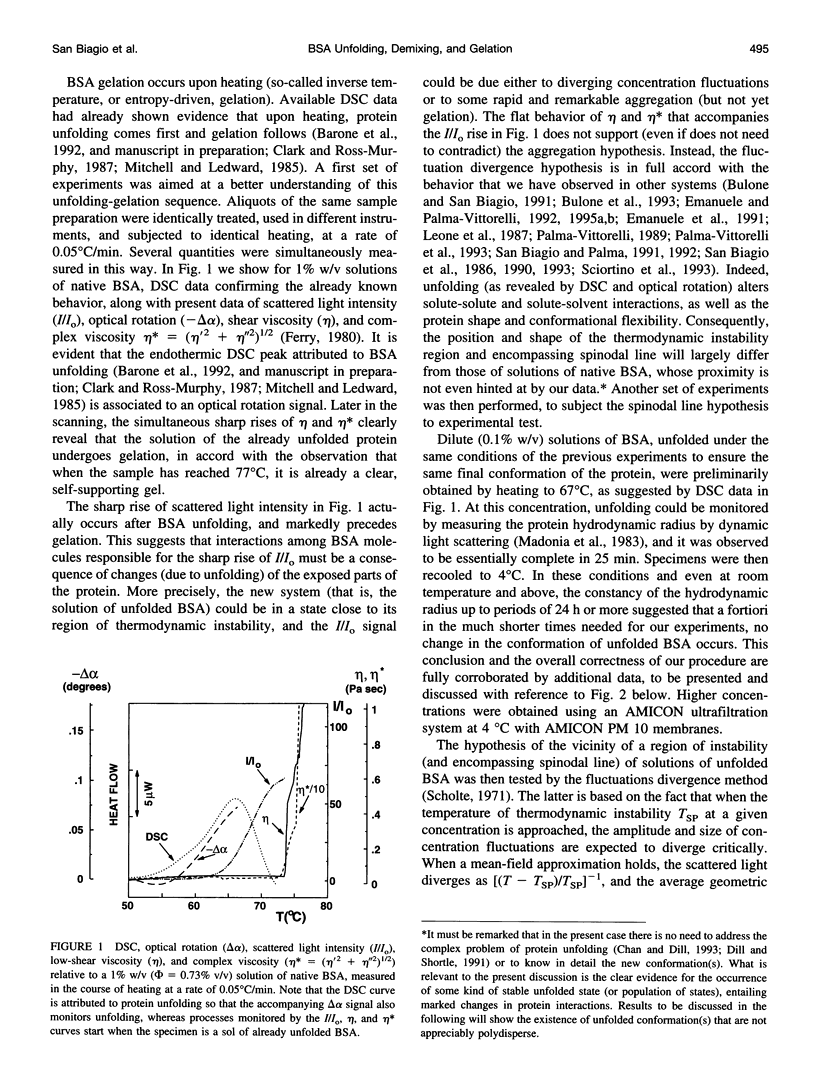
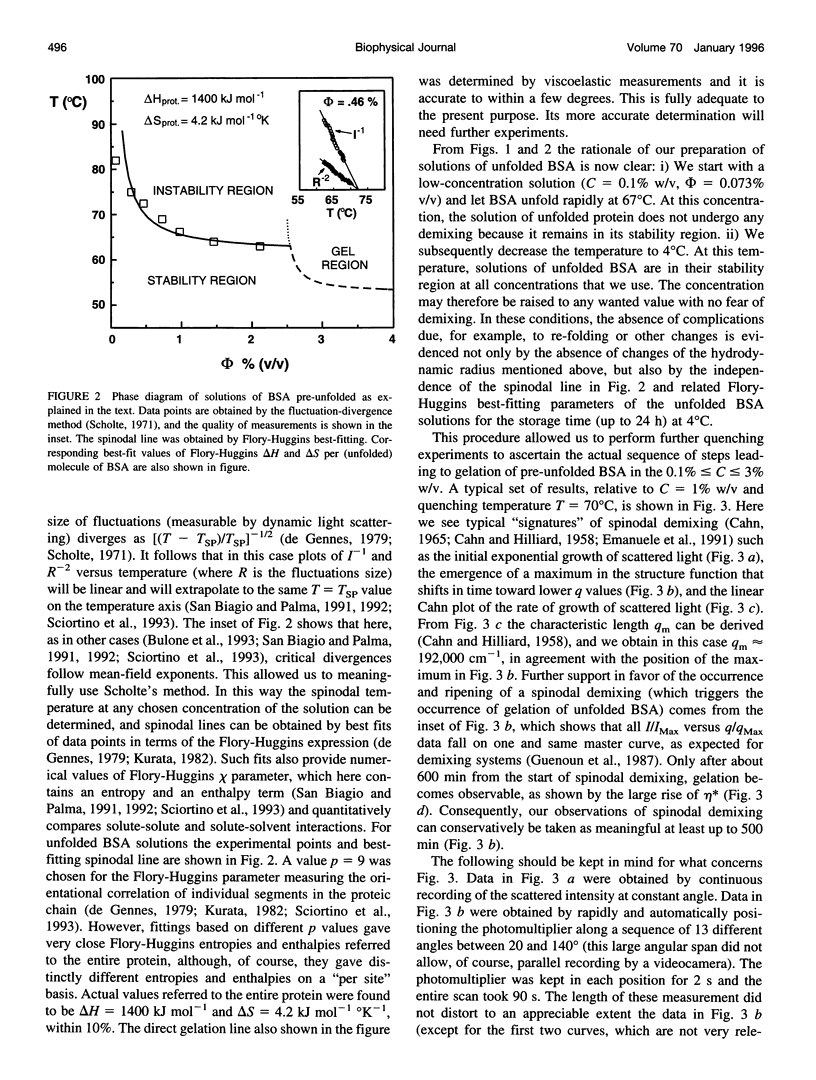
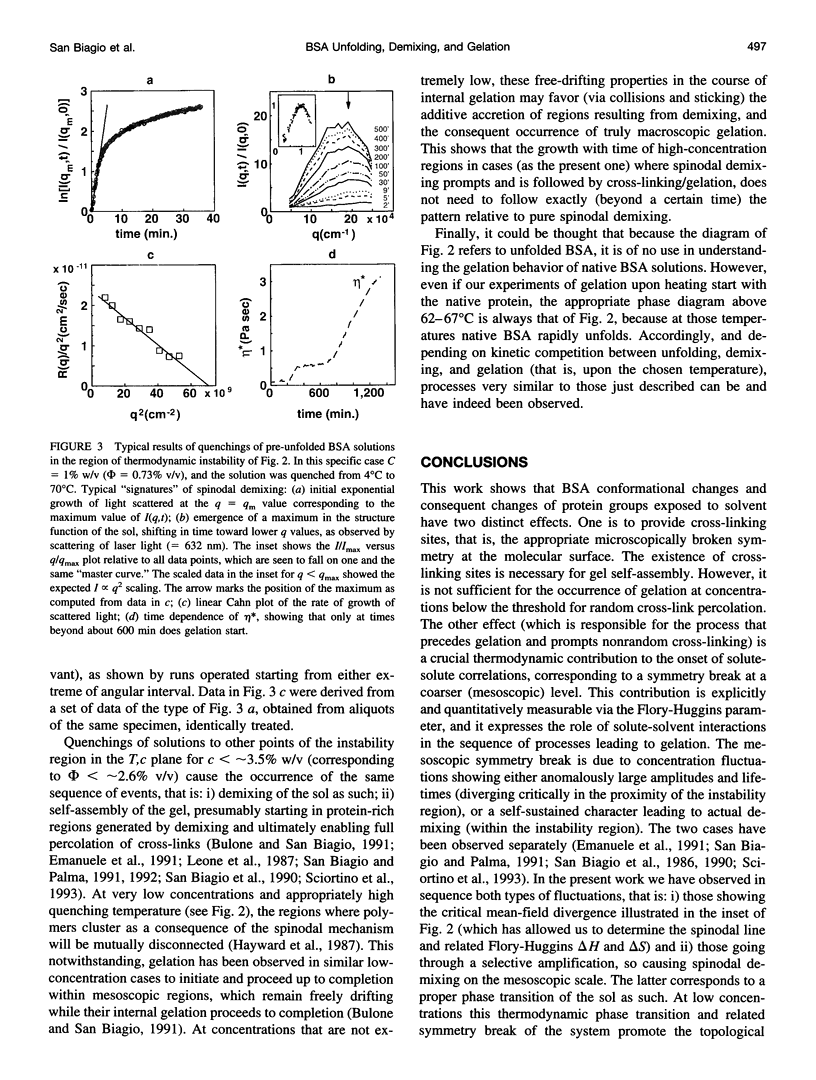
Abstract
Self-assembly of extended structures via cross-linking of individual biomolecules often occurs in solutions at concentrations well below the estimated threshold for random cross-link percolation. This requires solute-solute correlations. Here we study bovine serum albumin. Its unfolding causes the appearance of an instability region of the sol, not observed for native bovine serum albumin. As a consequence, spinodal demixing of the sol is observed. The thermodynamic phase transition corresponding to this demixing is the determinative symmetry-breaking step allowing the subsequent occurrence of (correlated) cross-linking and its progress up to the topological phase transition of gelation. The occurrence of this sequence is of marked interest to theories of spontaneous symmetry-breaking leading to morphogenesis, as well as to percolation theories. The present results extend the validity of conclusions drawn from our previous studies of other systems, by showing in one single case, system features that we have hitherto observed separately in different systems. Time-resolved experimental observations of the present type also bring kinetic and diffusional processes and solute-solvent interactions into the picture of cross-link percolation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelos MA, Kolb M. Crosslinked biopolymers: Experimental evidence for scalar percolation theory. Phys Rev Lett. 1990 Mar 19;64(12):1457–1460. doi: 10.1103/PhysRevLett.64.1457. [DOI] [PubMed] [Google Scholar]
- Binder S., Sanderson L. M. The role of the epidemiologist in natural disasters. Ann Emerg Med. 1987 Sep;16(9):1081–1084. doi: 10.1016/s0196-0644(87)80763-1. [DOI] [PubMed] [Google Scholar]
- Bulone D., San Biagio P. L., Palma-Vittorelli M. B., Palma M. U. The role of water in hemoglobin function and stability. Science. 1993 Feb 26;259(5099):1335–1336. doi: 10.1126/science.8446903. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Shortle D. Denatured states of proteins. Annu Rev Biochem. 1991;60:795–825. doi: 10.1146/annurev.bi.60.070191.004051. [DOI] [PubMed] [Google Scholar]
- Emanuele A, Palma-Vittorelli MB. Time-resolved experimental study of shear viscosity in the course of spinodal demixing. Phys Rev Lett. 1992 Jul 6;69(1):81–84. doi: 10.1103/PhysRevLett.69.81. [DOI] [PubMed] [Google Scholar]
- Guenoun P, Gastaud R, Perrot F, Beysens D. Spinodal decomposition patterns in an isodensity critical binary fluid: Direct-visualization and light-scattering analyses. Phys Rev A Gen Phys. 1987 Nov 15;36(10):4876–4890. doi: 10.1103/physreva.36.4876. [DOI] [PubMed] [Google Scholar]
- Madonia F., San Biagio P. L., Palma M. U., Schiliro' G., Musumeci S., Russo G. Photon scattering as a probe of microviscosity and channel size in gels such as sickle haemoglobin. 1983 Mar 31-Apr 6Nature. 302(5907):412–415. doi: 10.1038/302412a0. [DOI] [PubMed] [Google Scholar]
- San Biagio P. L., Palma M. U. Spinodal lines and Flory-Huggins free-energies for solutions of human hemoglobins HbS and HbA. Biophys J. 1991 Aug;60(2):508–512. doi: 10.1016/S0006-3495(91)82078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino F., Prasad K. U., Urry D. W., Palma M. U. Self-assembly of bioelastomeric structures from solutions: mean-field critical behavior and Flory-Huggins free energy of interactions. Biopolymers. 1993 May;33(5):743–752. doi: 10.1002/bip.360330504. [DOI] [PubMed] [Google Scholar]