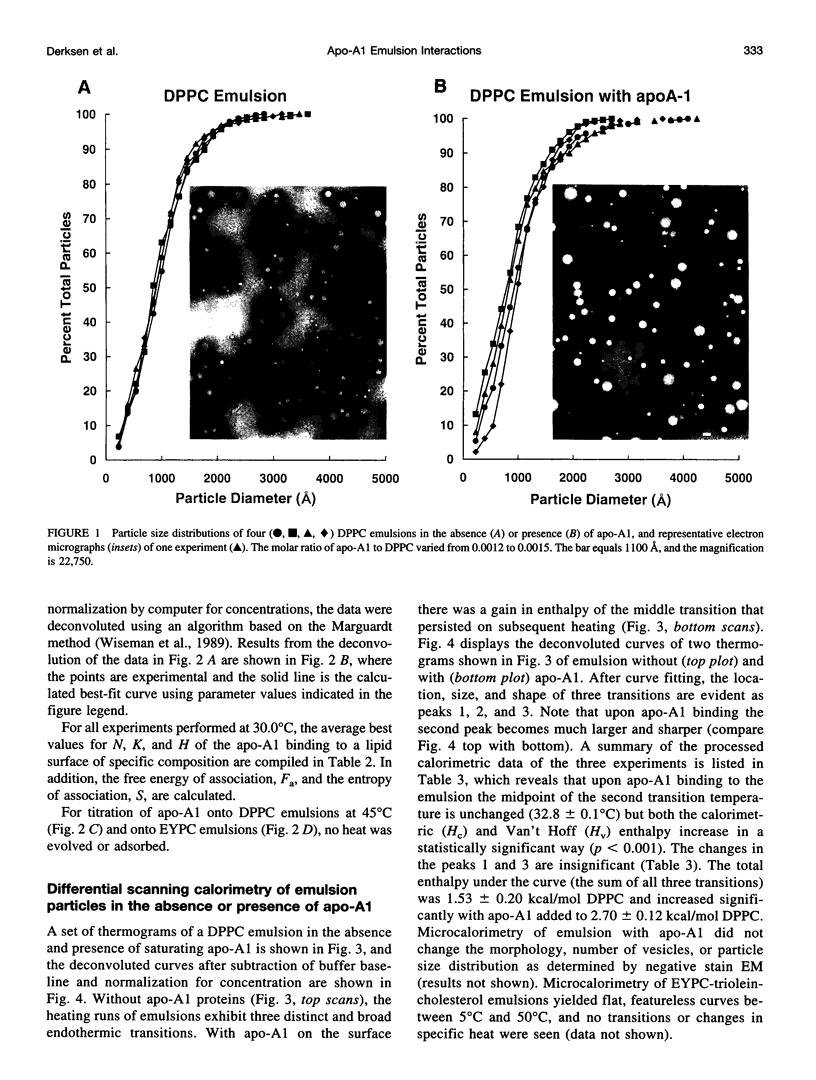
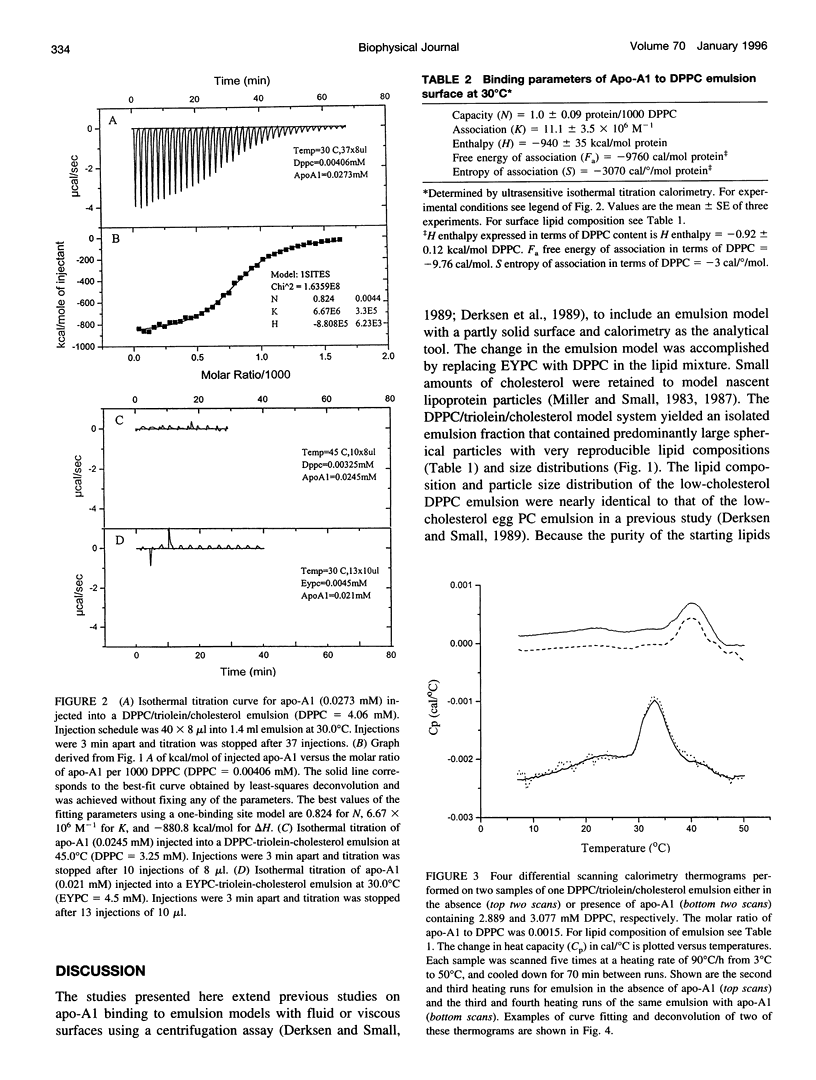
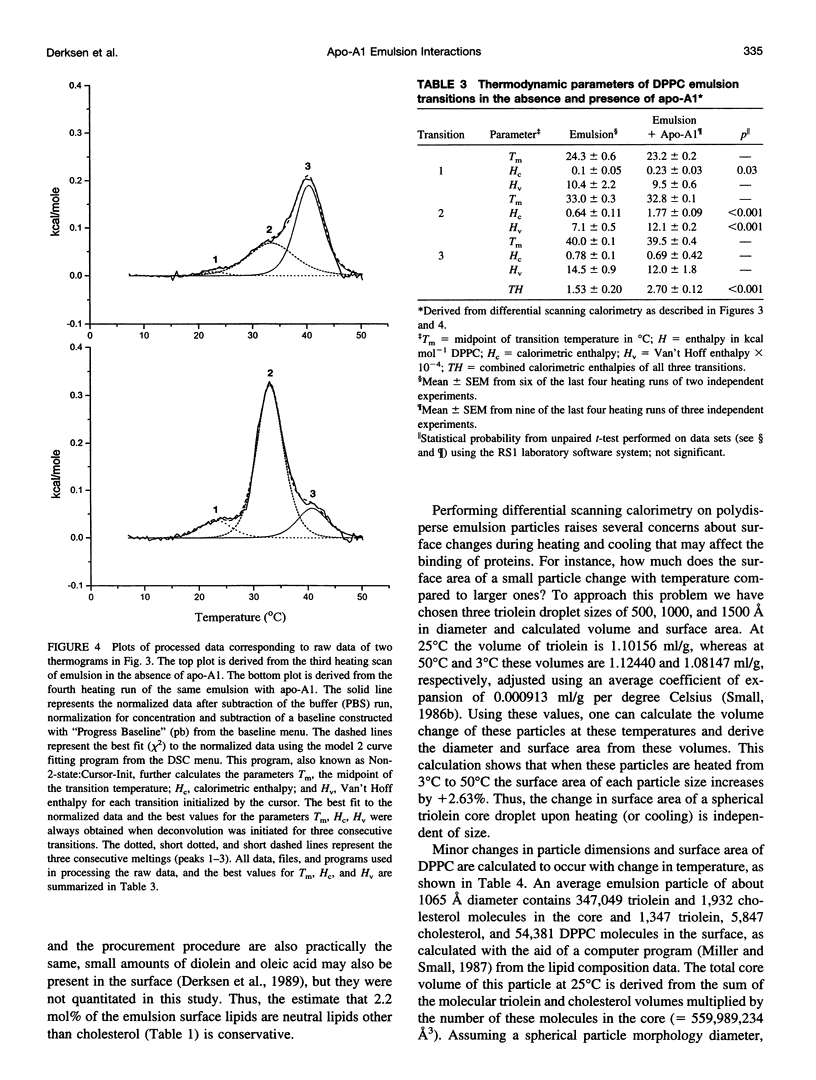
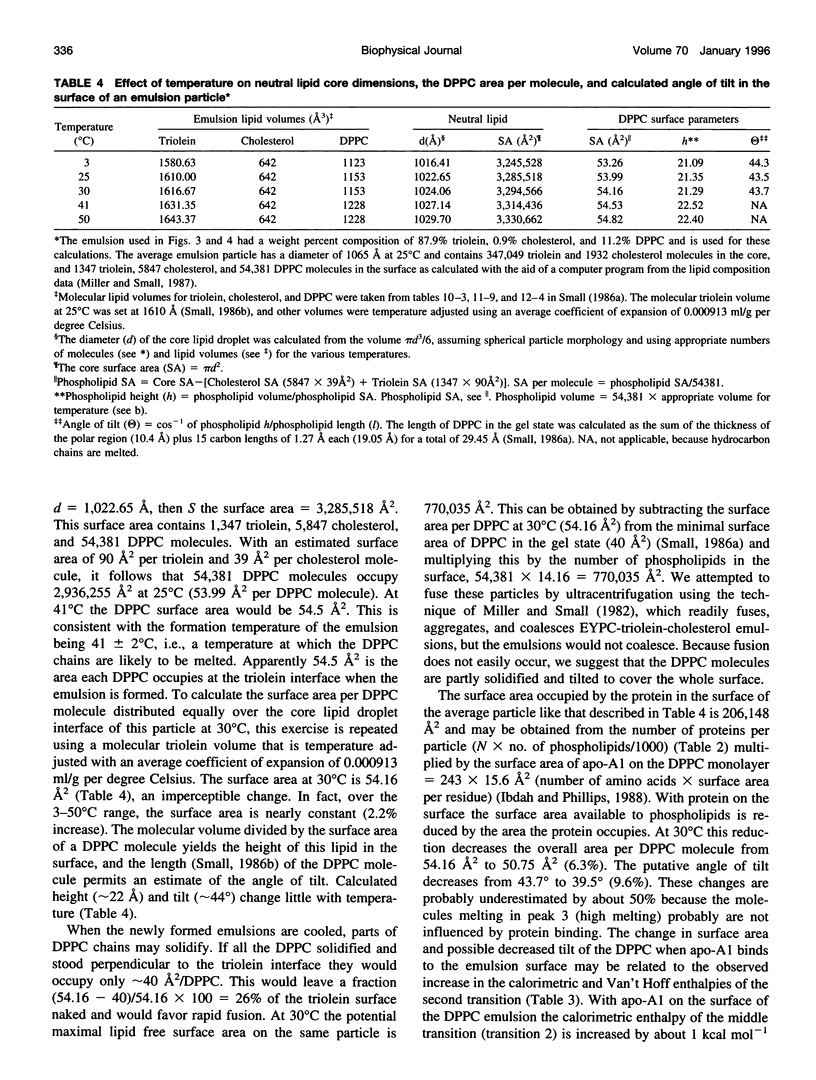
The thermotropic properties of triolein-rich, low-cholesterol dipalmitoyl phosphatidylcholine (DPPC) emulsion particles with well-defined chemical compositions (approximately 88% triolein, 1% cholesterol, 11% diacyl phosphatidylcholine) and particle size distributions (mean diameter, approximately 1000-1100 A) were studied in the absence and presence of apolipoprotein-A1 by a combination of differential scanning and titration calorimetry. The results are compared to egg yolk PC emulsions of similar composition and size. Isothermal titration calorimetry at 30 degrees C was used to saturate the emulsion surface with apo-A1 and rapidly quantitate the binding constants (affinity Ka = 11.1 +/- 3.5 x 10(6) M-1 and capacity N = 1.0 +/- 0.09 apo-A1 per 1000 DPPC) and heats of binding (enthalpy H = -940 +/- 35 kcal mol-1 apo-A1 or -0.92 +/- 0.12 kcal mol-1 DPPC). The entropy of association is -3070 cal deg-1 mol-1 protein or -3 cal deg-1 mol-1 DPPC. Without protein on the surface, the differential scanning calorimetry heating curve of the emulsion showed three endothermic transitions at 24.3 degrees C, 33.0 degrees C, and 40.0 degrees C with a combined enthalpy of 1.53 +/- 0.2 kcal mol-1 DPPC. With apo-A1 on the surface, the heating curve showed the three transitions more clearly, in particular, the second transition became more prominent by significant increases in both the calorimetric and Van't Hoff enthalpies. The combined enthalpy was 2.70 +/- 0.12 kcal mol-1 DPPC and remained constant upon repeated heating and cooling. Indicating that the newly formed DPPC emulsion-Apo-A1 complex is thermally reversible during calorimetry. Thus there is an increase in delta H of 1.17 kcal mol-1 DPPC after apo-A1 is bound, which is roughly balanced by the heat released during binding (-0.92 kcal) of apo-A1. The melting entropy increase, +3.8 cal deg-1 mol-1 DPPC of the three transitions after apo-A1 binds, also roughly balances the entropy (-3 cal deg-1 mol-1 DPPC) of association of apo-A1. These changes indicate that apo-A1 increases the amount of ordered gel-like phase on the surface of DPPC emulsions when added at 30 degrees C. From the stoichiometry of the emulsions we calculate that the mean area of DPPC at the triolein/DPPC interface is 54.5 A2 at 41 degrees C and 54.2 A2 at 30 degrees C. The binding of apo-A1 at 30 degrees C to the emulsion reduces the surface area per DPPC molecule from 54.2 A2 to 50.8 A2. At 30 degrees apo-A1 binds with high affinity and low capacity to the surface of DPPC emulsions and increases the packing density of the lipid domain to which it binds. Apo-A1 was also titrated onto DPPC emulsions at 45 degrees C. This temperature is above the gel liquid crystal transition. No heat was released or adsorbed. Furthermore, egg yolk phosphatidylcholine emulsions of nearly identical composition were also titrated at 30 degrees C with apo-A1 and were euthermic. Association constants were previously measured using a classical centrifugation assay and were used to calculate the entropy of apo-A1 binding (+28 cal deg-1 mol-1 apo-A1). This value indicates that apo-A1 binding to a fluid surface like egg yolk phosphatidylcholine or probably DPPC at 45 degrees C is hydrophobic and is consistent with hydrocarbon lipid or protein moities coming together and excluding water. Thus the binding of apo-A1 to partly crystalline surfaces is entropically negative and increases the order of the already partly ordered phases, whereas binding to liquid surfaces is mainly an entropically driven hydrophobic process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D., Small D. M. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu Rev Biophys Biophys Chem. 1986;15:403–456. doi: 10.1146/annurev.bb.15.060186.002155. [DOI] [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977 Jul;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. B., Tercyak A. M., Glander K. E. Plasma lipoproteins of free-ranging howling monkeys (Alouatta palliata). Comp Biochem Physiol B. 1987;88(3):729–735. doi: 10.1016/0305-0491(87)90236-7. [DOI] [PubMed] [Google Scholar]
- Derksen A., Ekman S., Small D. M. Oleic acid allows more apoprotein A-1 to bind with higher affinity to large emulsion particles saturated with cholesterol. J Biol Chem. 1989 Apr 25;264(12):6935–6940. [PubMed] [Google Scholar]
- Derksen A., Small D. M. Interaction of ApoA-1 and ApoE-3 with triglyceride-phospholipid emulsions containing increasing cholesterol concentrations. Model of triglyceride-rich nascent and remnant lipoproteins. Biochemistry. 1989 Jan 24;28(2):900–906. doi: 10.1021/bi00428a074. [DOI] [PubMed] [Google Scholar]
- Gantz D., Bennett Clark S., Derksen A., Small D. M. Size and shape determination of fixed chylomicrons and emulsions with fluid or solid surfaces by three-dimensional analysis of shadows. J Lipid Res. 1990 Jan;31(1):163–171. [PubMed] [Google Scholar]
- Gwynne J., Palumbo G., Brewer H. B., Jr, Edelhoch H. The interaction of apoA-I from human high density lipoprotein with lysolecithin. J Biol Chem. 1975 Sep 25;250(18):7300–7306. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A. Interactions of triglycerides with phospholipids: incorporation into the bilayer structure and formation of emulsions. Biochemistry. 1989 Mar 21;28(6):2514–2520. doi: 10.1021/bi00432a025. [DOI] [PubMed] [Google Scholar]
- Ibdah J. A., Phillips M. C. Effects of lipid composition and packing on the adsorption of apolipoprotein A-I to lipid monolayers. Biochemistry. 1988 Sep 6;27(18):7155–7162. doi: 10.1021/bi00418a073. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Hirz R., Shrager R. I., Gotto A. M. The influence of lipid on the conformation of human plasma high density apolipoproteins. J Biol Chem. 1972 Apr 25;247(8):2598–2606. [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. W., Small D. M. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J Biol Chem. 1983 Nov 25;258(22):13772–13784. [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104. [PubMed] [Google Scholar]
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Singer M. A., Finegold L. Cholesterol interacts with all of the lipid in bilayer membranes. Implications for models. Biophys J. 1990 Jan;57(1):153–156. doi: 10.1016/S0006-3495(90)82516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner P. J., Small D. M. Effect of free cholesterol on incorporation of triolein in phospholipid bilayers. Biochemistry. 1987 Sep 8;26(18):5820–5825. doi: 10.1021/bi00392a036. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Shipley G. G., Small D. M. Conformational and thermodynamic properties of apo A-1 of human plasma high density lipoproteins. J Biol Chem. 1976 Jun 25;251(12):3749–3755. [PubMed] [Google Scholar]
- Tall A. R., Small D. M., Deckelbaum R. J., Shipley G. G. Structure and thermodynamic properties of high density lipoprotein recombinants. J Biol Chem. 1977 Jul 10;252(13):4701–4711. [PubMed] [Google Scholar]
- Tall A. R., Small D. M., Shipley G. G., Lees R. S. Apoprotein stability and lipid-protein interactions in human plasma high density lipoproteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4940–4942. doi: 10.1073/pnas.72.12.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald J. H., Krul E. S., Jonas A. Structure of apolipoprotein A-I in three homogeneous, reconstituted high density lipoprotein particles. J Biol Chem. 1990 Nov 15;265(32):20037–20043. [PubMed] [Google Scholar]
- Wiseman T., Williston S., Brandts J. F., Lin L. N. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989 May 15;179(1):131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]



