Abstract
Longitudinally oriented axon collaterals of CA3 pyramidal cells may be critical for integrating distributed information in the hippocampus. To investigate the possible role of this pathway in the retention of spatial memory, we made a single transversely oriented cut through the dorsal CA3 region of each hippocampus. Although the lesion involved <3% of the hippocampal volume, it nonetheless disrupted memory retention in a water maze in preoperatively trained rats. New learning in a different water maze was attenuated. No significant impairment occurred in rats with longitudinally oriented cuts, or in animals with ibotenic acid-induced lesions of similar magnitude. To characterize the effect of a focal lesion on the integrity of longitudinally projecting axons, we stained degenerating cells and fibers in rats with unilateral CA3 transections by using FluoroJade-B. Degenerating terminals were seen across a wide region posterior to the cut, and were present in the strata of areas CA3 and CA1 that are innervated by CA3 pyramidal cells. These results suggest that the integrity of longitudinally oriented, translamellar axons of CA3 pyramidal cells may be necessary for efficient acquisition and retention of spatial memory.
Keywords: spatial learning‖memory‖hippocampus‖distributed‖recurrent collaterals
The hippocampus performs several critical memory operations including encoding, storage, consolidation, and retrieval (1–5). Spatial memory particularly depends on this structure (1, 5, 6). Lesions of the hippocampus severely impair acquisition and retention of spatial memory (5, 7–10), and the strongly location-specific activity of hippocampal pyramidal neurons (1) is influenced by recent experience (11–14), consistent with a role for these so-called “place cells” in spatial memory (15).
Several factors suggest that memory, under most circumstances, is encoded by a highly distributed process within the hippocampus. The organization of firing fields in hippocampal neurons is nontopographic, with local neuronal clusters representing the most of any spatial environment (1, 16). Retention of spatial memory requires the integrity of a large fraction of the hippocampal tissue (9). Together with the profound divergence of projections from the entorhinal cortex to the dentate gyrus and hippocampus (17–20), these factors suggest that spatial memory is usually stored as a highly distributed pattern. But how is the pattern integrated? The internal connectivity of the hippocampus provides some clues. Although much of the excitatory impulse transmission in the hippocampus is organized in the lamellar or transverse plane (21), mossy cells in the dentate hilus and pyramidal cells in the CA3 have associational and commissural axon collaterals that cover a significant portion of their subfields (17, 22–24). Each pyramidal cell in CA3 is contacted by ≈4% of the pyramidal cells within this subfield (25). This internal recurrent connectivity is probably sufficient to allow autoassociation, or association among individual elements of a patterned input (2, 26, 27). Presumably, CA3-CA3 connections, and the equally divergent connections from CA3 to area CA1 (22, 23), enable patterned information to be stored rapidly and efficiently (2, 26, 27). We tested the mnemonic role of longitudinal connections from area CA3 by measuring retention of a hippocampus-dependent memory task in a Morris water maze (5, 9, 28) after longitudinal impulse transmission was disrupted by a transversely oriented cut in the middle of the dorsal CA3. These lesions were compared with lesions that severed bypassing longitudinal fibers to a lesser extent (longitudinally oriented cuts and fiber-sparing excitotoxic lesions).
Materials and Methods
Subjects.
Naïve male Long Evans rats (250–400 g; n = 172) were housed in groups of 4 to 5 in transparent polycarbonate cages (59 × 38 × 20 cm) with food and water available ad libitum. They were kept on a 12-h light/12-h dark schedule and tested in the dark phase.
Five experimental groups participated in the retention study (n = 118). They received either a transversely oriented cut through area CA3 (n = 22), a longitudinal cut (n = 21) or an ibotenate lesion (n = 18) in the same location, a cut through the fimbria (n = 17), or sham surgery (n = 39). All lesions were bilateral. Training consisted of multiple balanced replications, each with 10–20 animals. The ibotenate group was tested separately with its own group of sham-operated control animals. An additional subset (n = 19) was used to describe the distribution of fiber degeneration. These animals received unilateral lesions that otherwise were similar to those in the behavioral study. To determine whether noninvasive CA3 damage could replicate the degeneration produced by the knife cuts, CA3 cells were destroyed by prolonged, seizure-inducing stimulation of the perforant path in three rats (29, 30). The remaining 33 rats were not operated on because of poor performance during initial training (see below) or problems with anesthesia.
Surgery.
The rats were anesthetized i.p. with Equithesin (1 ml/250 g body weight; Hjorten Apotek, Trondheim, Norway), a small hole was drilled above each hippocampus, and the dura was gently removed. Transverse cuts in area CA3 were made with a sterile 1-mm-wide razor blade mounted to the stereotaxic frame. On each side, the blade was oriented coronally 3.2 mm posterior and 3.9 mm lateral to bregma (central position of the blade). It was lowered slowly through the dorsal CA3 of each hippocampus and left in place for 2 min before it was retracted. Longitudinal cuts were made with the blade turned 45° relative to the midline (center of blade: 3.2 mm posterior and 3.9 mm lateral to bregma) and the front of the blade on the medial side. Cuts aimed at the fimbria were made in the coronal plane (2.4 mm posterior and 3.8 mm lateral to bregma). All cuts were deep (5.0 mm below dura for CA3 lesions and 5.8 mm below dura for fimbria lesions) to compensate for tissue inertia. Fiber-sparing lesions were made bilaterally by injection of ibotenic acid at a single position on each side (Sigma; 10 mg/ml, pH 7.4; 0.10 μl; 3.3 mm posterior and 4.1 mm lateral to bregma; 3.4 mm below dura) with a 1-μl Hamilton syringe mounted to the stereotaxic frame (31). The needle was retracted 2 min after the injection. In the sham-operated group, holes were drilled, and a blade or a needle was inserted into the neocortex.
Behavioral Training.
All rats were trained in a Morris water maze before surgery. The maze was a white cylindrical polyvinyl chloride tank (198 cm diameter, 50 cm deep) with opaque water (Latex liquid) at 25 ± 2°C. A pneumatically controlled escape platform (10-cm diameter) was located at a fixed position midway between the center and wall. The platform was usually 1.5 cm below the water surface, but on retention trials, the depth was 22 cm for the first 60 s. The position of the hooded rat was identified and stored at 10 Hz by a tracking system (9).
The rats were trained to asymptotic performance. Training consisted of 12 blocks of four consecutive trials (6 days × 2 blocks), as described (9). On day 7, retention for the platform location was probed by withholding the platform for 60 s. The rat was then allowed to escape onto it. The animals were ranked, matched, and assigned to surgery groups according to the proportion of time they spent in a zone around the platform (radius, 35 cm; chance level, 12.5%). If a rat spent less than 20% in the target zone on this probe trial, it was not operated on, and the rat was excluded from further study. Surgery was performed on the same day as the probe trial (day 7). Seven days after completion of pretraining and surgery (on day 14), the rats received another retention test. All rats except those with ibotenate lesions were then retrained in a new water maze in a different room on the same day (five blocks of two trials at 30-min intervals). Thirty minutes after the last block, retention was probed (60 s).
Evaluation of Lesions.
All rats were killed with an overdose of Equithesin and perfused intracardially with saline and 4% formaldehyde. The brains were stored in formaldehyde for ≥1 week. Frozen sections were cut coronally (30 μm). Every second section in the lesioned area was retained and stained with cresyl violet. Outside the lesion area, every tenth section was retained. The volume of the lesion was determined by placing the sections under a microscope attached to a digital camera (Olympus DP10; Olympus, New Hyde Park, NY) and a PC. Images were taken into Canvas (Deneba Systems, Miami, FL), and for each section, outlines of the hippocampus and the lesioned area in the hippocampus were traced. The areas were measured, and the volume of the lesion was calculated (9) and expressed as a percentage of the total hippocampal volume in the same rat.
Fiber Degeneration.
To characterize the lesion further, we stained a separate group of brains with the degeneration stain FluoroJade-B (Histo-Chem, Jefferson, AR) (32, 33). The rats were perfused 4 days after surgery. Sections were cut on a Vibratome at a thickness of 40 μm and stained with Fluoro-Jade B, as described (33). Brightfield and fluorescent images were acquired digitally on a Nikon E800M microscope with a Hamamatsu C5180 camera (Hamamatsu, Middlesex, NJ) with ADOBE PHOTOSHOP 5.0. This program was used to optimize contrast and brightness, but not to remove blemishes, or to enhance or change the image content in any way. All comparison photographs were taken under identical conditions of image acquisition, and all adjustments of brightness and contrast were made uniformly to all images.
Results
Experimental Groups.
The main experimental group received cuts in CA3 that were oriented in the transverse plane. This orientation was chosen to sever a maximal number of fibers projecting in the longitudinal direction. To the extent that the transverse cuts affected retention, the effect could reflect either disruption of bypassing longitudinal fibers or merely the loss of CA3 neurons in the cut area. To distinguish between these possibilities, we made additional lesions that were intended to sever fewer bypassing fibers without reducing neuronal loss at the target location in CA3. In one group, the cut was placed parallel with the longitudinal axis; in the other, we injected the fiber-sparing neurotoxin ibotenic acid. To examine whether impairments after fiber cuts in CA3 reflected inadvertent damage to the adjacent fimbria, we also added a group with lesions targeted at the fimbria.
Lesions.
Rats were only accepted for further analysis if they had selective bilateral lesions of the target area. Unilateral lesions or CA3 lesions that included the fimbria or the CA1 were not accepted. Seven rats with ibotenate lesions in CA3 were discarded because the lesions were too small (<1%). With this criterion, 39 sham-operated rats remained: 15 with transverse cuts, 10 with longitudinal cuts, 7 with ibotenate lesions, and 11 with cuts in the fimbria.
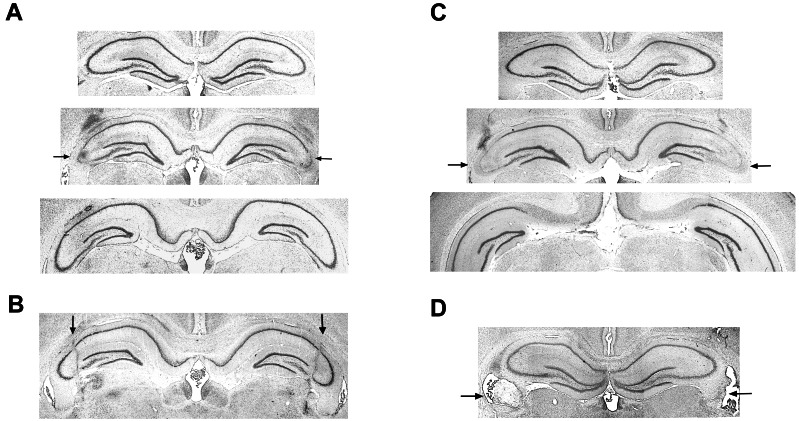
The transverse cuts damaged neuronal cell bodies throughout the CA3 at a restricted septotemporal level (Fig. 1A). Although CA3 pyramidal cells had degenerated at the cut position (Middle), no obvious cell loss was seen >0.5 mm anterior and posterior to the cut (Fig. 1A Top and Bottom). The damaged area encompassed 1.9 ± 0.2% of the total hippocampal tissue (means ± SE). The border between damaged and healthy tissue was usually sharp. In a few animals, the density of intact cells was reduced adjacent to the lesion. When these borderline areas were included, the total volume of the transverse lesion became 3.2 ± 0.5%. In rats with cuts in the longitudinal plane, the lesion covered a longer proportion of the dorsal CA3, but the extent of the damage in the mediolateral plane was small (Fig. 1B). The total volume of the lesion in this group was 1.3 ± 0.2% (2.0 ± 0.4% including areas with reduced cell density). Ibotenate lesions of the same region gave extensive and selective damage in the middle of the dorsal CA3 (Fig. 1C; n = 7). These lesions were generally larger than in the rats with transverse cuts (4.5 ± 0.4%). Cuts in the fimbria were mostly partial (Fig. 1D), but nonetheless more extensive than the likely damage to this structure in the CA3 groups.
Figure 1.
Cresyl violet stains showing representative lesions. Arrows indicate lesioned tissue. The images show a transversely oriented cut through CA3 (A), a longitudinally oriented cut in CA3 (B), an excitotoxic CA3 lesion made with ibotenic acid (C), and a partial cut through the fimbria (D). The images in A and C Top show the first section without apparent neuronal loss anterior to the lesion (0.6 and 0.7 mm anterior to the cut, respectively), and at Bottom, images show the first fully intact sections on the posterior side (0.8 and 1.4 mm posterior to the cut, respectively).
Behavior.
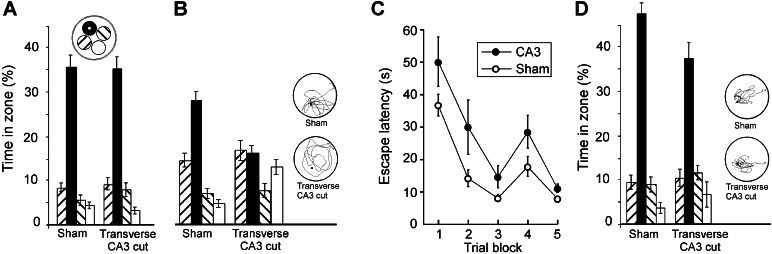
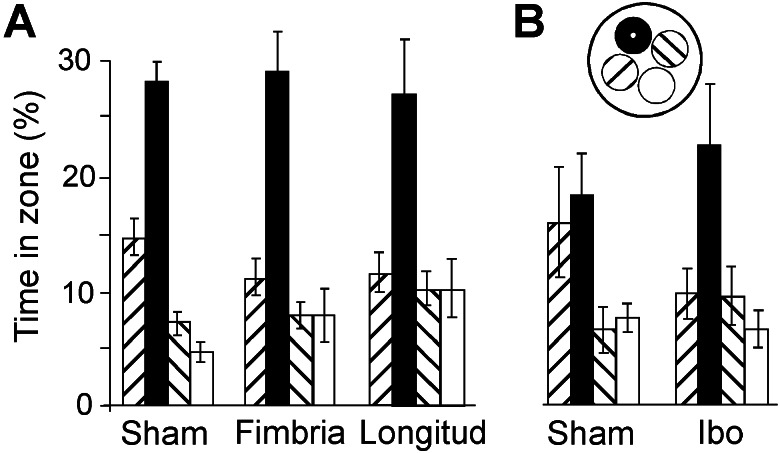
All rats learned to swim directly to the hidden platform well before the pretraining was completed. Escape latencies stabilized at 8–10 s on days 3–4. In general, the animals showed a clear bias toward the platform area on the retention test before surgery (Fig. 2A). Retention was probed again 7 days after pretraining and surgery (Fig. 2B). Whereas the sham-operated group still exhibited a clear preference for the platform region, the rats with transverse cuts in CA3 failed to search differentially. Rats with longitudinal cuts or cuts in the fimbria were not impaired (Fig. 3A). An analysis of variance of time spent around the platform (radius, 35 cm) and in corresponding zones of the other quadrants showed a significant effect of groups × zones [F (9,165) = 4.5, P < 0.001]. A one-way analysis of time in the target zone revealed an overall group difference [F (3,58) = 4.8, P < 0.005]. Post hoc analyses showed that the group with transverse CA3 cuts was significantly impaired relative to the sham group and the fimbria-lesioned group (Tukey's highest significant difference test; P < 0.01 and P < 0.05, respectively), whereas the difference between the transverse and longitudinal groups was nonsignificant with this test (P = 0.07). No difference occurred between rats with longitudinal cuts, fimbria cuts, or sham surgery. In animals with transverse cuts, a significant negative correlation existed between lesion volume and time spent in the target region (Pearson r = −0.70, n = 15, P < 0.005). Rats with lesions >2–3% generally searched randomly. In a separate experiment, we estimated retention in animals with local ibotenate lesions in CA3. Retention was preserved in these animals (Fig. 3B), even though the cell loss was larger than in the group with transverse fiber cuts. A significant effect of zones [F (3,45) = 6.2, P < 0.001] occurred, but not of groups × zones (F < 1).
Figure 2.
Impaired memory retention in a Morris water maze after a transversely oriented cut through the CA3. (A, B) Retention of probe trials on which the platform was unavailable for 60 s. The percentage of time (means ± SE) spent by lesioned and sham-operated animals around the platform is shown (A Inset, black circle) and in corresponding zones of the three other quadrants before surgery (A) and 7 days after surgery (B). (C) Latency to find the hidden platform during postoperative training in a new water maze 7 days after surgery. (D) Retention on a final probe trial in the new environment. Insets B and D show typical swim paths. Each zone covered 12.5% of the pool surface.
Figure 3.
Preserved memory retention after a longitudinal cut in CA3 or a partial cut in the fimbria (A) or a local ibotenate lesion in CA3 (B). Rats with ibotenate lesions showed a clear bias toward the platform location. The increased time spent by the sham group in the zone adjacent to the target zone was largely because of one rat that searched consistently in the wrong quadrant. Symbols are as in Fig. 2.
To estimate new learning, we trained the same rats in another spatial environment. The training protocol was sensitive to hippocampal lesions (9). The rats with transverse cuts in CA3 learned to find the platform at its new location, but their performance was still inferior to that of the other groups. Although all groups had final escape latencies of 12 s or less, the latency to find the platform was generally longer after transverse CA3 cuts (Fig. 2C). A significant group effect on escape latency occurred [F (3,54) = 6.6, P < 0.001]. Post hoc analyses showed that the transverse group had longer latencies than each of the other groups (longitudinal, fimbria, sham) (all P values <0.01). No differences existed among the three control groups. A similar pattern was seen on the final probe trial (Fig. 2D). Animals with transverse CA3 cuts performed above chance and showed clear spatial bias, but were less accurate than the other groups (groups × zones: F (9,156) = 2.1, P < 0.05). A one-way analysis of the group difference in the target zone gave a significant effect of groups [F (3,55) = 3.1, P < 0.05]. The only significant pairwise comparison (P < 0.05) was between the group with transverse cuts and the sham-operated animals.
Fiber Degeneration.
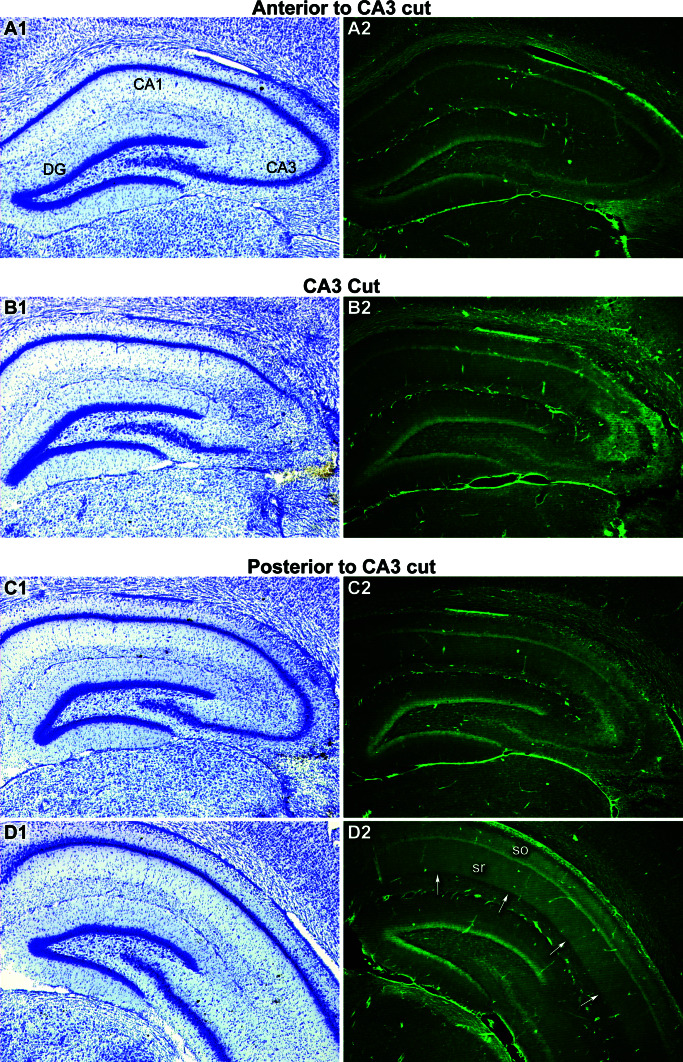
To determine whether the bilateral CA3 lesions produced disruption of longitudinal axon projections, we stained sections from brains with unilateral CA3 cuts by using the fluorescent anionic dye FluoroJade-B, which apparently stains basic proteins produced by degenerating cells (32, 33). Extensive terminal degeneration was apparent across wide parts of areas CA3 and CA1 posterior to the cut after both transverse and longitudinal lesions (Fig. 4). Degeneration was observed primarily in strata oriens and radiatum, which are the laminae innervated by CA3 pyramidal cells (22, 23). FluoroJade-B staining stopped abruptly at the CA1/subiculum border, suggesting that CA1 pyramidal cells, which innervate the subiculum, were spared. Little staining was detected on the anterior side of the cut, consistent with the fact that fewer pyramidal cell axons from the affected CA3 area have an anterior orientation than a posterior orientation (21–23).
Figure 4.
Degeneration of caudally directed axons of CA3 pyramidal cells after a transverse cut through area CA3. Sections from the same brain were stained with cresyl violet (Left) or FluoroJade-B (Right). (A1, C1) A minimal distance anterior and posterior, respectively, to the knife cut shown in B1, no obvious histological disruption was apparent. (A2, B2, C2) Close to the focal CA3 knife cut the fluorescence mainly reflected staining of damaged CA3 cells (B2). (D2) At a distance in the caudal direction from the knife cut, FluoroJade-B staining produced a diffuse band of fluorescence throughout the stratum radiatum (sr) and stratum oriens (so) of area CA1 (arrows) and CA3 (not shown). This pattern of degeneration suggests that a highly localized lesion of area CA3 causes degeneration of longitudinally projecting axons that terminate diffusely throughout the dendritic layers of areas CA1 and CA3. The pattern of FluoroJade-B fluorescence was limited to the precise laminae innervated by CA3 pyramidal cell axons.
In three rats, the CA3 was lesioned noninvasively by prolonged stimulation of the perforant path. Afferent stimulation outside the hippocampus causes granule cell discharges that release glutamate and destroy CA3 pyramidal cells and hilar neurons throughout the dorsal hippocampus (29, 30). This method was used to determine whether a lesion that does not physically sever fibers of passage in CA3 would reproduce the pattern of axonal degeneration caused by highly focal invasive lesions in this area. Perforant path stimulation produced CA3 cell loss in the dorsal hippocampus, and resulted in a wide band of staining in strata oriens and radiatum of posterior CA3 and CA1 that was almost identical with that caused by the knife cuts in area CA3. A similar pattern of degeneration was observed in these strata after local infusion of ibotenic acid in CA3 (data not shown). The equal pattern of degeneration in CA3 after fiber cuts, excitotoxic lesions, and perforant-path stimulation suggests that the staining observed after transverse cuts in area CA3 was the result of transection of CA3 pyramidal cell axons traveling longitudinally in the posterior direction.
Discussion
First, we have concluded that memory retention in the water maze depends critically on the integrity of hippocampal area CA3, because the neuronal loss was limited to the CA3 subfield. Fiber degeneration was observed both in strata oriens and radiatum of areas CA3 and CA1, consistent with the termination pattern of CA3 pyramidal cell axons (22, 23). A lack of staining in the subiculum suggested that area CA1 was largely spared. Lesions targeting the adjacent fimbria failed to disrupt retention. The results are consistent with research demonstrating impaired spatial learning after excitotoxic CA3 lesions (34, 35), but add a role for this subregion in retention and possibly in retrieval of such memory. The weak effect on new learning in the present study is probably a consequence of leaving large areas of CA3 intact (9, 36). The results are therefore consistent with the proposal that area CA3, with its high plasticity and extensive recurrent connections, may act as an autoassociative matrix capable of associating and storing patterned information projected to the hippocampus from the entorhinal cortex (2, 26, 27).
Second, we have concluded that successful retention depends on the integrity of longitudinal impulse transmission in the hippocampus. Although rats can remember successfully with only a small block of hippocampal tissue remaining if learning takes place after surgery (9, 36), retention was impaired after cuts that damaged less than 3% of the hippocampal tissue, provided that the lesion was placed in CA3 and in the transverse plane. Longitudinal cuts failed to cause a similar impairment, probably because longitudinally coursing fibers were minimally cut. Retention was preserved also after fiber-sparing lesions caused by ibotenic acid, even when the neuronal loss was equivalent, suggesting that retention was primarily determined by fiber disruption rather than limited CA3 cell loss. Thus, the results suggest that longitudinal transmission in the CA3-CA3 and CA3-CA1 system is essential for normal memory retention. The resolution of our techniques does not permit us to differentiate between the functions of associational fibers in CA3 and the equally divergent Schaffer collaterals projecting from CA3 pyramidal cells to CA1 pyramidal cells, because both were severed. The relative contribution of excitatory and inhibitory longitudinal connections also remains to be resolved. Some inhibitory interneurons in CA3 have divergent axon collaterals (37), and their integrity may play a role in memory retention.
Acknowledgments
We thank I. Hammer, K. Haugen, J. Hoff, K. Jenssen, H. T. Skiri, and R. Ulriksen for technical assistance. This work was supported by Norwegian Research Council Grants 139786/300 and 133958/420 (to E.I.M. and M.B.M.) and National Institutes of Health, National Institute of Neurological Disorders and Stroke Grant NS18201 (to R.S.S.).
References
- 1.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 2.McNaughton B L, Morris R G M. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 3.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H. Nat Rev Neurosci. 2001;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 5.Riedel G, Micheau J, Lam A G, Roloff E v, Martin S J, Bridge H, Hoz L d, Poeschel B, McCulloch J, Morris R G M. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 6.Maguire E A, Burgess N, Donnett J G, Frackowiak R S, Frith C D, O'Keefe J. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 7.Olton D S, Walker J A, Gage F H. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- 8.Morris R G M, Garrud P, Rawlins J N P, O'Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 9.Morris R G M, Schenk F, Tweedie F, Jarrard L E. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 10.Moser M-B, Moser E I. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Keefe J, Speakman A. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- 12.Quirk G J, Muller R U, Kubie J L. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood E R, Dudchenko P A, Eichenbaum H. Nature (London) 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 14.Hollup S A, Molden S, Donnett J G, Moser M B, Moser E I. J Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser E I, Paulsen O. Curr Opin Neurobiol. 2001;11:745–751. doi: 10.1016/s0959-4388(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 16.Wilson M A, McNaughton B L. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 17.Amaral D G, Witter M P. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 18.Tamamaki N, Nojyo Y. Hippocampus. 1993;3:471–480. doi: 10.1002/hipo.450030408. [DOI] [PubMed] [Google Scholar]
- 19.Dolorfo C L, Amaral D G. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- 20.Naber P A, Lopes da Silva F H, Witter M P. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- 21.Andersen P, Bliss T V P, Skrede K K. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka N, Weber J, Amaral D G. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 23.Li X G, Somogyi P, Ylinen A, Buzsaki G. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 24.Zappone C A, Sloviter R S. J Comp Neurol. 2001;441:324–344. doi: 10.1002/cne.1415. [DOI] [PubMed] [Google Scholar]
- 25.Amaral D G, Ishizuka N, Claiborne B. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 26.Marr D. Philos Trans R Soc Lond B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 27.Treves A, Rolls E T. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 28.Morris R G M, Hagan J J, Rawlins J N P. Q J Exp Psychol B. 1986;38:365–395. [PubMed] [Google Scholar]
- 29.Sloviter R S. Brain Res Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- 30.Sloviter R S. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 31.Jarrard L E. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 32.Schmued L C, Alberson C, Slikker W. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 33.Schmued L C, Hopkins K J. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 34.Handelmann G E, Olton D S. Brain Res. 1981;217:41–58. doi: 10.1016/0006-8993(81)90183-9. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland R J, Whishaw I Q, Kolb B. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 36.Moser M-B, Moser E I, Forrest E, Andersen P, Morris R G M. Proc Natl Acad Sci USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund T F, Buzsaki G. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]