Abstract
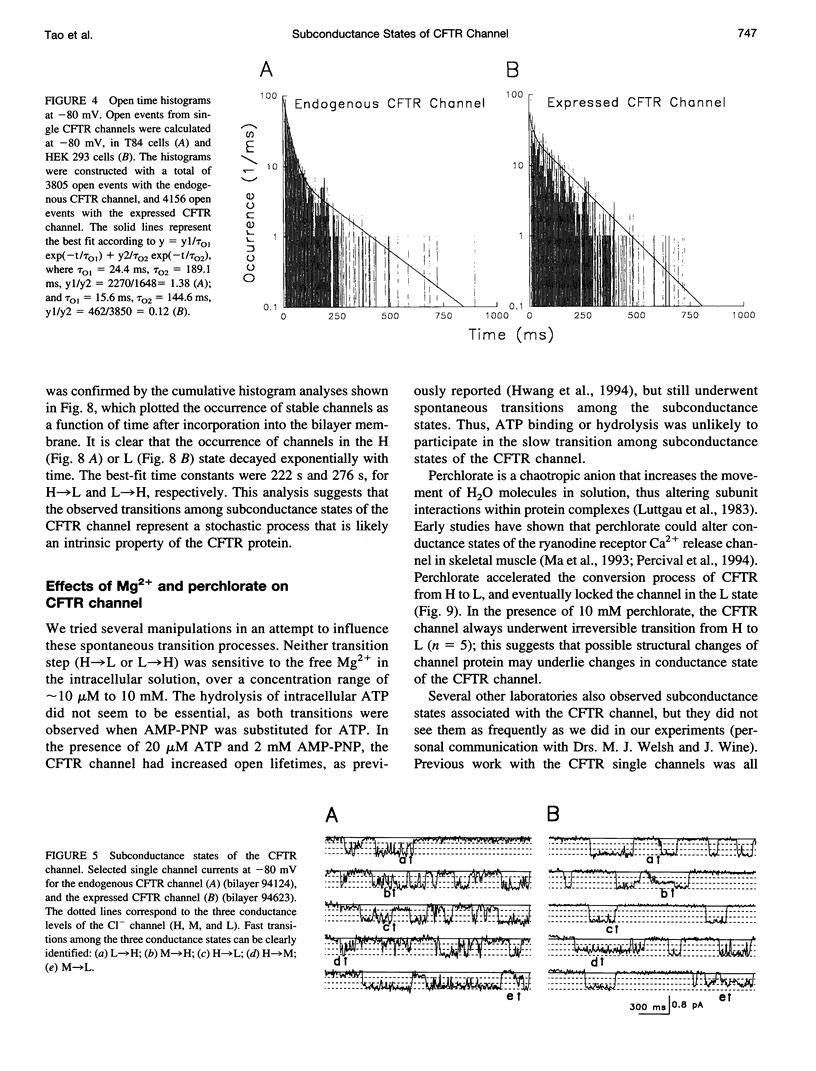
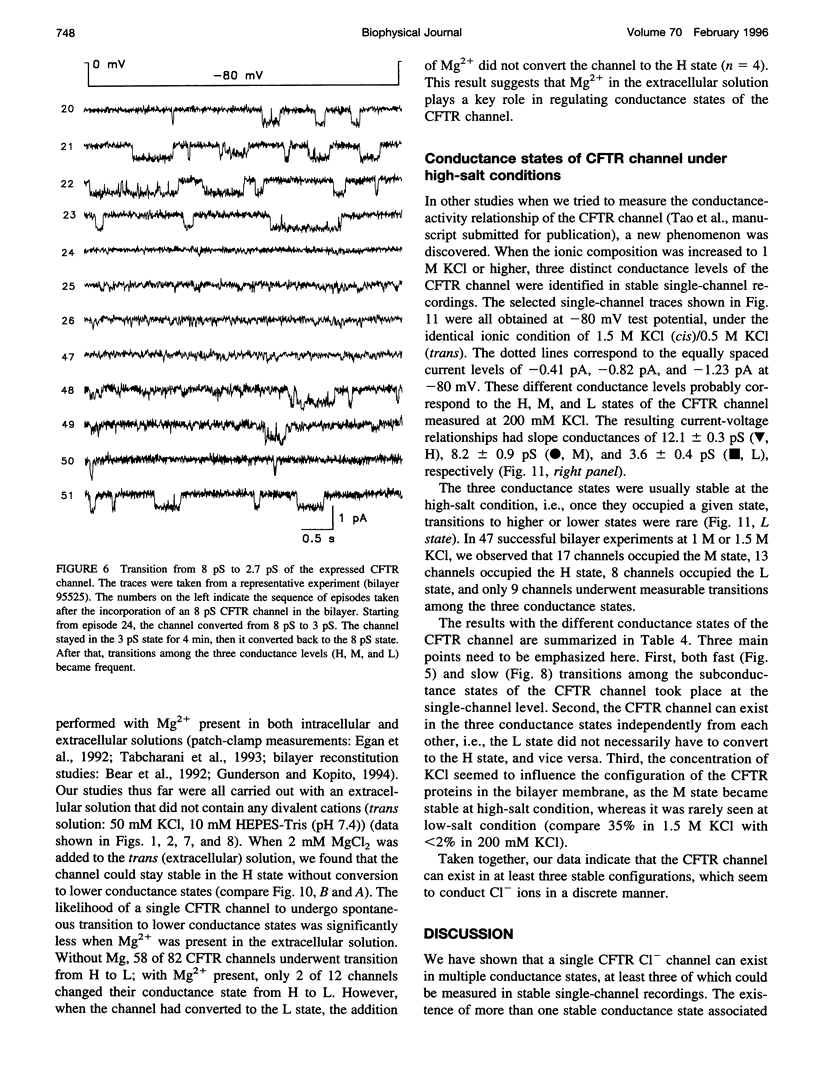
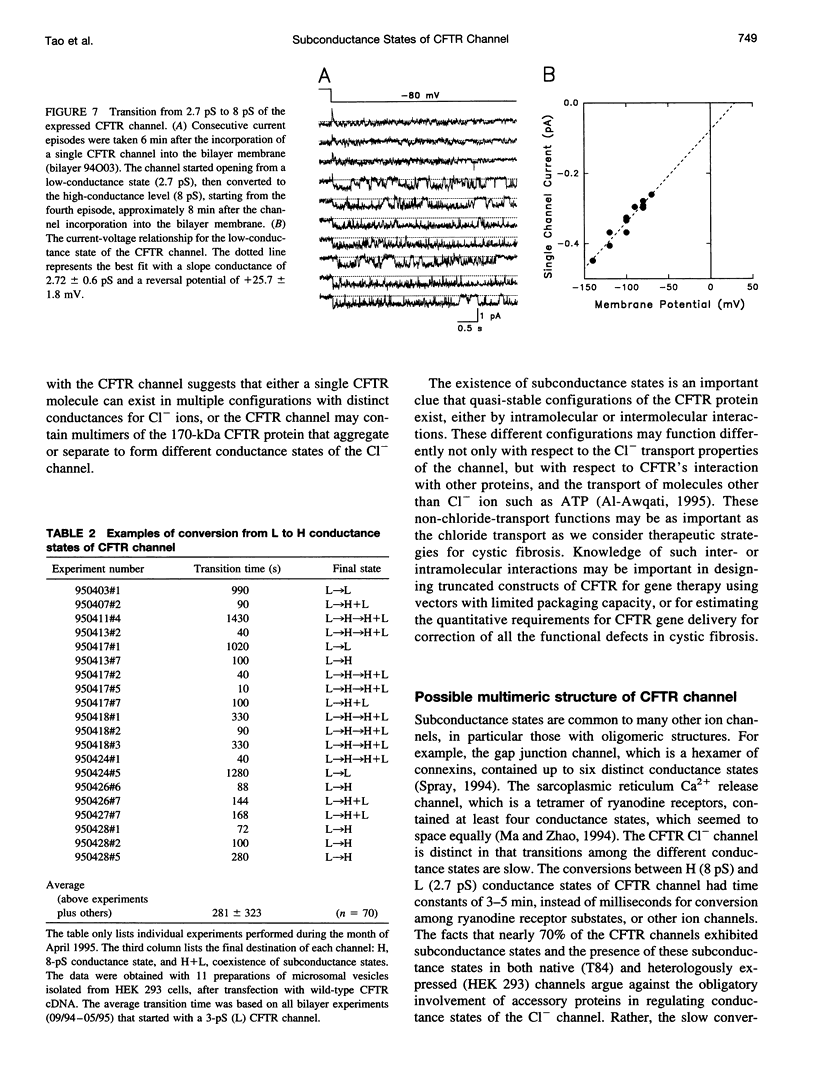
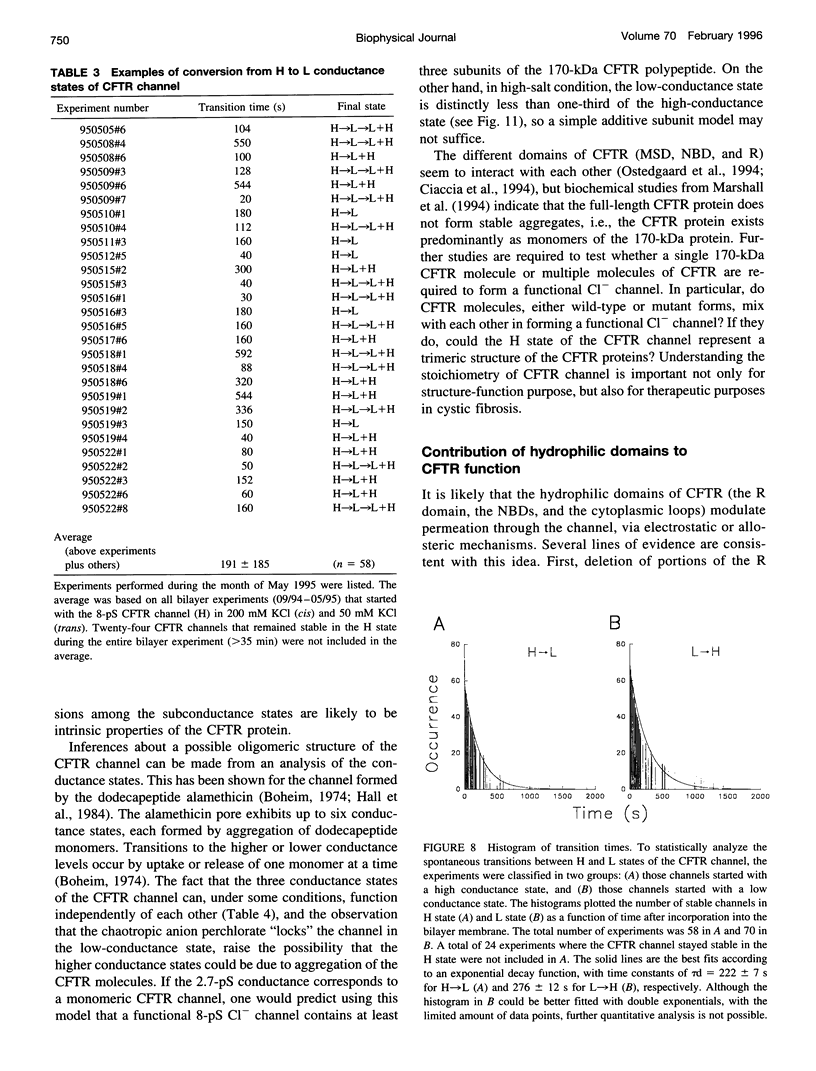
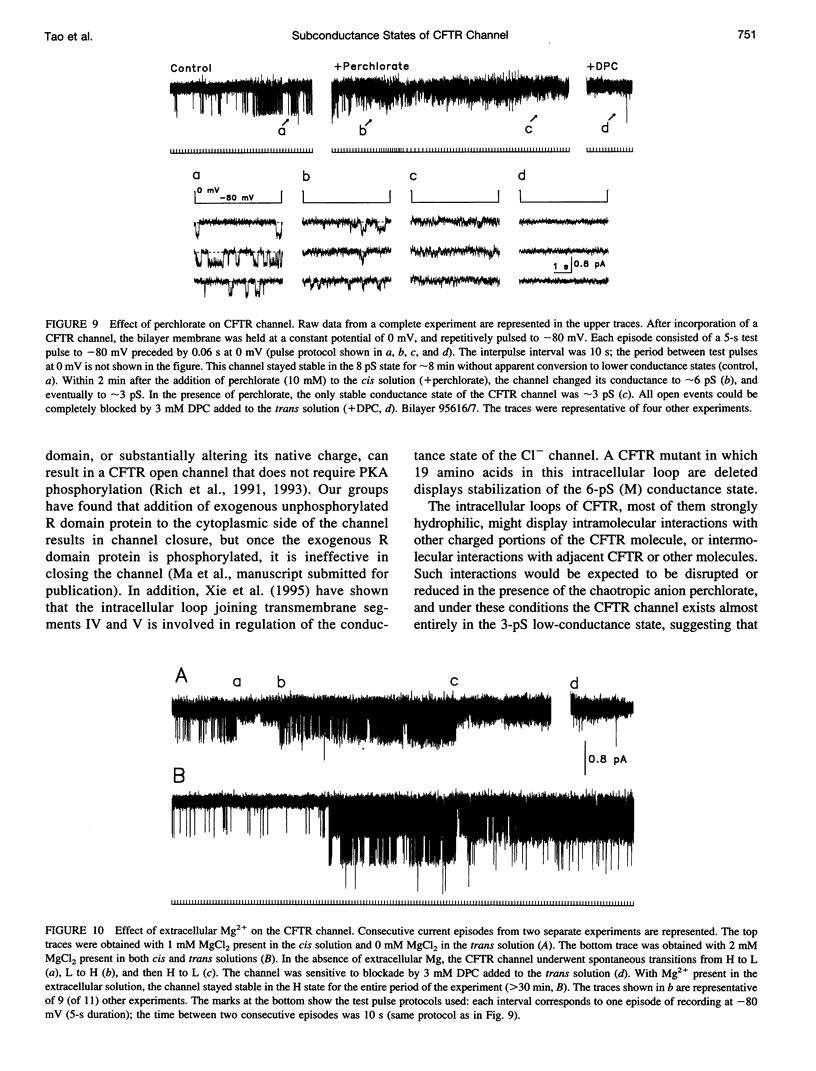
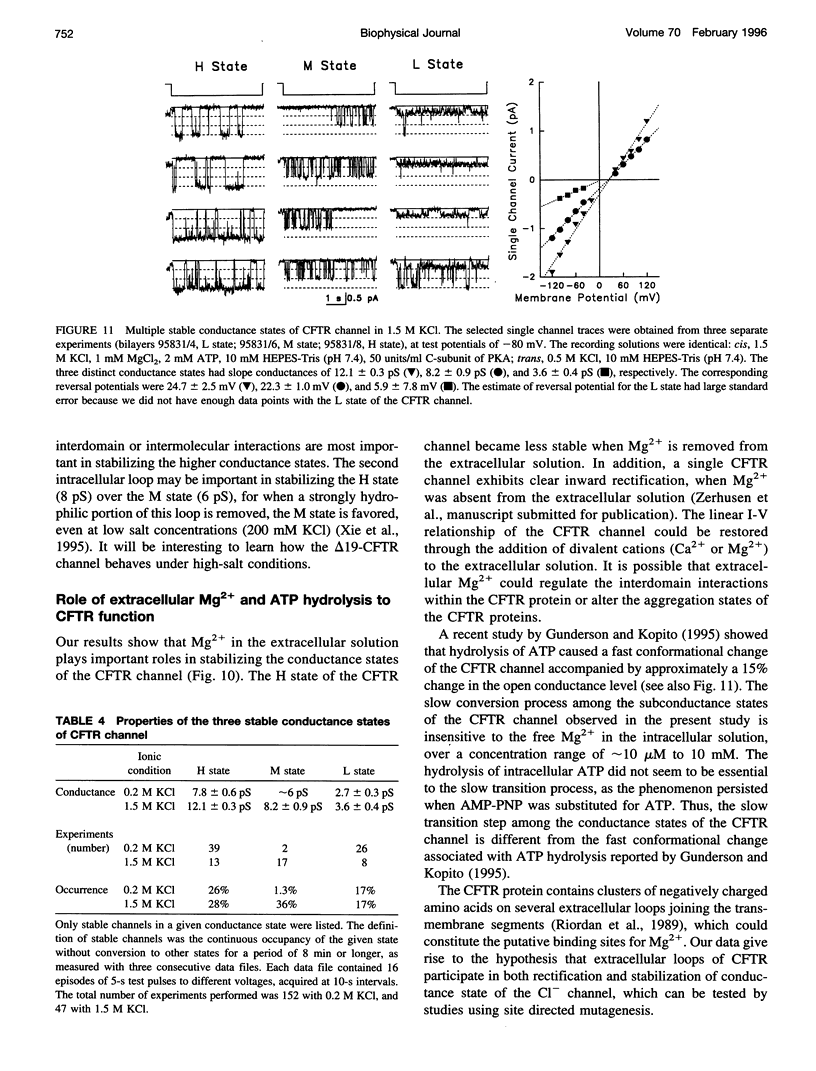
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel exhibits multiple subconductance states. To study the regulation of conductance states of the CFTR channel, we expressed the wild-type CFTR protein in HEK 293 cells, and isolated microsomal membrane vesicles for reconstitution studies in lipid bilayer membranes. A single CFTR channel had a dominant conductance of 7.8 pS (H), plus two sub-open states with conductances of approximately 6 pS (M) and 2.7 pS (L) in 200 mM KCl with 1 mM MgCl2 (intracellular) and 50 mM KCl with no MgCl2 (extracellular), with pH maintained at 7.4 by 10 mM HEPES-Tris on both sides of the channel. In 200 mM KCl, both H and L states could be measured in stable single-channel recordings, whereas M could not. Spontaneous transitions between H and L were slow; it took 4.5 min for L-->H, and 3.2 min for H-->L. These slow conversions among subconductance states of the CFTR channel were affected by extracellular Mg; in the presence of millimolar Mg, the channel remained stable in the H state. Similar phenomena were also observed with endogenous CFTR channels in T84 cells. In high-salt conditions (1.5 M KCl), all three conductance states of the expressed CFTR channel, 12.1 pS, 8.2 pS, and 3.6 pS, became stable and seemed to gate independently from each other. The existence of multiple stable conductance states associated with the CFTR channel suggests two possibilities: either a single CFTR molecule can exist in multiple configurations with different conductance values, or the CFTR channel may contain multimers of the 170-kDa CFTR protein, and different conductance states are due to different aggregation states of the CFTR protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Berger H. A., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991 Nov 15;67(4):775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Chang X. B., Tabcharani J. A., Hou Y. X., Jensen T. J., Kartner N., Alon N., Hanrahan J. W., Riordan J. R. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993 May 25;268(15):11304–11311. [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Egan M., Flotte T., Afione S., Solow R., Zeitlin P. L., Carter B. J., Guggino W. B. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S. E., Price E. M., Boucher R. C., Stutts M. J. Small linear chloride channels are endogenous to nonepithelial cells. Am J Physiol. 1992 Sep;263(3 Pt 1):C708–C713. doi: 10.1152/ajpcell.1992.263.3.C708. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Nairn A. C. Regulation of CFTR channel gating. Trends Biochem Sci. 1994 Nov;19(11):513–518. doi: 10.1016/0968-0004(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Gunderson K. L., Kopito R. R. Conformational states of CFTR associated with channel gating: the role ATP binding and hydrolysis. Cell. 1995 Jul 28;82(2):231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Gunderson K. L., Kopito R. R. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994 Jul 29;269(30):19349–19353. [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws C., Krouse M. E., Xia Y., Gruenert D. C., Wine J. J. CFTR channels in immortalized human airway cells. Am J Physiol. 1992 Dec;263(6 Pt 1):L692–L707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Nagel G., Nairn A. C., Gadsby D. C. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Gottschalk G., Kovács L., Fuxreiter M. How perchlorate improves excitation-contraction coupling in skeletal muscle fibers. Biophys J. 1983 Aug;43(2):247–249. doi: 10.1016/S0006-3495(83)84346-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Anderson K., Shirokov R., Levis R., González A., Karhanek M., Hosey M. M., Meissner G., Ríos E. Effects of perchlorate on the molecules of excitation-contraction coupling of skeletal and cardiac muscle. J Gen Physiol. 1993 Sep;102(3):423–448. doi: 10.1085/jgp.102.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Zhao J. Highly cooperative and hysteretic response of the skeletal muscle ryanodine receptor to changes in proton concentrations. Biophys J. 1994 Aug;67(2):626–633. doi: 10.1016/S0006-3495(94)80522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S., Davidson N., Lester H. A., McCarty N. A. Novel pore-lining residues in CFTR that govern permeation and open-channel block. Neuron. 1994 Sep;13(3):623–634. doi: 10.1016/0896-6273(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Percival A. L., Williams A. J., Kenyon J. L., Grinsell M. M., Airey J. A., Sutko J. L. Chicken skeletal muscle ryanodine receptor isoforms: ion channel properties. Biophys J. 1994 Nov;67(5):1834–1850. doi: 10.1016/S0006-3495(94)80665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Reddy M. M. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature. 1992 Nov 5;360(6399):79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Berger H. A., Cheng S. H., Travis S. M., Saxena M., Smith A. E., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by negative charge in the R domain. J Biol Chem. 1993 Sep 25;268(27):20259–20267. [PubMed] [Google Scholar]
- Rich D. P., Gregory R. J., Anderson M. P., Manavalan P., Smith A. E., Welsh M. J. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991 Jul 12;253(5016):205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Riordan J. R. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Spray D. C. Gap junction channels: yes, there are substates, but what does that mean? Biophys J. 1994 Aug;67(2):491–492. doi: 10.1016/S0006-3495(94)80510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Rommens J. M., Hou Y. X., Chang X. B., Tsui L. C., Riordan J. R., Hanrahan J. W. Multi-ion pore behaviour in the CFTR chloride channel. Nature. 1993 Nov 4;366(6450):79–82. doi: 10.1038/366079a0. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., Winter M. C., Ostedgaard L. S., O'Riordan C., Smith A. E., Welsh M. J. Cyclic AMP-dependent protein kinase activation of cystic fibrosis transmembrane conductance regulator chloride channels in planar lipid bilayers. J Biol Chem. 1992 May 15;267(14):9470–9473. [PubMed] [Google Scholar]
- Tsui L. C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992 Nov;8(11):392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993 Jul 2;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- Xie J., Drumm M. L., Ma J., Davis P. B. Intracellular loop between transmembrane segments IV and V of cystic fibrosis transmembrane conductance regulator is involved in regulation of chloride channel conductance state. J Biol Chem. 1995 Nov 24;270(47):28084–28091. doi: 10.1074/jbc.270.47.28084. [DOI] [PubMed] [Google Scholar]
- al-Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995 Aug 11;269(5225):805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]