Abstract
We use the lipophilic fluorescence probe Laurdan to study cell membranes. The generalized polarization (GP) of Laurdan-labeled cells contains useful information about membrane fluidity and polarity. A high GP is usually associated with low fluidity, low polarity, or high cholesterol content of the membranes, and a low GP is the opposite. We have combined the GP method and two-photon fluorescence microscopy to provide an alternative approach to study cell membranes. Using two-photon excitation in a conventional microscope offers great advantages for studying biological samples. These advantages include efficient background rejection, low photodamage, and improved depth discrimination. We performed GP measurements on mouse fibroblast cells and observed that both intensity and GP images are not spatially uniform. We tested for possible GP artifacts arising from cellular autofluorescence and lifetime quenching, using a procedure for background fluorescence subtraction and by direct lifetime measurements in the microscope. GP measured in a single cell displays a broad distribution, and the GP of 40 different cells grown on the same cover glass is also statistically distributed. The correlations between intensity and GP images were analyzed, and no monotonic dependence between the two was found. By digitally separating high and low GP values, we found that high GP values often associate with the regions of the plasma membrane and low GP values link with the nuclear membranes. Our results also show local GP variations within the plasma and nuclear membranes.
Full text
PDF


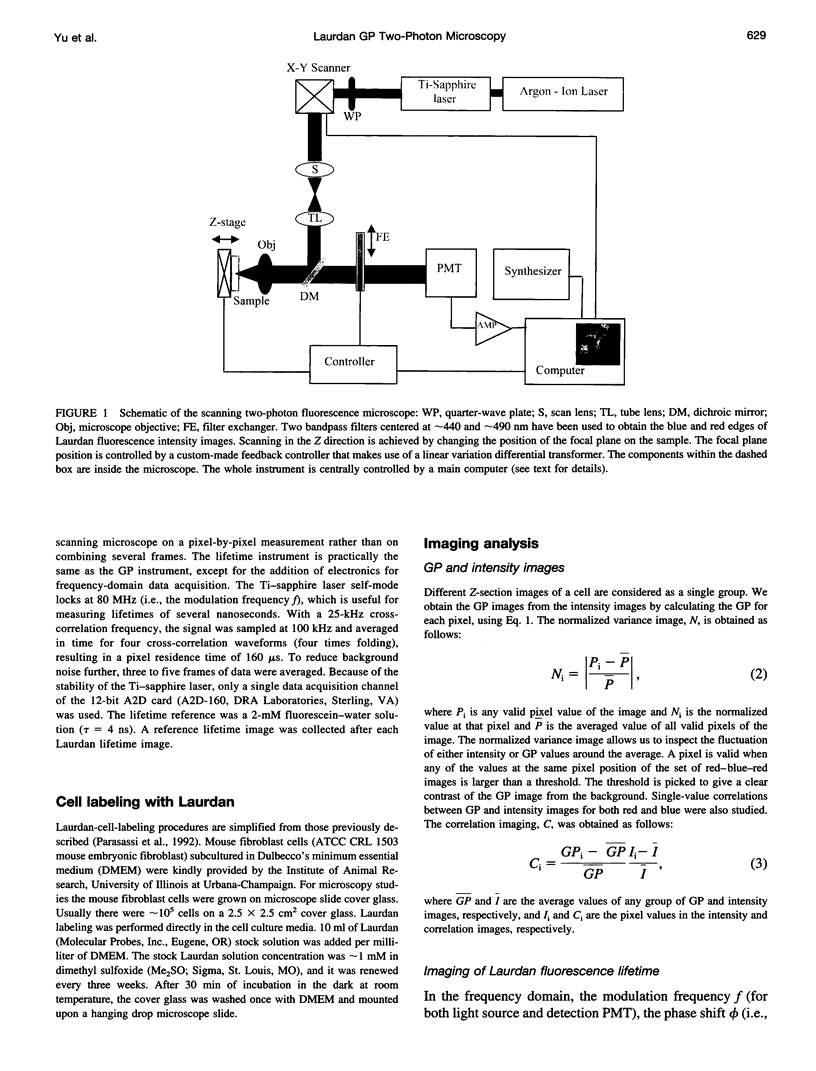
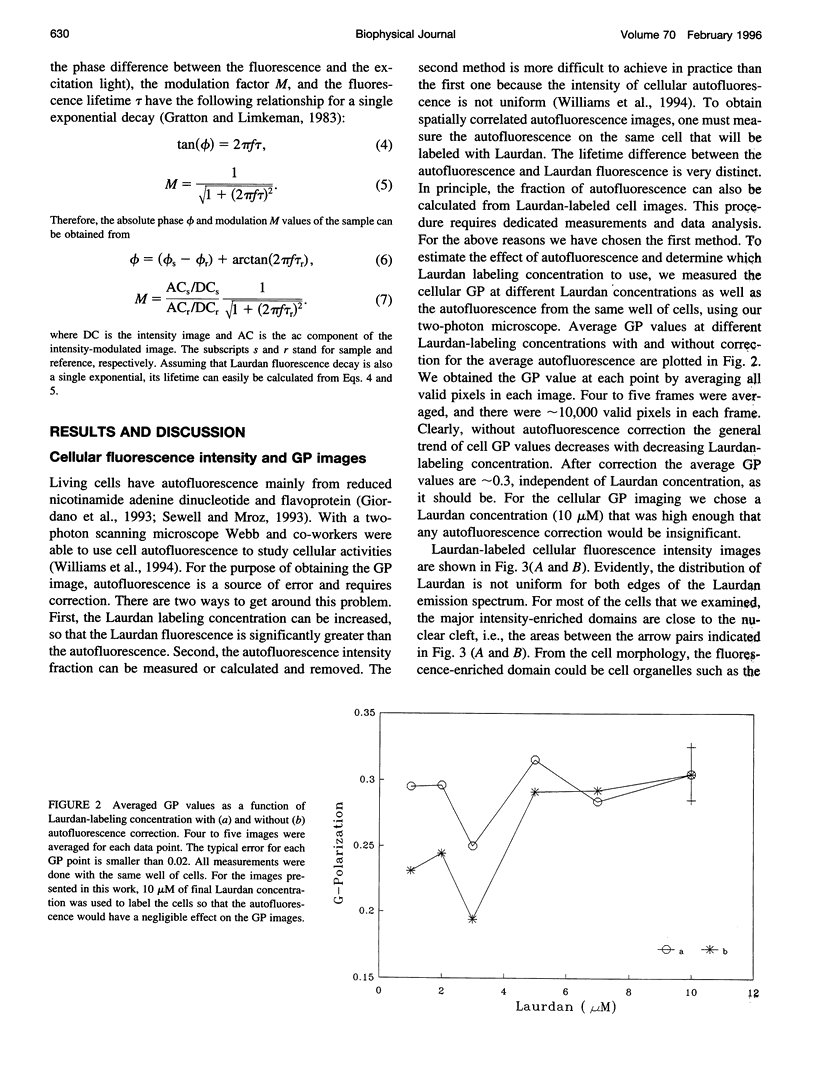
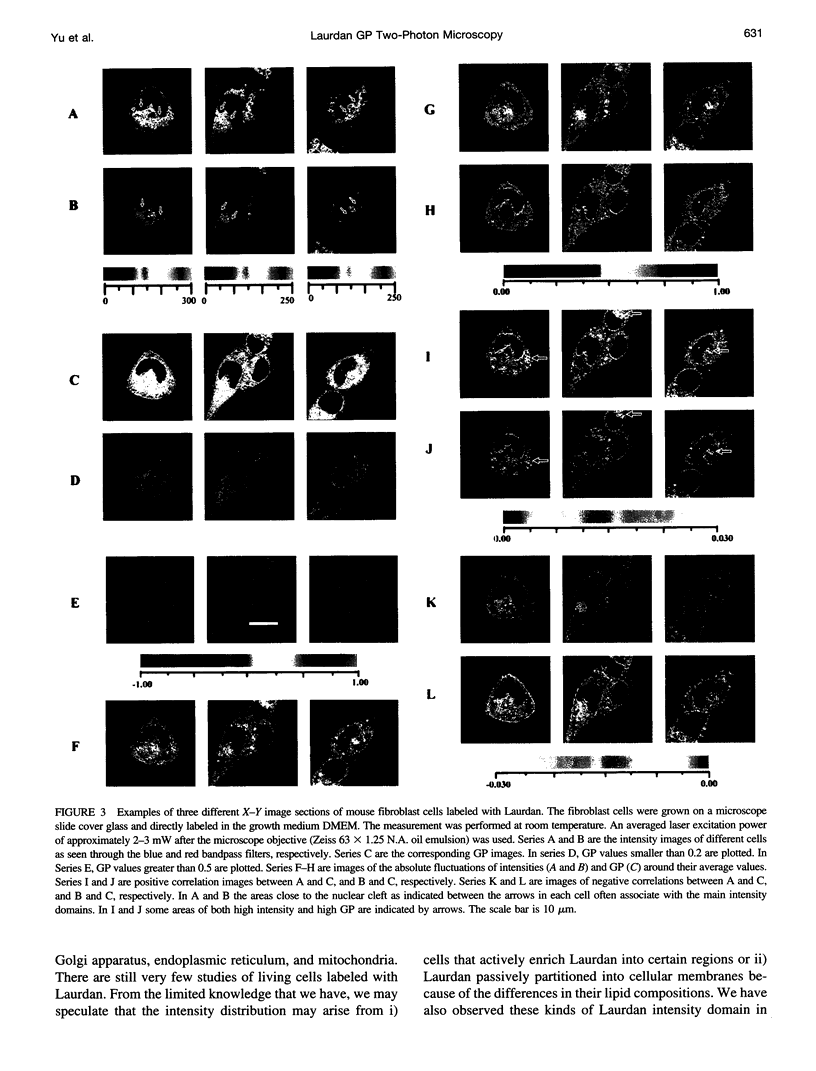
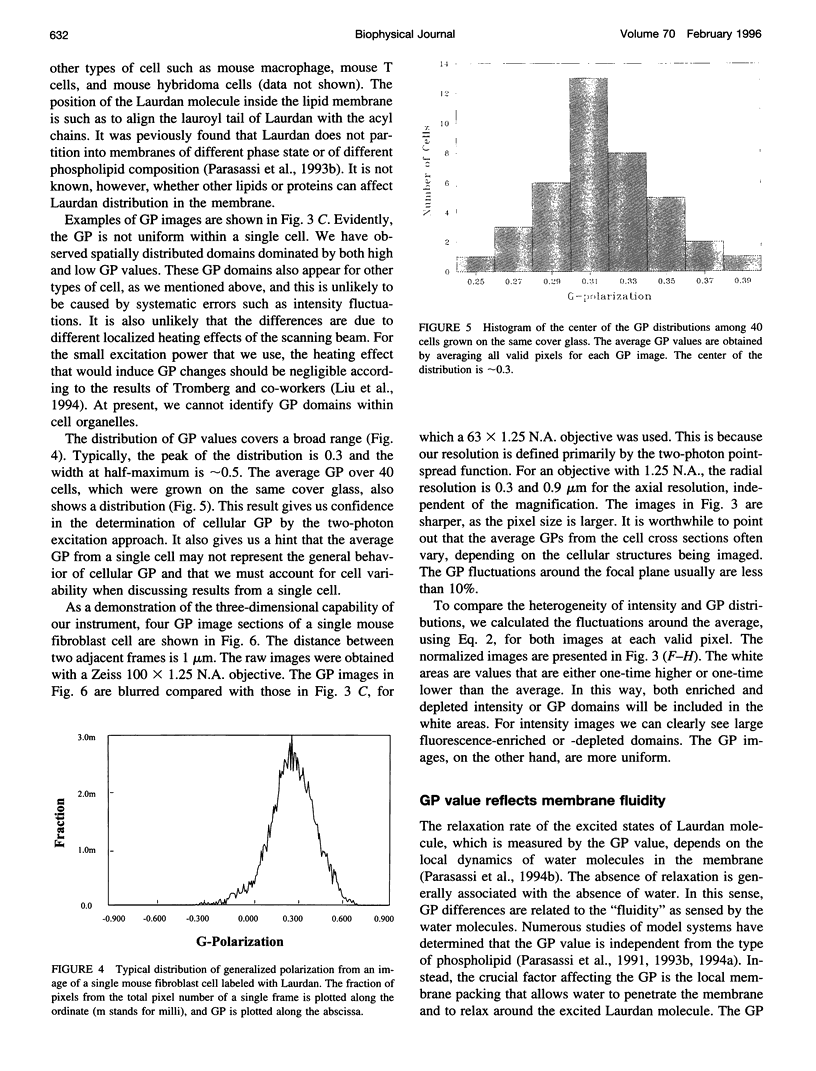



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R., Pak C. C., Raviv Y., Krumbiegel M., Bergelson L. D., Morris S. J., Lowy R. J. Transient domains induced by influenza haemagglutinin during membrane fusion. Mol Membr Biol. 1995 Jan-Mar;12(1):135–142. doi: 10.3109/09687689509038509. [DOI] [PubMed] [Google Scholar]
- Denk W., Strickler J. H., Webb W. W. Two-photon laser scanning fluorescence microscopy. Science. 1990 Apr 6;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Divecha N., Irvine R. F. Phospholipid signaling. Cell. 1995 Jan 27;80(2):269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Edidin M., Stroynowski I. Differences between the lateral organization of conventional and inositol phospholipid-anchored membrane proteins. A further definition of micrometer scale membrane domains. J Cell Biol. 1991 Mar;112(6):1143–1150. doi: 10.1083/jcb.112.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini R., Curatola G., Kantar A., Giorgi P. L., Gratton E. Use of Laurdan fluorescence in studying plasma membrane organization of polymorphonuclear leukocytes during the respiratory burst. Photochem Photobiol. 1993 Mar;57(3):438–441. doi: 10.1111/j.1751-1097.1993.tb02315.x. [DOI] [PubMed] [Google Scholar]
- Florine-Casteel K. Phospholipid order in gel- and fluid-phase cell-size liposomes measured by digitized video fluorescence polarization microscopy. Biophys J. 1990 Jun;57(6):1199–1215. doi: 10.1016/S0006-3495(90)82639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquaud C., Wong P. Mechanism of interaction between actin and membrane lipids: a pressure-tuning infrared spectroscopy study. Biochem J. 1994 Nov 1;303(Pt 3):769–774. doi: 10.1042/bj3030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano E., Cirulli V., Bosco D., Rouiller D., Halban P., Meda P. B-cell size influences glucose-stimulated insulin secretion. Am J Physiol. 1993 Aug;265(2 Pt 1):C358–C364. doi: 10.1152/ajpcell.1993.265.2.C358. [DOI] [PubMed] [Google Scholar]
- Gordon G. W., Chazotte B., Wang X. F., Herman B. Analysis of simulated and experimental fluorescence recovery after photobleaching. Data for two diffusing components. Biophys J. 1995 Mar;68(3):766–778. doi: 10.1016/S0006-3495(95)80251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton E., Limkeman M. A continuously variable frequency cross-correlation phase fluorometer with picosecond resolution. Biophys J. 1983 Dec;44(3):315–324. doi: 10.1016/S0006-3495(83)84305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajoo A., Dube A., Bharti S. Mg(2+)-induced lipid phase transition in thylakoid membranes is reversed by anions. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1724–1730. doi: 10.1006/bbrc.1994.2134. [DOI] [PubMed] [Google Scholar]
- Levi M., Wilson P. V., Cooper O. J., Gratton E. Lipid phases in renal brush border membranes revealed by Laurdan fluorescence. Photochem Photobiol. 1993 Mar;57(3):420–425. doi: 10.1111/j.1751-1097.1993.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Maresca B., Cossins A. R. Cell physiology. Fatty feedback and fluidity. Nature. 1993 Oct 14;365(6447):606–607. doi: 10.1038/365606a0. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Lateral mobility of Na,K-ATPase and membrane lipids in renal cells. Importance of cytoskeletal integrity. J Membr Biol. 1994 Oct;142(1):127–135. doi: 10.1007/BF00233390. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Conti F., Gratton E. Time-resolved fluorescence emission spectra of Laurdan in phospholipid vesicles by multifrequency phase and modulation fluorometry. Cell Mol Biol. 1986;32(1):103–108. [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990 Jun;57(6):1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe. Biophys J. 1994 Mar;66(3 Pt 1):763–768. doi: 10.1016/s0006-3495(94)80852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J. 1994 Jan;66(1):120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Ravagnan G., Sapora O., Gratton E. Membrane aging during cell growth ascertained by Laurdan generalized polarization. Exp Cell Res. 1992 Oct;202(2):432–439. doi: 10.1016/0014-4827(92)90096-q. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Loiero M., Raimondi M., Ravagnan G., Gratton E. Absence of lipid gel-phase domains in seven mammalian cell lines and in four primary cell types. Biochim Biophys Acta. 1993 Dec 12;1153(2):143–154. doi: 10.1016/0005-2736(93)90399-k. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Ravagnan G., Rusch R. M., Gratton E. Modulation and dynamics of phase properties in phospholipid mixtures detected by Laurdan fluorescence. Photochem Photobiol. 1993 Mar;57(3):403–410. doi: 10.1111/j.1751-1097.1993.tb02309.x. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Joo F., Vigh L. The role of unsaturated lipids in membrane structure and stability. Prog Biophys Mol Biol. 1989;53(2):71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Distributions of proteins and lipids in the erythrocyte membrane. Biochemistry. 1993 Nov 30;32(47):12591–12598. doi: 10.1021/bi00210a007. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Gierasch L. M., Thompson T. E. Reorganization of lipid domain structure in membranes by a transmembrane peptide: an ESR spin label study on the effect of the Escherichia coli outer membrane protein A signal peptide on the fluid lipid domain connectivity in binary mixtures of dimyristoyl phosphatidylcholine and distearoyl phosphatidylcholine. Biophys J. 1994 Jun;66(6):1959–1968. doi: 10.1016/S0006-3495(94)80989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Marsh D., Thompson T. E. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers. An electron spin resonance spin label study. Biophys J. 1992 Aug;63(2):340–349. doi: 10.1016/S0006-3495(92)81619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B., Cimini A., Sette M., Sartori C. 31P-NMR of liver peroxisome membranes from normal and clofibrate-treated rats. Cell Mol Biol (Noisy-le-grand) 1993 Jul;39(5):479–489. [PubMed] [Google Scholar]
- Sewell W. F., Mroz E. A. Flavin adenine dinucleotide is a major endogenous fluorophore in the inner ear. Hear Res. 1993 Nov;70(2):131–138. doi: 10.1016/0378-5955(93)90150-y. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tanaka Y., Nakajima Y., Hirano K., Itoh H., Miyata H., Hayakawa T., Kinosita K., Jr Spatiotemporal relationships among early events of fertilization in sea urchin eggs revealed by multiview microscopy. Biophys J. 1995 Mar;68(3):739–748. doi: 10.1016/S0006-3495(95)80289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L., Los D. A., Horváth I., Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hollingsworth R. I. A solvent system for the high-resolution proton nuclear magnetic resonance spectroscopy of membrane lipids. Anal Biochem. 1995 Mar 1;225(2):242–251. doi: 10.1006/abio.1995.1149. [DOI] [PubMed] [Google Scholar]
- Weber G., Farris F. J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979 Jul 10;18(14):3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- Williams R. M., Piston D. W., Webb W. W. Two-photon molecular excitation provides intrinsic 3-dimensional resolution for laser-based microscopy and microphotochemistry. FASEB J. 1994 Aug;8(11):804–813. doi: 10.1096/fasebj.8.11.8070629. [DOI] [PubMed] [Google Scholar]
- Wolf D. E., Maynard V. M., McKinnon C. A., Melchior D. L. Lipid domains in the ram sperm plasma membrane demonstrated by differential scanning calorimetry. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6893–6896. doi: 10.1073/pnas.87.17.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Lee G. M., Jacobson K. Protein lateral mobility as a reflection of membrane microstructure. Bioessays. 1993 Sep;15(9):579–588. doi: 10.1002/bies.950150903. [DOI] [PubMed] [Google Scholar]








