Abstract
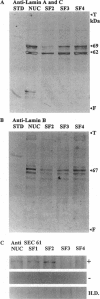
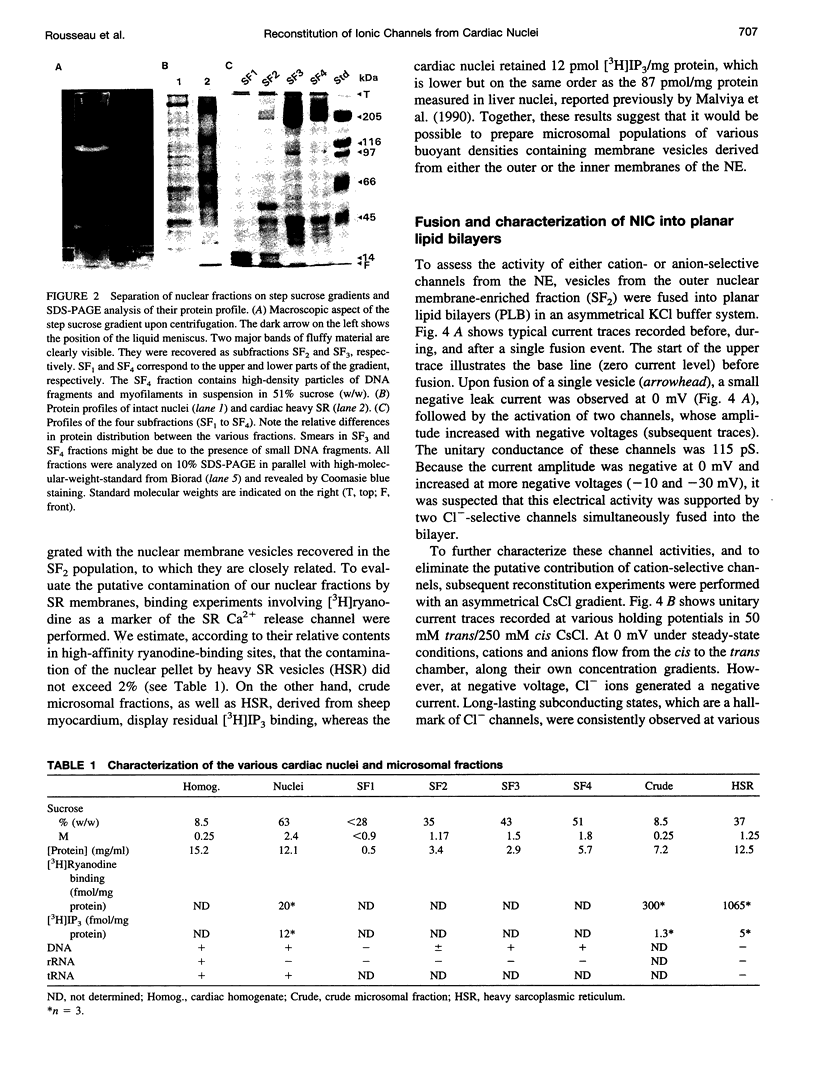
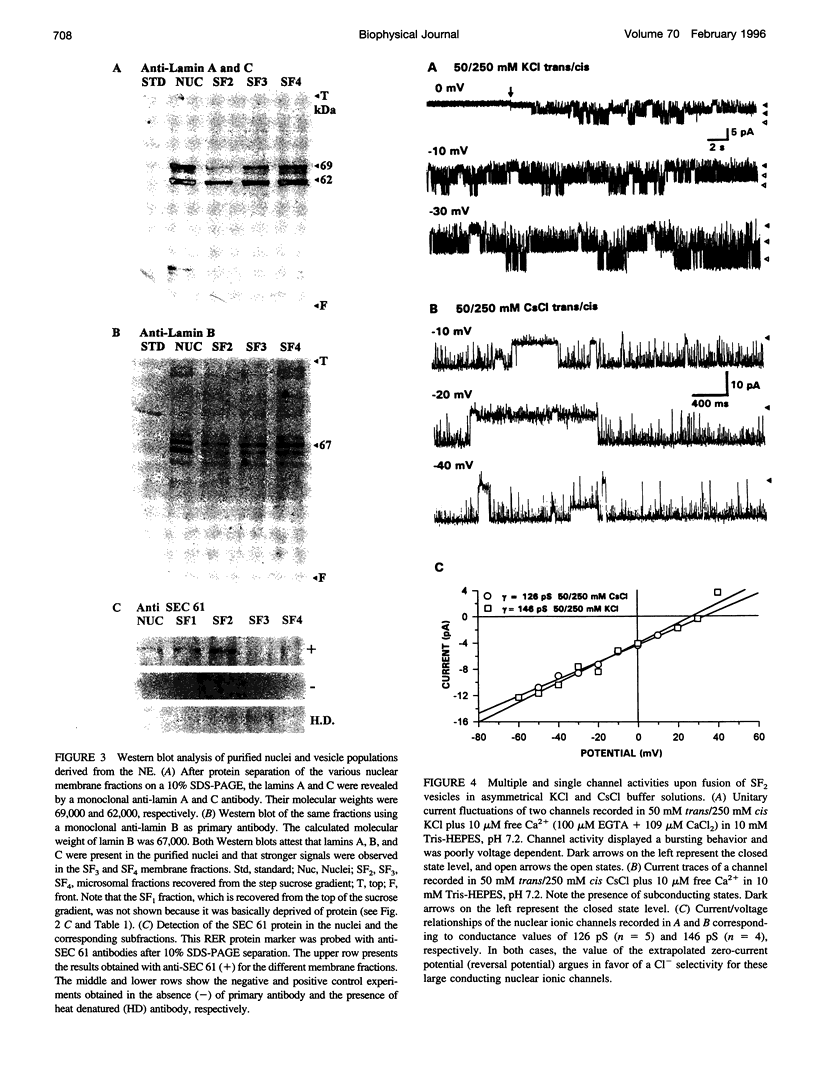
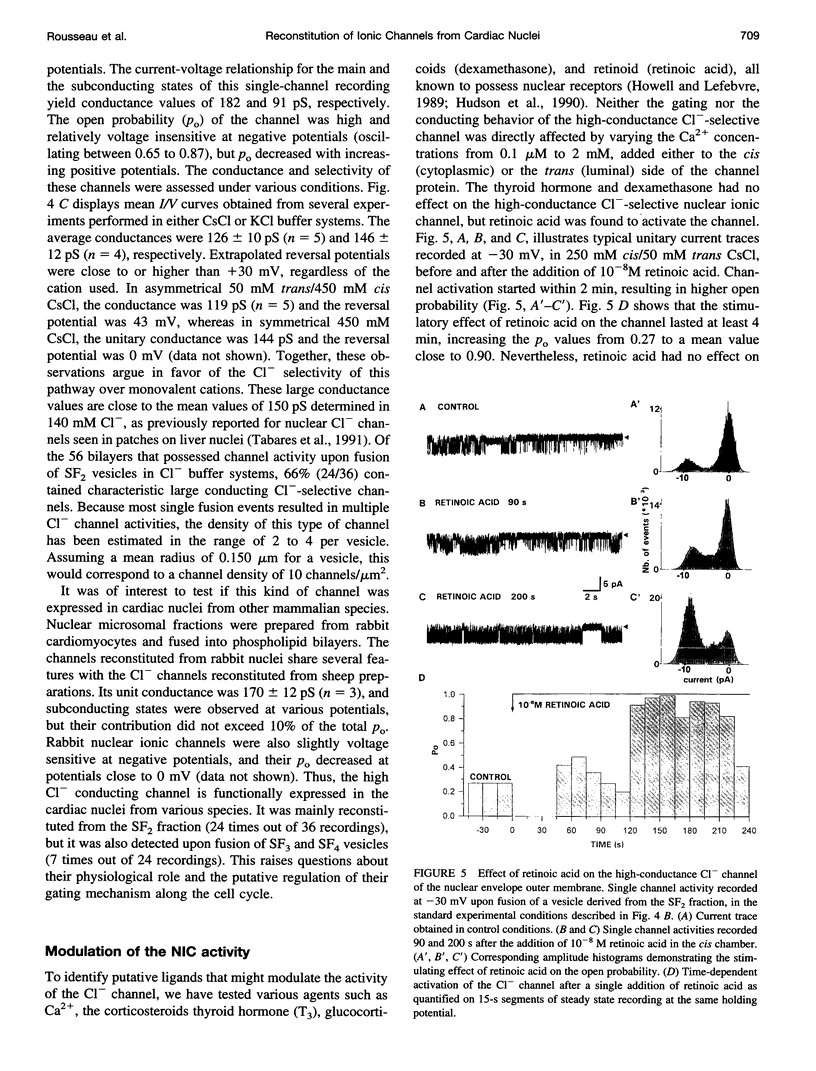
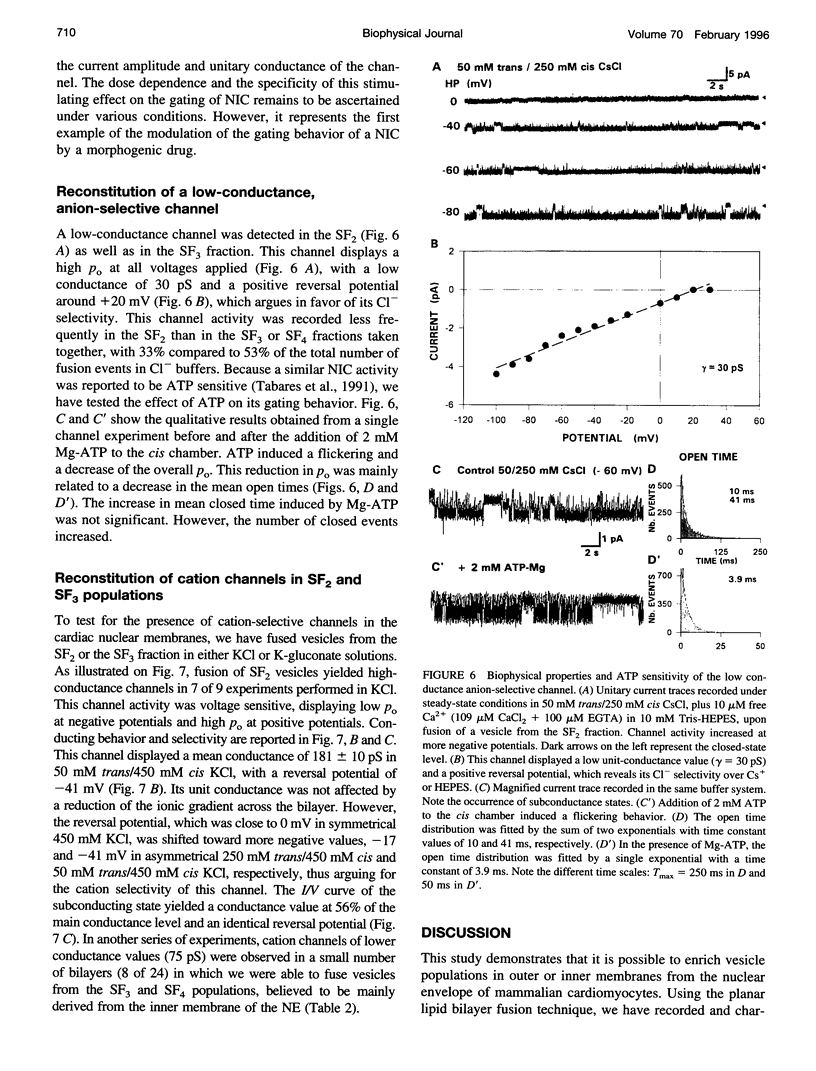
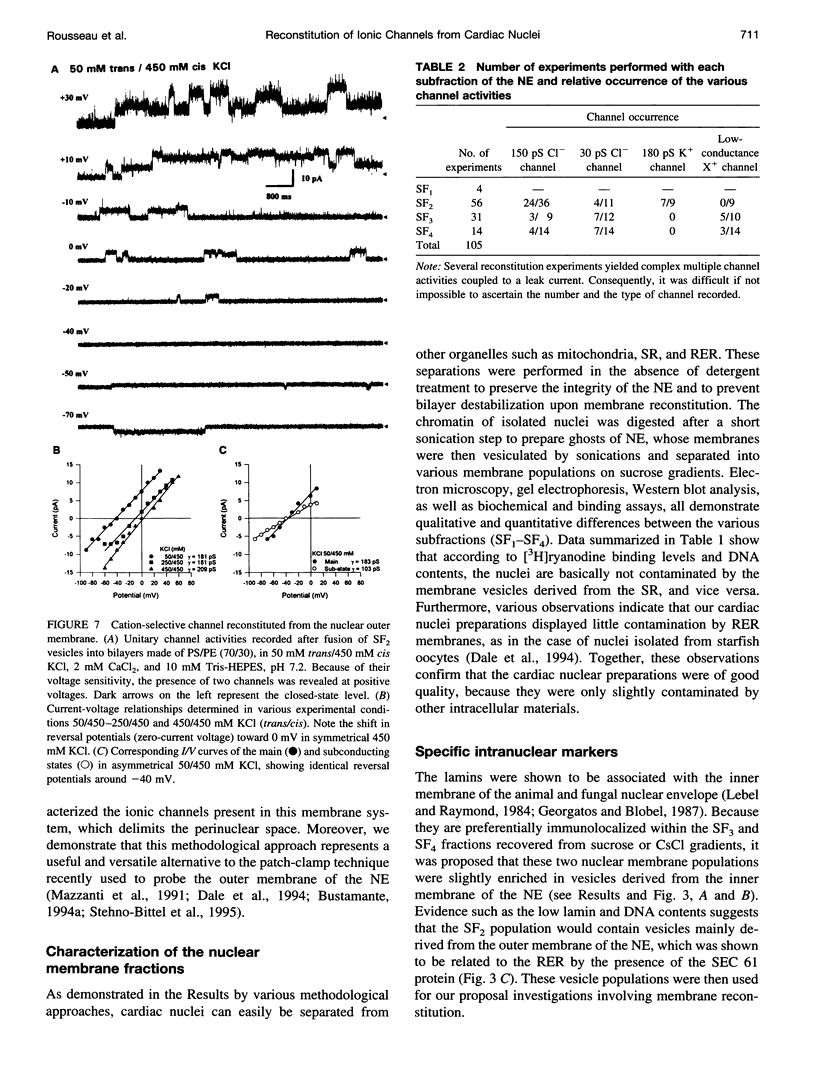
Recent reports suggest that the nuclear envelope possesses specific ion transport mechanisms that regulate the electrolyte concentrations within the nucleoplasm and perinuclear space. In this work, intact nuclei were isolated from sheep cardiac cells. After chromatin digestion, the nuclear envelopes were sonicated and four nuclear vesicle populations were separated by sucrose step gradients (SF1-SF4). These fractions were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and their protein content was analyzed by Western blot, using lamin and SEC 61 antibodies. The lamins, which are associated with the inner nuclear membrane, were present in three fractions, SF2, SF3, and SF4, with a lower amount in SF2. The SEC 61 protein, a marker of the rough endoplasmic reticulum, was detected in small amounts in SF1 and SF2. Upon fusion of vesicles into bilayers, the activities of nuclear ionic channels were recorded in 50 mM trans/250 mM cis KCl or CsCl, pH 7.2. Two types of Cl- selective channels were recorded: a large conducting 150-180-pS channel displaying substates, and a low conducting channel of 30 pS. They were both spontaneously active into bilayers, and their open probability was poorly voltage dependent at negative voltages. Retinoic acid (10(-8) M) increases the po of the large Cl- conducting channel, whereas ATP modifies the kinetics of the low conductance anion selective channel. Our data also suggest that this anionic channel is mainly present in the SF3 and SF4 population. The presence of a 181 +/- 10 pS cation-selective channel was consistently observed in the SF2 population. The behavior of this channel was voltage dependent in the voltage range -80 to +60 mV. Furthermore, we report for the first time the activity of a channel exclusively present in the SF3 and SF4 fractions, shown to contain mainly inner membrane vesicles. This cation selective channel displays a 75-pS conductance in 50 mM trans/250 mM cis K-gluconate. It is concluded that the bilayer reconstitution technique is an attractive approach to studying the electrophysiological properties of the inner and outer membranes of the nuclear envelope.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aris J. P., Blobel G. Yeast nuclear envelope proteins cross react with an antibody against mammalian pore complex proteins. J Cell Biol. 1989 Jun;108(6):2059–2067. doi: 10.1083/jcb.108.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachs O., Agell N., Carafoli E. Calcium and calmodulin function in the cell nucleus. Biochim Biophys Acta. 1992 Aug 14;1113(2):259–270. doi: 10.1016/0304-4157(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Nuclear electrophysiology. J Membr Biol. 1994 Mar;138(2):105–112. doi: 10.1007/BF00232638. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Nuclear ion channels in cardiac myocytes. Pflugers Arch. 1992 Aug;421(5):473–485. doi: 10.1007/BF00370259. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Open states of nuclear envelope ion channels in cardiac myocytes. J Membr Biol. 1994 Feb;138(1):77–89. doi: 10.1007/BF00211071. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Restricted ion flow at the nuclear envelope of cardiac myocytes. Biophys J. 1993 Jun;64(6):1735–1749. doi: 10.1016/S0006-3495(93)81545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégault B., Anagnostopoulos T., Edelman A. ATP-regulated chloride conductance in endoplasmic reticulum (ER)-enriched pig pancreas microsomes. Biochim Biophys Acta. 1993 Nov 7;1152(2):319–327. doi: 10.1016/0005-2736(93)90264-z. [DOI] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986 Jun 6;45(5):699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Davis L. I. Control of nucleocytoplasmic transport. Curr Opin Cell Biol. 1992 Jun;4(3):424–429. doi: 10.1016/0955-0674(92)90007-y. [DOI] [PubMed] [Google Scholar]
- Dessev G. N. Nuclear envelope structure. Curr Opin Cell Biol. 1992 Jun;4(3):430–435. doi: 10.1016/0955-0674(92)90008-z. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. The nuclear membrane. Science. 1992 Nov 6;258(5084):942–947. doi: 10.1126/science.1439805. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Witter R. Z., Nash P. B., Young J. D., Ojcius D. M. Cytolysis mediated by ionophores and pore-forming agents: role of intracellular calcium in apoptosis. FASEB J. 1994 Feb;8(2):237–246. doi: 10.1096/fasebj.8.2.8119494. [DOI] [PubMed] [Google Scholar]
- Eggena P., Zhu J. H., Clegg K., Barrett J. D. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension. 1993 Oct;22(4):496–501. doi: 10.1161/01.hyp.22.4.496. [DOI] [PubMed] [Google Scholar]
- Foisner R., Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993 Jul 2;73(7):1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987 Jul;105(1):117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J. S., Pierce G. N. Identification and purification of a calcium-binding protein in hepatic nuclear membranes. J Biol Chem. 1993 Feb 25;268(6):4291–4299. [PubMed] [Google Scholar]
- Guillemette G., Lamontagne S., Boulay G., Mouillac B. Differential effects of heparin on inositol 1,4,5-trisphosphate binding, metabolism, and calcium release activity in the bovine adrenal cortex. Mol Pharmacol. 1989 Mar;35(3):339–344. [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Howell G. M., Lefebvre Y. A. Characterization of high affinity and low affinity dexamethasone binding sites on male rat liver nuclear envelopes. J Steroid Biochem. 1989 Nov;33(5):977–986. doi: 10.1016/0022-4731(89)90249-5. [DOI] [PubMed] [Google Scholar]
- Hudson L. G., Santon J. B., Glass C. K., Gill G. N. Ligand-activated thyroid hormone and retinoic acid receptors inhibit growth factor receptor promoter expression. Cell. 1990 Sep 21;62(6):1165–1175. doi: 10.1016/0092-8674(90)90393-s. [DOI] [PubMed] [Google Scholar]
- Jans D. A. Nuclear signaling pathways for polypeptide ligands and their membrane receptors? FASEB J. 1994 Aug;8(11):841–847. doi: 10.1096/fasebj.8.11.8070633. [DOI] [PubMed] [Google Scholar]
- Kowarski D., Shuman H., Somlyo A. P., Somlyo A. V. Calcium release by noradrenaline from central sarcoplasmic reticulum in rabbit main pulmonary artery smooth muscle. J Physiol. 1985 Sep;366:153–175. doi: 10.1113/jphysiol.1985.sp015790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. The electrical conductance and potential across the membrane of some cell nuclei. J Cell Biol. 1963 Feb;16:421–425. doi: 10.1083/jcb.16.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanini L., Bachs O., Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem. 1992 Jun 5;267(16):11548–11552. [PubMed] [Google Scholar]
- Lebel S., Raymond Y. Lamin B from rat liver nuclei exists both as a lamina protein and as an intrinsic membrane protein. J Biol Chem. 1984 Mar 10;259(5):2693–2696. [PubMed] [Google Scholar]
- Leno G. H., Munshi R. Initiation of DNA replication in nuclei from quiescent cells requires permeabilization of the nuclear membrane. J Cell Biol. 1994 Oct;127(1):5–14. doi: 10.1083/jcb.127.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of a nuclear membrane: changes during normal development and changes induced by growth hormone. Science. 1965 Nov 12;150(3698):909–910. doi: 10.1126/science.150.3698.909. [DOI] [PubMed] [Google Scholar]
- Mak D. O., Foskett J. K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J Biol Chem. 1994 Nov 25;269(47):29375–29378. [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Cohn J., Malter H. Ion channels in the nuclear envelope. Nature. 1990 Feb 22;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Smith E. F. Ion channels in murine nuclei during early development and in fully differentiated adult cells. J Membr Biol. 1991 Apr;121(2):189–198. doi: 10.1007/BF01870532. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., Innocenti B., Rigatelli M. ATP-dependent ionic permeability on nuclear envelope in in situ nuclei of Xenopus oocytes. FASEB J. 1994 Feb;8(2):231–236. doi: 10.1096/fasebj.8.2.7509760. [DOI] [PubMed] [Google Scholar]
- Meier J., Georgatos S. D. Type B lamins remain associated with the integral nuclear envelope protein p58 during mitosis: implications for nuclear reassembly. EMBO J. 1994 Apr 15;13(8):1888–1898. doi: 10.1002/j.1460-2075.1994.tb06458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Darling E., Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986 Jan 14;25(1):236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Newcomer M. E. Retinoid-binding proteins: structural determinants important for function. FASEB J. 1995 Feb;9(2):229–239. doi: 10.1096/fasebj.9.2.7781925. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Pinkos J., Savaria D. Functional sensitivity of the native skeletal Ca(2+)-release channel to divalent cations and the Mg-ATP complex. Can J Physiol Pharmacol. 1992 Mar;70(3):394–402. doi: 10.1139/y92-049. [DOI] [PubMed] [Google Scholar]
- Smith S., Blobel G. The first membrane spanning region of the lamin B receptor is sufficient for sorting to the inner nuclear membrane. J Cell Biol. 1993 Feb;120(3):631–637. doi: 10.1083/jcb.120.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L., Lückhoff A., Clapham D. E. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995 Jan;14(1):163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Busa W. B., Wilson K. L. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993 Jul 2;73(7):1411–1422. doi: 10.1016/0092-8674(93)90366-x. [DOI] [PubMed] [Google Scholar]
- Tabares L., Mazzanti M., Clapham D. E. Chloride channels in the nuclear membrane. J Membr Biol. 1991 Jul;123(1):49–54. doi: 10.1007/BF01993962. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers G. P., Lohka M. J. A distinct vesicle population targets membranes and pore complexes to the nuclear envelope in Xenopus eggs. J Cell Biol. 1991 Feb;112(4):545–556. doi: 10.1083/jcb.112.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D. J. Making synaptic vesicles fuse with lipid bilayers. Biophys J. 1993 Aug;65(2):973–975. doi: 10.1016/S0006-3495(93)81130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]