Abstract
Serotonin is implicated in the regulation of complex sensory, motor, affective, and cognitive functions. However, the biochemical mechanisms whereby this neurotransmitter exerts its actions remain largely unknown. DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of molecular weight 32,000) is a phosphoprotein that has primarily been characterized in relation to dopaminergic neurotransmission. Here we report that serotonin regulates DARPP-32 phosphorylation both in vitro and in vivo. Stimulation of 5-hydroxy-tryptamine (5-HT4 and 5-HT6) receptors causes an increased phosphorylation state at Thr34–DARPP-32, the protein kinase A site, and a decreased phosphorylation state at Thr75–DARPP-32, the cyclin-dependent kinase 5 site. Furthermore, stimulation of 5-HT2 receptors increases the phosphorylation state of Ser137–DARPP-32, the casein kinase-1 site. Behavioral and gene transcriptional effects induced by compounds that selectively release serotonin were greatly reduced in DARPP-32 knockout mice. Our data indicate that DARPP-32 is essential not only for dopaminergic but also for serotonergic neurotransmission.
The serotonergic and dopaminergic neurotransmitter systems regulate emotion, mood, reward, and cognition. Pertubations of these neurotransmitter systems are thought to contribute to the etiology of several common neuropsychiatric disorders, such as schizophrenia, bipolar disorder, depression, and drug addiction. Indeed, the serotonergic and the dopaminergic systems appear to be the primary targets for most of the current medications used for the treatment of psychiatric disorders. To better understand the etiology of these disorders and to improve therapeutic options, there is a considerable interest in elucidating the biochemical mechanisms that underlie the behavioral effects of serotonin and dopamine, in particular in prefrontal cortex and striatum, two forebrain areas that receive converging serotonergic and dopaminergic innervation (1, 2). Most reports favor the notion that serotonin and dopamine interact in a cooperative manner to exert biochemical (3, 4) and electrophysiological (5) actions, but there are also numerous publications reporting opposing actions of these neurotransmitter systems (for a recent review see ref. 6).
One well-characterized mediator of the biochemical, electrophysiological, transcriptional, and behavioral effects of dopamine is DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of molecular weight 32,000) (7–9). DARPP-32 is highly enriched in prefrontal cortex and striatum. Activation of dopamine D1 receptors, by means of stimulation of protein kinase A (PKA), phosphorylates DARPP-32 at Thr34 and thereby converts DARPP-32 into a potent inhibitor of protein phosphatase-1 (PP-1) (10). This effect is counteracted by activation of D2 receptors, by means of inhibition of PKA (11) and stimulation of the Ca2+/protein phosphatase-2B (PP-2B) signaling cascade, which dephosphorylates Thr34–DARPP-32 (12). Activation of D1 receptors also decreases the phosphorylation state of DARPP-32 at Thr75 (13). This site is phosphorylated by cyclin-dependent kinase 5 (Cdk5) (14) and dephosphorylated by protein phosphatase 2A (PP-2A) (13). The dopaminergic regulation of the phosphorylation state at Thr75–DARPP-32 is mediated by means of a PKA/PP-2A signaling cascade (13). Thus, enhanced dopaminergic transmission by means of D1 receptors leads to a decreased phosphorylation of Thr75–DARPP-32, which reduces inhibition of PKA (14), and thereby facilitates transmission by means of the PKA/Thr34–DARPP-32/PP-1 signaling cascade. The efficacy of this signaling cascade also is regulated by the phosphorylation state of DARPP-32 at Ser137, because an increased phosphorylation at Ser137–DARPP-32, by casein kinase-1 (CK-1), prevents the dephosphorylation at Thr34–DARPP-32 by protein PP-2B and thereby potentiates the PKA/Thr34–DARPP-32/PP-1 cascade (15).
In view of the critical role for serotonin in modulating mental functions, we have conducted a detailed characterization of the regulation of DARPP-32 phosphorylation by serotonin in vitro and in vivo. By the use of DARPP-32 knockout (KO) mice, we also have evaluated the possible involvement of DARPP-32 in mediating biochemical and behavioral effects of compounds that selectively release serotonin.
Materials and Methods
Preparation and Treatment of Brain Slices.
Slices (300 μm) from the neostriatum (dorsal striatum), nucleus accumbens (ventral striatum), and prefrontal cortex were prepared from adult male C57/Bl6 mice as described (12). The slices were preincubated in Krebs buffer (118 mM NaCl/4.7 mM KCl/1.5 mM Mg2SO4/1.2 mM KH2PO4/25 mM NaHCO3/11.7 mM glucose/1.3 mM CaCl2) at 30°C under constant oxygenation (95% O2/5% CO2) for 60 min, with a change of buffer after 30 min. To prevent reuptake of serotonin into nerve terminals, slices were pretreated with the serotonin reuptake inhibitor, fluoxetine (10 μM), 2 min before the application of serotonin. The slices were thereafter treated with serotonin and other drugs as specified in each experiment. After drug treatment, the buffer was removed and the slices were rapidly frozen on dry ice and stored at −80°C until immunoblotted.
Whole Animal Studies.
In experiments examining the effects of para-chloroamphetamine (PCA) and 5-hydroxytryptophan (5-HTP) on DARPP-32 phosphorylation, adult male C57/Bl6 mice were injected i.p. with saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg) and killed 15 min after the injection by focused microwave irradiation (4.5–5 kW for 1.4 s), using a small animal microwave (Muromachi Kikai, Tokyo). In these and all other experiments with 5-HTP, carbidopa (50 mg/kg) was administered 30 min before 5-HTP. The brains were rapidly removed, and striata and frontal cortices were dissected out and stored at −80°C until assayed.
Immunoblotting.
Immunoblot procedures were as described (16). In experiments using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-pretreated mice, immunoblotting was also carried out with an antibody against tyrosine hydroxylase (Chemicon). Data on protein phosphorylation are expressed as percentage of control.
In situ hybridization wild type (WT) mice and DARPP-32 KO mice (7) were given an i.p. injection with either saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg) and killed 20 min postinjection by decapitation. In situ hybridization experiments against c-fos mRNA were carried out as described (16).
Locomotor Activity Measurements.
Locomotor activity of WT and DARPP-32 KO mice was measured in activity monitors (43 × 44 × 45 cm) equipped with both horizontal and vertical sensors (Opto-Varimex, Columbus Instruments, Columbus, OH). Mice were acclimated to the boxes for 1 h, 2–4 days before the day of the experiment. The day of the experiment, mice were placed in the boxes for 20 min (habituation period) before drug administration. Saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg; 30 min after 50 mg/kg carbidopa i.p.) was injected i.p., and locomotor activity was measured for 50 min. In some experiments, adult male C57/Bl6 mice were pretreated with clozapine (0.3 mg/kg) 10 min before the administration of saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg). Locomotor activity parameters (distance traveled and stereotypic movements) were quantified by using the AutoTrack System (Columbus Instruments).
Results
Regulation by Serotonin of DARPP-32 Phosphorylation in Brain Slices.
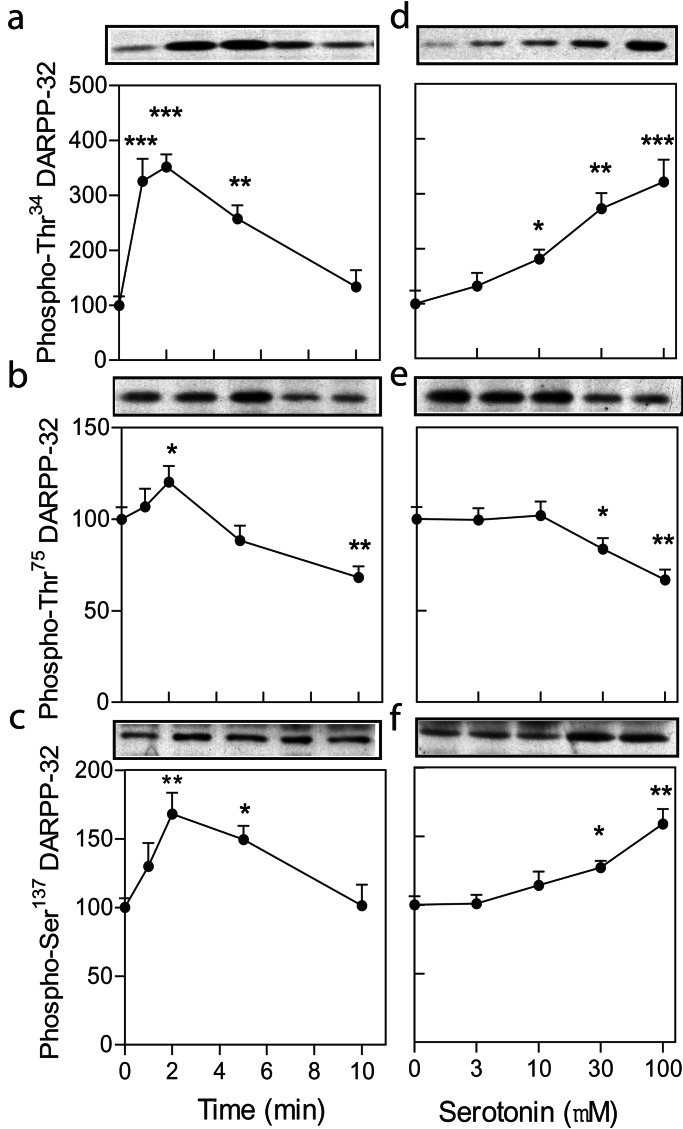
It was shown that serotonin regulates the phosphorylation of DARPP-32 at Thr34 (the PKA site), Thr75 (the Cdk5 site), and Ser137 (the CK-1 site) (16). Here we have studied this phenomenon in greater detail, using slices prepared from neostriatum (dorsal striatum), nucleus accumbens (ventral striatum), and prefrontal cortex. Time-course experiments in neostriatal slices showed that serotonin (100 μM) caused a rapid increase in DARPP-32 phosphorylation at all three phosphorylation sites (Fig. 1 a–c), with the effect at Thr34 being the most pronounced. The state of phosphorylation of DARPP-32 at Thr34 and Ser137 returned to basal levels within 10 min (Fig. 1 a–c) and remained there at 30 min (not shown). In contrast, the small, but significant, increase in DARPP-32 phosphorylation at Thr75 was followed by a pronounced decrease in the state of phosphorylation at this site. No changes in the total levels of DARPP-32 were found at any time point. Based on these data, slices were incubated for 2 min for studies of phospho-Thr34–DARPP-32 and phospho-Ser137–DARPP-32, and for 10 min for studies of phospho-Thr75–DARPP-32 in all subsequent experiments. A lower concentration of serotonin was required to regulate DARPP-32 phosphorylation at Thr34 than at Thr75 and Ser137 (Fig. 1 d–f). The EC50 values for the effects of serotonin on DARPP-32 phosphorylation at Thr34, Thr75, and Ser137 were 13.5, 29.6, and 37.6 μM, respectively.
Figure 1.
Regulation by serotonin of DARPP-32 phosphorylation in slices of neostriatum. (a–c) Time course and (d–f) dose–response studies of regulation by serotonin of phosphorylation of DARPP-32 at (a and d) Thr34, (b and e) Thr75, and (c and f) Ser137 in neostriatum. (a–c) Serotonin was used at 100 μM. (d–f) Slices were incubated for 2 min for studies of phospho-Thr34–DARPP-32 and phospho-Ser137–DARPP-32 and for 10 min for studies of phospho-Thr75–DARPP-32. Data represent means (± SE) (n = 6–10). *, P < 0 05; **, P <0.01; ***, P < 0.001 compared with control; one-way ANOVA followed by Dunnett's test.
Using slices prepared from nucleus accumbens, it was found that 2 min of incubation with serotonin (100 μM) increased phosphorylation at Thr34 (307 ± 25%, n = 12) and Ser137 (143 ± 7%, n = 12), and that 10 min of incubation with serotonin decreased phosphorylation at Thr75 (75 ± 6%, n = 12). Experiments carried out in slices from the prefrontal cortex showed that 2 min of incubation with serotonin increased phospho-Thr34–DARPP-32 (185 ± 15%, n = 6) and that 10 min of incubation with serotonin decreased phospho-Thr75–DARPP-32 (85 ± 3%, n = 6). Because of a low signal in prefrontal cortex, the level of phospho-Ser137–DARPP-32 could not be accurately assayed in this area. Thus, the regulation of phosphorylation of DARPP-32 by serotonin was similar in all three brain regions. Because more slices can be collected from neostriatum than from nucleus accumbens, slices made from neostriatum were used in most of the subsequent experiments.
Effects of 5-Hydroxytryptamine (5-HT) Receptor Agonists on DARPP-32 Phosphorylation in Neostriatal and Cortical Slices.
Several serotonin receptors, most notably 5-HT1B, 5-HT2A/C, 5-HT3, 5-HT4, and 5-HT6, are expressed throughout the striatum. The identity of the serotonin receptors involved in the serotonin-mediated regulation of DARPP-32 phosphorylation was determined by the use of selective agonists and antagonists. Results obtained with various serotonin agonists in neostriatal slices are shown in Table 1. 2-[1-(4-Piperonyl)piperazinyl]benzothiazole (PPB, a 5-HT4 receptor agonist) and 2-ethyl-5-methoxy-N,N-dimethyltryptamine (EMDT, a 5-HT6 receptor agonist), alone or together, qualitatively mimicked the action of serotonin at phospho-Thr34–DARPP-32 and phospho-Thr75–DARPP-32, but not at phospho-Ser137–DARPP-32. The EC50 values for the actions of PPB and EMDT at phospho-Thr34–DARPP-32 were 27.3 and 20.5 μM, and at phospho-Thr75–DARPP-32 were 45.5 and 27.4 μM, respectively. In addition, 5-methoxytryptamine (100 μM), which has a high affinity as an agonist at 5-HT4 and 5-HT6 receptors, increased phospho-Thr34–DARPP-32 (261 ± 31%, n = 12) and decreased phospho-Thr75–DARPP-32 (76 ± 7%, n = 12). Conversely, DOI [(±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride; (±)-1-(2,5 dimethoxy-4-iodophenyl)-2-aminopropane], a 5-HT2A/C agonist, mimicked the action of serotonin at phospho-Ser137–DARPP-32, but not at phospho-Thr34–DARPP-32 and phospho-Thr75–DARPP-32. The EC50 value for DOI at phospho-Ser137–DARPP-32 was 18.2 μM. The 5-HT1B receptor agonist, anpirtoline (presumably by decreasing cAMP), and the 5-HT3 agonist, SR57227A (presumably by increasing Ca2+), decreased phospho-Thr34–DARPP-32, but were without significant effects on phospho-Thr75–DARPP-32 and phospho-Ser137–DARPP-32.
Table 1.
Regulation of DARPP-32 phosphorylation in vitro by 5-HT receptor agonists and antagonists
| Thr34 | Thr75 | Ser137 | |
|---|---|---|---|
| Control | 100 (17.0) | 100 (5.0) | 100 (3.9) |
| Serotonin | 342 (26.7)** | 76 (7.5)** | 141 (6.0)** |
| 5-HT receptor agonists | |||
| PPB (5HT4 agonist) | 218 (36.1)* | 79 (8.8)* | 107 (2.0) |
| EMDT (5HT6 agonist) | 301 (39.5)** | 76 (7.6)** | 103 (5.5) |
| PPB + EMDT | 397 (41.0)** | 70 (3.8)** | 105 (7.9) |
| DOI (5HT2 agonist) | 144 (10.7) | 105 (4.5) | 158 (8.3)** |
| Anpirtoline (5HT1B agonist) | 86 (3.7)* | 102 (22.2) | 93 (10.0) |
| SR 57227 (5HT3 agonist) | 78 (3.5)* | 88 (6.8) | 105 (9.6) |
| 5-HT receptor antagonists | |||
| SDZ 205,557 (5HT4 antagonist) | 94 (16.7) | 103 (17.5) | 102 (8.8) |
| SDZ + serotonin | 257 (34.4)**† | 95 (11.5)† | 129 (5.0)* |
| Ro 04-6790 (5HT6 antagonist) | 90 (8.7) | 101 (12.5) | 105 (7.3) |
| Ro + serotonin | 225 (28.5)*† | 96 (8.4)† | 131 (7.2)* |
| Ro + SDZ | 84 (6.5) | 110 (11.5) | 107 (6.2) |
| SDZ + Ro + serotonin | 198 (44.5)*‡ | 98 (6.1)‡ | 134 (18.1)* |
| Ketanserin (5HT2 antagonist) | 94 (2.9) | 105 (8.9) | 97 (4.8) |
| Ketanserin + serotonin | 322 (28.4)** | 78 (12.1)* | 101 (9.1)† |
| Clozapine (5HT2/6 antagonist) | 124 (19.8) | 101 (13.4) | 105 (5.5) |
| Clozapine + serotonin | 156 (15.4)*‡ | 98 (5.2)‡ | 110 (4.9)† |
Effects of serotonin (100 μM), PPB (100 μM), a 5-HT4 receptor agonist, EMDT (100 μM), a 5-HT6 receptor agonist, DOI (100 μM), a 5-HT2 receptor agonist, anpirtoline, (100 μM), a 5-HT1B receptor agonist, and SR 57227 (100 μM), a 5-HT3 receptor agonist, on Thr34, Thr75, and Ser137. In antagonist experiments, ketanserin (10 μM), a 5-HT2 receptor antagonist, SDZ 205,557 (10 μM), a 5-HT4 receptor antagonist, Ro 04-6790 (10 μM), a 5-HT6 receptor antagonist, or clozapine (10 μM), a multireceptor antagonist with high affinity for 5-HT2 and 5-HT6 receptors, was applied to striatal slices 10 min before serotonin (100 μM). Data represent means (± SEM) (n = 12–30).
, P < 0.05;
, P < 0.01;
, P < 0.001 compared with control;
, P < 0.05;
, P < 0.01 compared with serotonin alone; one-way ANOVA followed by Dunnett's test.
Using slices prepared from the prefrontal cortex, it was found that 2 min of incubation with the 5-HT4 receptor agonist, PPB, or the 5-HT6 receptor agonist, EMDT, increased phospho-Thr34–DARPP-32 (203 ± 15%, n = 6, and 185 ± 19%, n = 6, respectively), whereas 10 min of incubation with PPB or EMDT decreased phospho-Thr75–DARPP-32 (84 ± 4%, n = 6, and 80 ± 7%, n = 6, respectively).
Effects of 5-HT Receptor Antagonists on Serotonin-Mediated DARPP-32 Phosphorylation in Neostriatal Slices.
The results obtained with selective serotonin receptor antagonists (Table 1) were consistent with the results obtained with agonists. Thus, SDZ 205,557, a 5-HT4 receptor antagonist, and Ro 04–6790, a 5-HT6 receptor antagonist, alone or together, reduced the effects of serotonin on phospho-Thr34–DARPP-32 and phospho-Thr75–DARPP-32, but not on phospho-Ser137–DARPP-32. Conversely, ketanserin, a 5-HT2A/C receptor antagonist, reduced the effects of serotonin on phospho-Ser137–DARPP-32 phosphorylation, but not on phospho-Thr34–DARPP-32 or phospho-Thr75–DARPP-32. The multireceptor antagonist, clozapine, which has a particularly high affinity for 5-HT2 and 5-HT6 receptors (17), significantly counteracted serotonin-mediated phosphorylation of DARPP-32 at all sites examined. Taken together, the results obtained with serotonin receptor agonists and antagonists indicate that the effects of serotonin on DARPP-32 phosphorylation can be accounted for primarily by activation of 5-HT2, 5-HT4, and 5-HT6 receptors.
Effects of Signal Transduction Modulators on Serotonin-Mediated DARPP-32 Phosphorylation in Neostriatal Slices.
To investigate whether the effects of serotonin on DARPP-32 phosphorylation were mediated through a direct action on DARPP-32-containing neurons, slices were pretreated with tetrodotoxin (TTX), which inhibits action potential-dependent synaptic transmission by blocking voltage-gated sodium channels. The actions of serotonin on DARPP-32 phosphorylation were unaffected by TTX, indicating a direct action (Table 2). The principal signal transduction pathways used by 5-HT2 and 5-HT4/5-HT6 receptors involve activation of phospholipase C and PKA, respectively. To investigate the contributions of these signaling pathways to the action of serotonin, striatal slices were pretreated with either U-73122, a selective phospholipase C inhibitor, or Rp-cAMPS, a selective PKA inhibitor, before the application of serotonin. As shown in Table 2, U-73122 blocked the effect of serotonin on phospho-Ser137–DARPP-32, but not on phospho-Thr34–DARPP-32 or phospho-Thr75–DARPP-32. Conversely, Rp-cAMPS inhibited the action of serotonin on phospho-Thr34–DARPP-32 and phospho-Thr75–DARPP-32, but not on phospho-Ser137–DARPP-32.
Table 2.
Regulation of serotonin-mediated DARPP-32 phosphorylation in vitro by signal transduction modulators
| Thr34 | Thr75 | Ser137 | |
|---|---|---|---|
| Control | 100 (17.0) | 100 (5.0) | 100 (3.9) |
| Serotonin | 342 (26.7)** | 76 (7.5)** | 141 (6.0)** |
| TTX | 92 (5.8) | 103 (3.5) | 98 (12.5) |
| TTX + serotonin | 348 (63.2)** | 83 (8.1)* | 144 (3.3)** |
| U-73122 (PLC inhibitor) | 117 (10.9) | 90 (6.6) | 86 (6.2) |
| U-73122 + serotonin | 297 (34.0)** | 70 (6.1)** | 89 (3.7)‡ |
| RpcAMPs (PKA inhibitor) | 88 (13.1) | 105 (7.2) | 108 (10.0) |
| RpcAMPs + serotonin | 150 (44.7)‡ | 102 (9.5)† | 147 (4.0)** |
TTX (2 μM), an inhibitor of voltage-gated sodium channels, U-73122 (10 μM), a phospholipase C inhibitor, or Rp-cAMPS (300 μM), a PKA inhibitor, were applied to striatal slices 10 min before serotonin (100 μM). Data represent means (± SEM) (n = 10–30).
, P < 0.05;
, P < 0.01;
, P < 0.001 compared with no serotonin;
, P < 0.05;
, P < 0.01 compared with serotonin alone; one-way ANOVA followed by Dunnett's test.
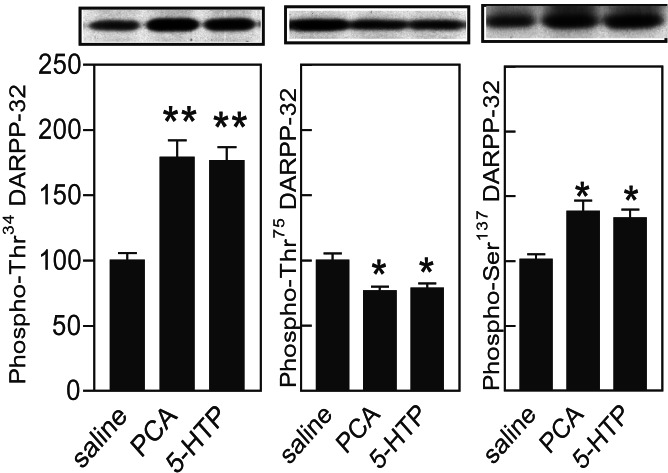
Regulation of DARPP-32 Phosphorylation in Vivo by PCA and 5-HTP.
Mice were injected i.p. with the serotonin releaser, PCA (4 mg/kg), or the serotonin precursor, 5-HTP (50 mg/kg). The results shown in Fig. 2 demonstrate that in striatum PCA and 5-HTP cause a common pattern of alteration of DARPP-32 phosphorylation, i.e., an increase at phospho-Thr-34–DARPP-32 and phospho-Ser137–DARPP-32 and a decrease at phospho-Thr75–DARPP-32. Furthermore, in prefrontal cortex PCA and 5-HTP increased phospho-Thr34–DARPP-32 (147 ± 4.8%, n = 5 mice, and 144 ± 8.3%, n = 5 mice, respectively), but decreased phospho-Thr75–DARPP-32 (79 ± 3.8%, n = 5 mice, and 74 ± 8.0%, n = 5, respectively). Thus, serotonin regulates DARPP-32 phosphorylation in a qualitatively similar fashion both in vitro and in vivo.
Figure 2.
Regulation of DARPP-32 phosphorylation in vivo by PCA and 5-HTP. Mice were injected i.p. with saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg). Fifteen minutes later mice were killed by focused microwave irradiation. Data represent means ± SE for 5–10 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with saline-treated mice; one-way ANOVA followed by Dunnett's test.
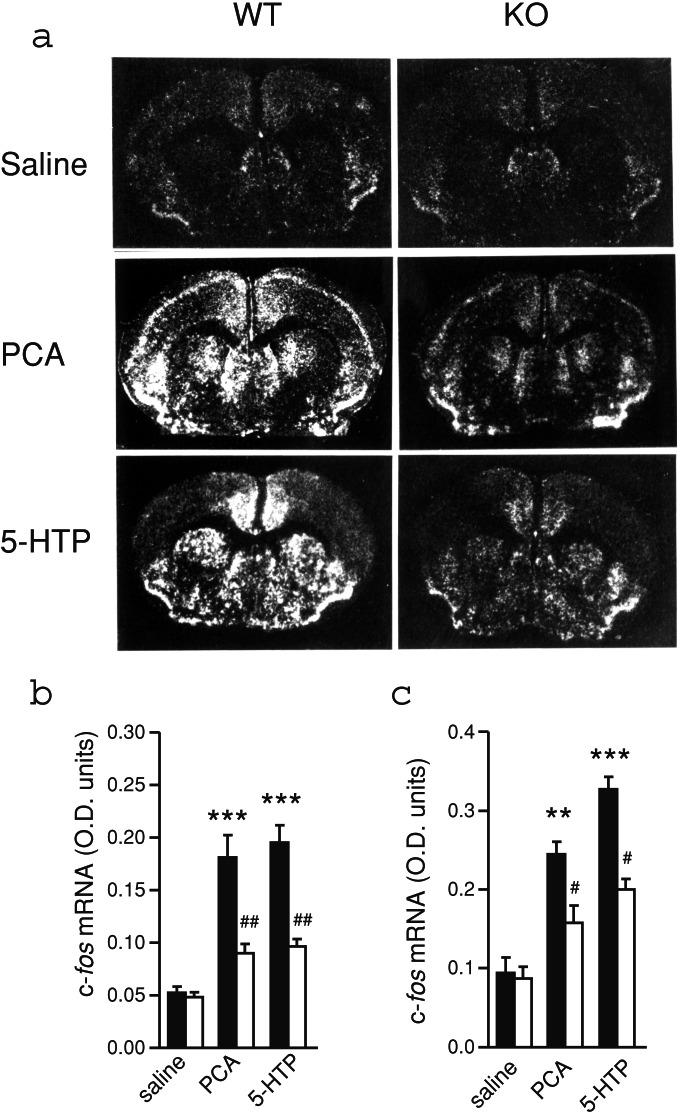
Involvement of DARPP-32 in the Regulation of c-fos mRNA Expression by PCA and 5-HTP.
To evaluate the relevance of DARPP-32 in mediating actions of PCA and 5-HTP on gene transcription, WT and DARPP-32 KO mice were injected with PCA or 5-HTP, and the effects on c-fos mRNA expression were investigated. As illustrated in Fig. 3a, both PCA and 5-HTP induced a strong expression of c-fos mRNA throughout striatum and cerebral cortex of WT mice. This effect, which was quantitated in the periventricular region of striatum (Fig. 3b) and in the cingulate cortex (Fig. 3c), was strongly attenuated in DARPP-32 KO mice.
Figure 3.
Regulation of c-fos mRNA expression by PCA and 5-HTP in WT and DARPP-32 KO mice. (a) Dark-field autoradiograms showing the expression of c-fos mRNA 20 min after treatment with saline, PCA (4 mg/kg), or 5-HTP (50 mg/kg) in WT and DARPP-32 KO mice. (Magnification: ×5.) (b and c) Histograms show quantification of the expression of c-fos mRNA in (b) periventricular area of striatum and (c) cingulate cortex after each treatment. WT, filled bars; DARPP-32 KO, open bars. Data represent means ± SE for 5–8 mice per group. **, P < 0.01; ***, P < 0.001 compared with saline-treated mice; #, P < 0.05; ##, P < 0.01 compared with WT mice given the same treatment; one-way ANOVA followed by Dunnett's test.
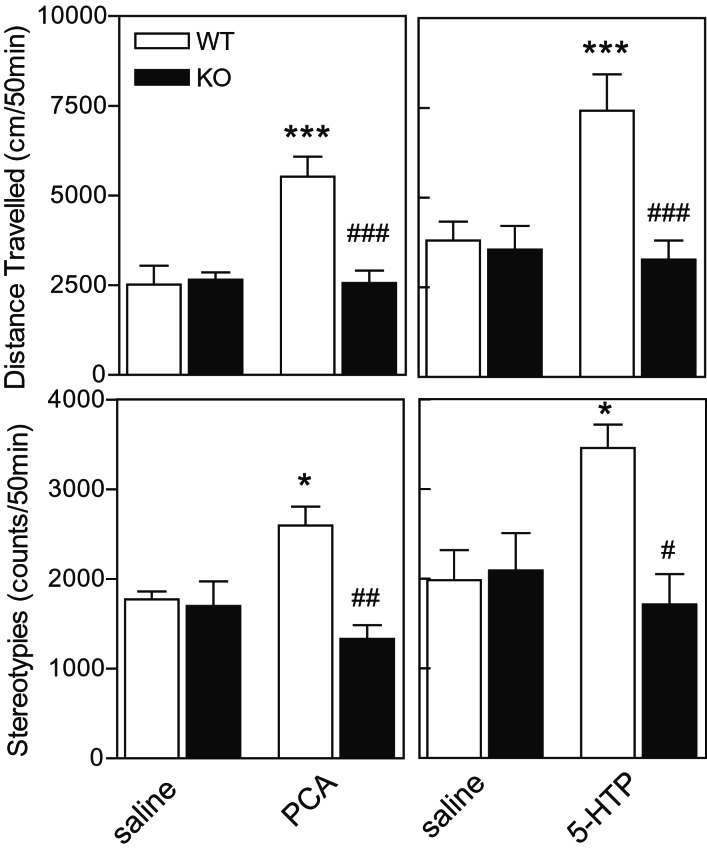
Involvement of DARPP-32 in the Behavioral Effects of PCA and 5-HTP.
To investigate the role of DARPP-32 in mediating behavioral actions exerted by an enhanced release of serotonin, the effects of PCA and 5-HTP on locomotor activity and stereotypic behaviors were examined in WT and DARPP-32 KO mice. PCA as well as 5-HTP caused a robust increase both in locomotor activity and stereotypic behavior in WT mice (Fig. 4). The actions on both of these behavioral parameters were abolished in DARPP-32 KO mice. The attenuated responsiveness of DARPP-32 KO mice to PCA and 5-HTP cannot be explained by a loss of ability of these mice to be hyperactive. In experiments in which drug-naive animals were exposed to the novel environment represented by the test cages, we found no difference in locomotor activity scores between WT (6,133 ± 293 cm/20 min) and DARPP-32 KO (6,268 ± 418 cm/20 min) mice.
Figure 4.
Behavioral responses to treatment with PCA and 5-HTP in WT and DARPP-32 KO mice. Effects of (Left) PCA (4 mg/kg) and (Right) 5-HTP (50 mg/kg) on total locomotion (Upper) and stereotypic movements (Lower) in WT and DARPP-32 KO mice. Data represent means ± SE for 6–10 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with saline-treated mice; ###, P < 0.001; ##, P < 0.01; #, P < 0.05 compared with WT mice given the same treatment; two-way ANOVA followed by Duncan's test.
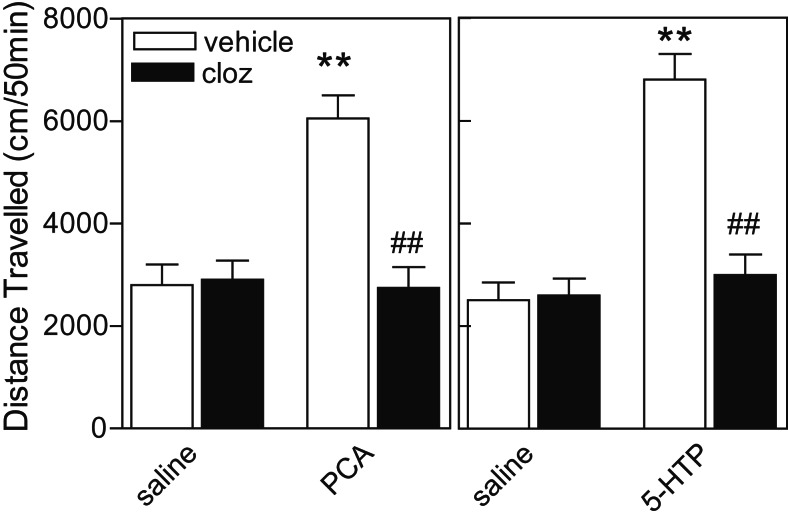
Blockade by Clozapine of Behavioral Effects of PCA and 5-HTP.
As demonstrated above, the atypical neuroleptic agent, clozapine, which has a very high affinity at 5-HT2 and 5-HT6 receptors, strongly counteracted the effects of serotonin on DARPP-32 phosphorylation. We therefore evaluated the effects of pretreatment with a relatively low dose of clozapine (0.3 mg/kg) on PCA- and 5-HTP-induced behaviors. The results shown in Fig. 5 demonstrate that clozapine abolished the hyperactivity induced by PCA or 5-HTP. It should be noted that clozapine also has a moderate affinity for several dopamine receptors (17). However, previous work has shown that at least 10 times higher concentrations (2–10 mg/kg) of clozapine are necessary to inhibit dopamine-dependent hyperactivity (17).
Figure 5.
Effect of clozapine on behavioral responses to treatment with PCA and 5-HTP in WT mice. Effect of pretreatment with clozapine (0.3 mg/kg) on locomotion induced by (Left) PCA (4 mg/kg) or (Right) 5-HTP (50 mg/kg). Data represent means ± SE for 6–10 mice per group. **, P < 0.01 compared with saline-treated mice; ##, P < 0.01 compared with PCA-treated or 5-HTP-treated mice; two-way ANOVA followed by Duncan's test.
Characterization of Serotonin-Dopamine Interactions in the Regulation of DARPP-32 Phosphorylation.
To determine whether the effects of serotonin on DARPP-32 phosphorylation were mediated through modulation of dopaminergic signaling pathways, several types of experiments were carried out (Table 3). Serotonin and SKF 82958, a D1 receptor-selective agonist, had additive effects in increasing phospho-Thr34–DARPP-32 and decreasing phospho-Thr75–DARPP-32. Serotonin and quinpirole, a D2 receptor-selective agonist, had antagonistic effects on phospho-Thr34–DARPP-32 and phospho-Thr75–DARPP-32 and additive effects in increasing phosphorylation at phospho-Ser137–DARPP-32. The effects of serotonin on DARPP-32 phosphorylation were not significantly altered in slices from D1 receptor KO mice (unpublished results) or from mice pretreated with the dopaminergic neurotoxin, MPTP (Table 3), indicating that the effects of serotonin on DARPP-32 phosphorylation were not mediated through regulation of dopamine release.
Table 3.
Interactions of serotonin and dopamine signaling pathways on DARPP-32 phosphorylation in slices
| Thr34 | Thr75 | Ser137 | |
|---|---|---|---|
| Control | 100 (17.0) | 100 (5.0) | 100 (3.9) |
| Serotonin | 342 (26.7)** | 76 (7.5)** | 141 (6.0)** |
| SKF 82958 (D1 agonist) | 552 (59.1)** | 63 (1.7)** | 105 (14.7) |
| SKF 82958 + serotonin | 717 (42.4)**#§ | 56 (1.1)**#§ | 121 (14.4) |
| Quinpirole (D2 agonist) | 65 (4.7)** | 112 (8.5) | 135 (5.1)* |
| Quinpirole + serotonin | 125 (12.7)169† | 106 (13.2)167 | 186 (11.1)**#† |
| MPTP treatment | 93 (8.7) | 118 (9.3) | 104 (7.9) |
| MPTP-treatment + serotonin | 307 (44.1)** | 80 (9.2)* | 154 (21.1)** |
SKF 82958 (10 μM), a D1 receptor agonist, or quinpirole (10 μM), a D2 receptor agonist, was applied 10 min before serotonin (100 μM). MPTP (50 mg/kg, i.p.) was administered i.p. four times at 12-h intervals, 4 weeks before the slice experiment. This pretreatment caused a 85–93% reduction in the levels of tyrosine hydroxylase in striatum. Data represent means (± SEM) (n = 16–30).
, P < 0.05;
, P < 0.01;
, P < 0.001 compared with control;
, P < 0.05;
, P < 0.01;
, P < 0.001 compared with serotonin alone;
, P < 0.05 compared with SKF 82958 alone;
, P < 0.05 compared with quinpirole alone; one-way ANOVA followed by Dunnett's test.
Discussion
The present studies, and those described in ref. 16, have demonstrated that behavioral as well as biochemical effects induced by enhanced serotonergic neurotransmission are attenuated in DARPP-32 KO mice. The results show an important role for DARPP-32 in mediating the actions of serotonin in vivo. We have investigated the underlying molecular mechanisms.
Neostriatum, nucleus accumbens, and prefrontal cortex receive a moderate to high serotonergic innervation (1), which is further increased when the levels of dopamine in this region are decreased (18). Several serotonin receptors, most notably 5-HT1B, 5-HT1E, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, and 5-HT6, are expressed in striatum (6). All of them are metabotropic receptors, with the exception of 5-HT3 receptors, which are ionotropic. These serotonin receptors act primarily by means of the following second messenger systems: 5-HT1B/E receptors decrease cAMP formation; 5-HT2A/C receptors increase inositol triphosphate and diacylglycerol formation; 5-HT3 receptors increase Na+ and Ca2+ influx; and 5-HT4 and 5-HT6 receptors increase cAMP formation.
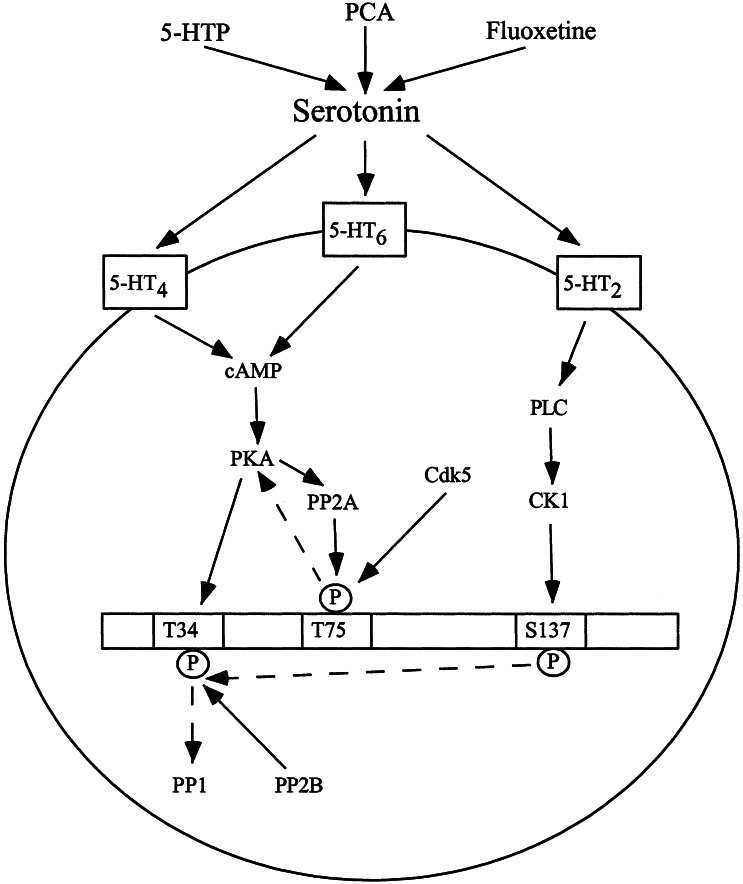
Serotonin caused an increased phosphorylation of DARPP-32 at Thr34 (the PKA site) and Ser137 (the CK-1 site) and a decreased phosphorylation at Thr75 (the Cdk5 site), in brain slices. The use of selective serotonin receptor agonists and antagonists, and other pharmacological reagents, including TTX, U-73122, and Rp-cAMPs, has enabled us to elucidate the underlying signaling pathways. The actions of serotonin in regulating DARPP-32 phosphorylation at Thr34 and Thr75 were mediated primarily by means of activation of 5-HT4 and 5-HT6 receptors, whereas the regulation at Ser137 was mediated primarily by means of 5-HT2 receptors. The three pathways appear to inhibit PP-1 through synergistic mechanisms (Fig. 6) and provide a model by which serotonin as well as compounds which increase serotonin transmission may produce their effects. These latter compounds include, in addition to PCA and 5-HTP, the selective serotonin reuptake inhibitor, fluoxetine, a widely used antidepressant agent.
Figure 6.
Model summarizing pathways by which enhanced serotonergic transmission regulates DARPP-32 phosphorylation at Thr34, Thr75, and Ser137. Activation of 5-HT4 or 5-HT6 receptors sequentially increases cAMP levels, activity of PKA, and phosphorylation of Thr34. Activated PKA also phosphorylates and activates protein phosphatase 2A (PP-2A) (13), causing dephosphorylation of the Cdk5 site, Thr75 (14), removing inhibition of PKA (14) and increasing phosphorylation of Thr34. 5-HT2 receptors activate PLC, increasing casein kinase-1 (CK1) activity (27), phosphorylation of Ser137, and inhibition of dephosphorylation by protein phosphatase-2B (PP2B) of Thr34 (15). These various actions of serotonin on DARPP-32 phosphorylation are synergistic, each leading to an increased state of phosphorylation of Thr34 and an increased inhibition of PP-1 (10). Solid lines indicate activation; dashed lines indicate inhibition.
The pattern of DARPP-32 phosphorylation induced by elevated serotonergic neurotransmission is very similar to that induced by elevated dopaminergic neurotransmission (13). However, the serotonin-mediated regulation of DARPP-32 phosphorylation is largely independent of altered dopaminergic neurotransmission (Table 3). Numerous psychoactive compounds act directly on both the serotonergic and dopaminergic systems. For example, cocaine inhibits the reuptake of both dopamine and serotonin from the synapse. It has recently been shown that the reinforcing properties of cocaine in the place preference test remain intact in dopamine transporter KO mice or serotonin transporter KO mice, but are severely impaired in mice that lack both transporters (19). These data imply that certain biochemical effects of cocaine can be mediated either by dopamine or serotonin. Our data indicate that signaling through the DARPP-32 pathway may be responsible for this redundancy. In support of this suggestion, the diminished responsiveness to the reinforcing properties of cocaine in the place preference test of the double transporter KO mice is mimicked by the DARPP-32 KO mice (20).
Evidence that the effects of serotonin on DARPP-32 phosphorylation observed in vitro are of physiological relevance came from studies in which we injected mice with PCA or 5-HTP and examined the effects on DARPP-32 phosphorylation in the striatum and prefrontal cortex. Both treatments led to a pattern of DARPP-32 phosphorylation that closely resembled that observed in slices in response to serotonin, namely increases at phospho-Thr34–DARPP-32 and phospho-Ser137–DARPP-32 and a decrease at phospho-Thr75–DARPP-32. It seemed possible that the effects of 5-HTP on DARPP-32 phosphorylation were attributable mainly to ectopically released serotonin produced in dopaminergic nerve terminals. However, the fact that PCA, which acts selectively on serotonergic nerve terminals, at the dose used, also had pronounced effects on DARPP-32 phosphorylation in the striatum and the prefrontal cortex, shows that an enhanced release of serotonin in these areas regulates DARPP-32 phosphorylation. The conclusion that DARPP-32 mediates the behavioral effects of serotonin is supported by experiments showing that 0.3 mg/kg of clozapine, a dose that antagonize responses by means of 5-HT2 and 5-HT6 receptors, counteracts the locomotor effects of PCA and 5-HTP.
Strong evidence for an involvement of DARPP-32 in the biochemical and behavioral actions of serotonin under in vivo conditions came from studies in which we examined effects of PCA and 5-HTP in WT and DARPP-32 KO mice. An induction of c-fos mRNA was found throughout striatum and cerebral cortex in WT mice treated with PCA or 5-HTP. The ability of both PCA and 5-HTP to induce c-fos mRNA expression was strongly reduced in DARPP-32 KO mice. It is well known that the transcription of c-fos mRNA is negatively regulated by PP-1 (21, 22). The critical involvement of DARPP-32 in PCA- and 5-HTP-mediated c-fos induction is likely explained, at least in part, by its ability to inhibit PP-1.
Previous work implicated serotonin in the regulation of locomotion and exploratory behavior (23). Depletion of serotonin caused a long-term reduction in exploration (24), whereas local application of serotonin into nucleus accumbens caused an increase in locomotion (25) in rats. It is generally agreed that agents that increase the release of serotonin, such as PCA, 5-HTP, and 3,4-methylenedioxymethamphetamine (“Ecstasy”) increase locomotor activity (23, 26). To further investigate the functional relevance of DARPP-32 phosphorylation in the actions of PCA and 5-HTP, the effects of these drugs on locomotor activity and stereotypic behaviors were examined in WT and DARPP-32 KO mice. As expected, both PCA and 5-HTP increased locomotor activity and stereotypic behaviors in WT mice. Both of these responses were greatly attenuated in DARPP-32 KO mice.
In conclusion, the studies described here, together with those described in ref. 16, indicate that DARPP-32 plays a central role in serotonergic signaling. Moreover, the data provide the outline of a molecular mechanism for the behavioral actions of those psychostimulants and antidepressant agents that achieve their effects through pertubation of serotonergic transmission.
Acknowledgments
We thank D. Nelson and A. Nishi for their critical reading of this manuscript. We thank G. L. Snyder, J. A. Bibb, and H. C. Hemmings Jr. for antibodies against phospho-Thr34 DARPP-32, phospho-Thr75 DARPP-32, and total DARPP-32 and S. Col, S. Galdi, and L. Lannebo for technical assistance. This work was supported by National Institute of Mental Health Grants MH-40899 and DA10044 (to P.G.). P.S. is supported by a postdoctoral fellowship from Stiftelsen för Internationalisering av Högre utbildning och forskning. 2-Ethyl-5-methoxy-N,N-dimethyltryptamine was synthesized by Eli Lilly & Company.
Abbreviations
- Cdk5
cyclin-dependent kinase 5
- CK-1
casein kinase 1
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of molecular weight 32,000
- EMDT
2-ethyl-5-methoxy-N,N-dimethyltryptamine
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
- KO
knockout
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PCA
para-chloroamphetamine
- PKA
protein kinase A
- PP-1
protein phosphatase-1
- PPB
2-[1-(4-piperonyl)piperazinyl]benzothiazole
- TTX
tetrodotoxin
- WT
wild type
References
- 1.Steinbusch H W. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Björklund A. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, editors. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- 3.Bhat R V, Baraban J M. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- 4.Genova L M, Hyman S E. Synapse. 1998;30:71–78. doi: 10.1002/(SICI)1098-2396(199809)30:1<71::AID-SYN9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.White F J, Hu X, Henry D J. J Pharmacol Exp Ther. 1993;266:1075–1084. [PubMed] [Google Scholar]
- 6.Barnes N M, Sharp T. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Fienberg A A, Hiroi N, Mermelstein P G, Song W J, Snyder G L, Nishi A, Cheramy A, OCallaghan J P, Miller D B, Cole D G, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 8.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 9.Greengard P. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 10.Hemmings H C, Jr, Greengard P, Tung H Y L, Cohen P. Nature (London) 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 11.Lindskog M, Svenningsson P, Fredholm B B, Greengard P, Fisone G. Neuroscience. 1998;88:1005–1008. doi: 10.1016/s0306-4522(98)00411-4. [DOI] [PubMed] [Google Scholar]
- 12.Nishi A, Snyder G L, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishi A, Bibb J A, Snyder G L, Higashi H, Nairn A C, Greengard P. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bibb J A, Snyder G L, Nishi A, Yan Z, Meijer L, Fienberg A A, Tsai L H, Kwon Y T, Girault J A, Czernik A J, et al. Nature (London) 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 15.Desdouits F, Siciliano J C, Greengard P, Girault J-A. Proc Natl Acad Sci USA. 1995;92:2682–2685. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svenningsson P, Tzavara E T, Witkin J M, Fienberg A A, Nomikos G G, Greengard P. Proc Natl Acad Sci USA. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leysen J E. In: Atypical Antipsychotics. Ellenbroek B A, Cools A R, editors. Basel: Birkhäuser; 2000. pp. 57–81. [Google Scholar]
- 18.Rozas G, Liste I, Guerra M J, Labandeira-Garcia J L. Neurosci Lett. 1998;245:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 19.Sora I, Hall S, Andrews A M, Itokawa M, Li X-F, Wei H-B, Wichems C, Lesch K-P, Murphy D L, Uhl G R. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariou, V., Benoit-Marandl, M., Allen, P. B., Ingrassia, P., Fienberg, A. A., Gonon, F., Greengard, P. & Picciotto, M. (2002) Biol. Psychiatry, in press. [DOI] [PubMed]
- 21.Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- 22.Liu F-C, Graybiel A M. Neuron. 1996;17:1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- 23.Geyer M A. Behav Brain Res. 1996;73:31–36. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.Lipska B K, Jaskiw G E, Arya A, Weinberger D R. Pharmacol Biochem Behav. 1992;43:1247–1252. doi: 10.1016/0091-3057(92)90510-m. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki-Adams D M, Kelley A E. Neuropsychopharmacology. 2001;25:440–452. doi: 10.1016/S0893-133X(01)00240-8. [DOI] [PubMed] [Google Scholar]
- 26.Modigh K. Psychopharmacology. 1972;23:48–54. [Google Scholar]
- 27.Liu F, Ma X H, Ule J, Bibb J A, Nishi A, DeMaggio A J, Yan Z, Nairn A C, Greengard P. Proc Natl Acad Sci USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]