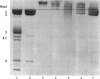
Abstract
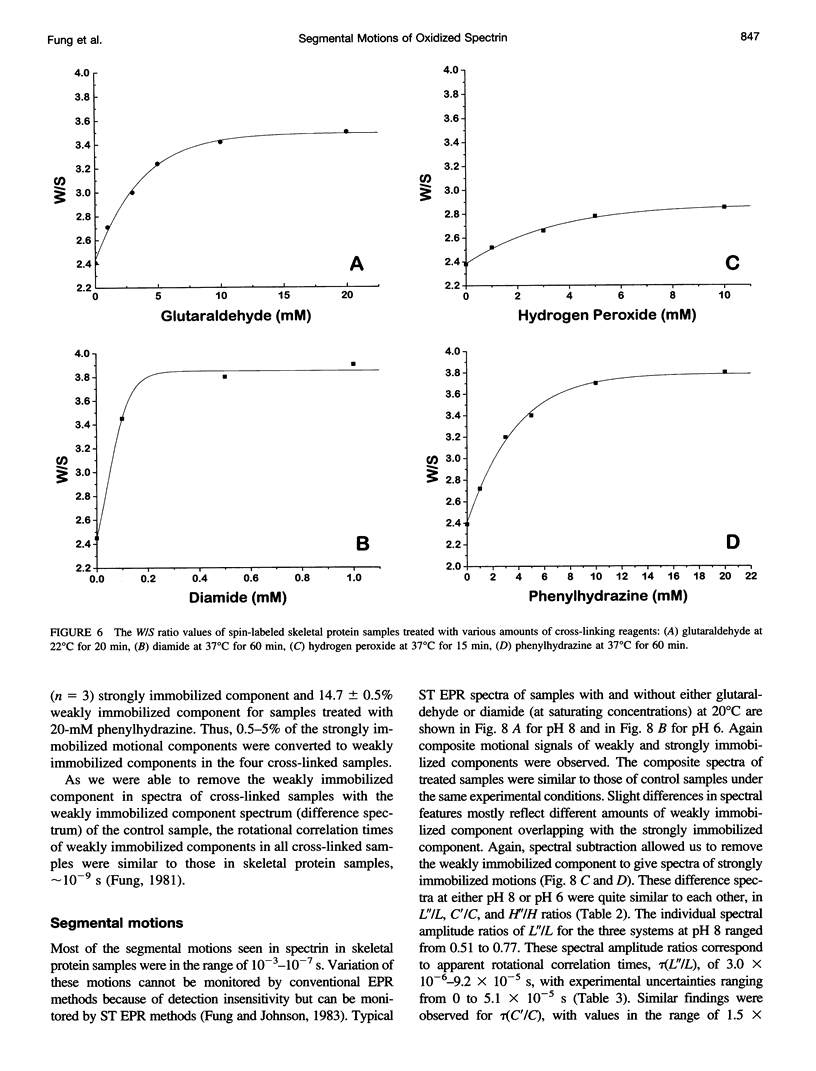
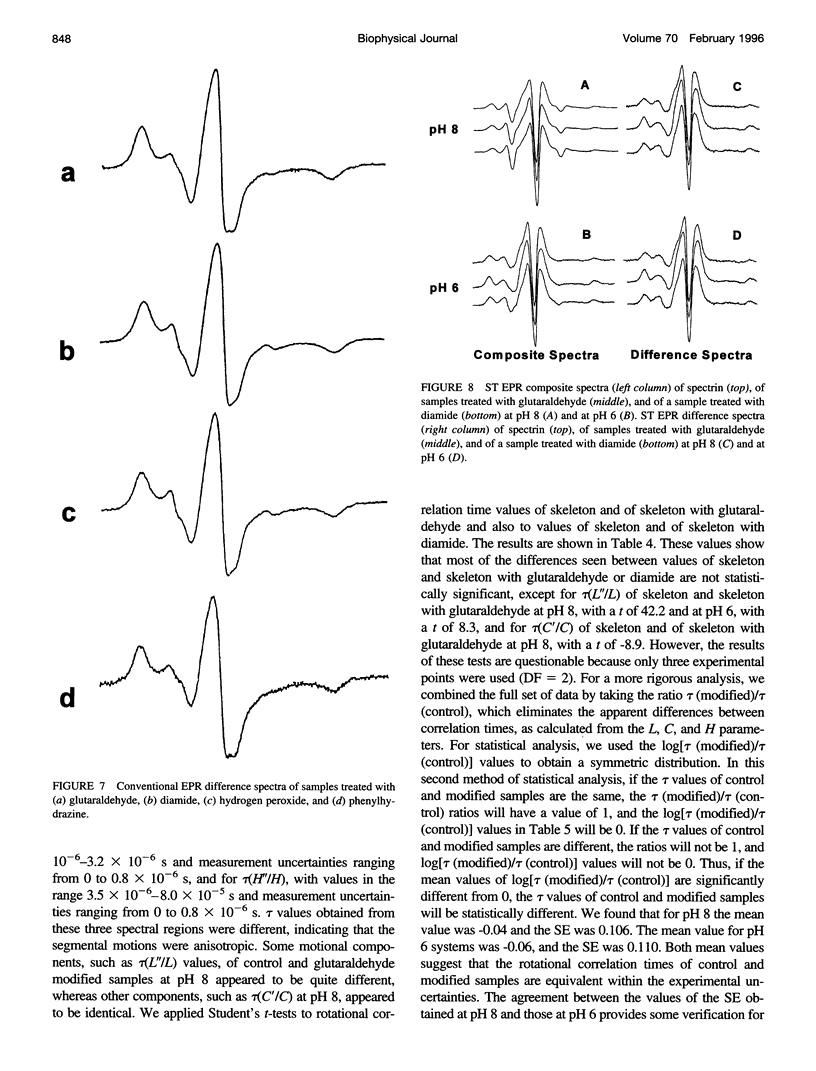
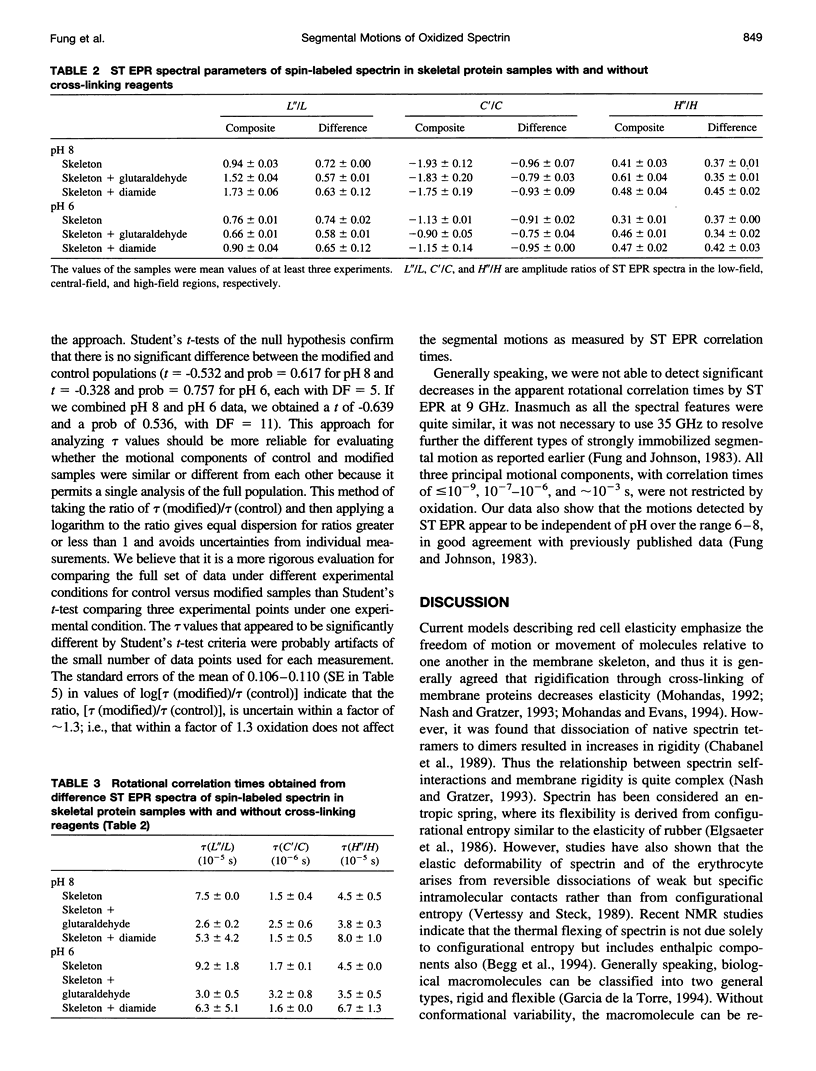
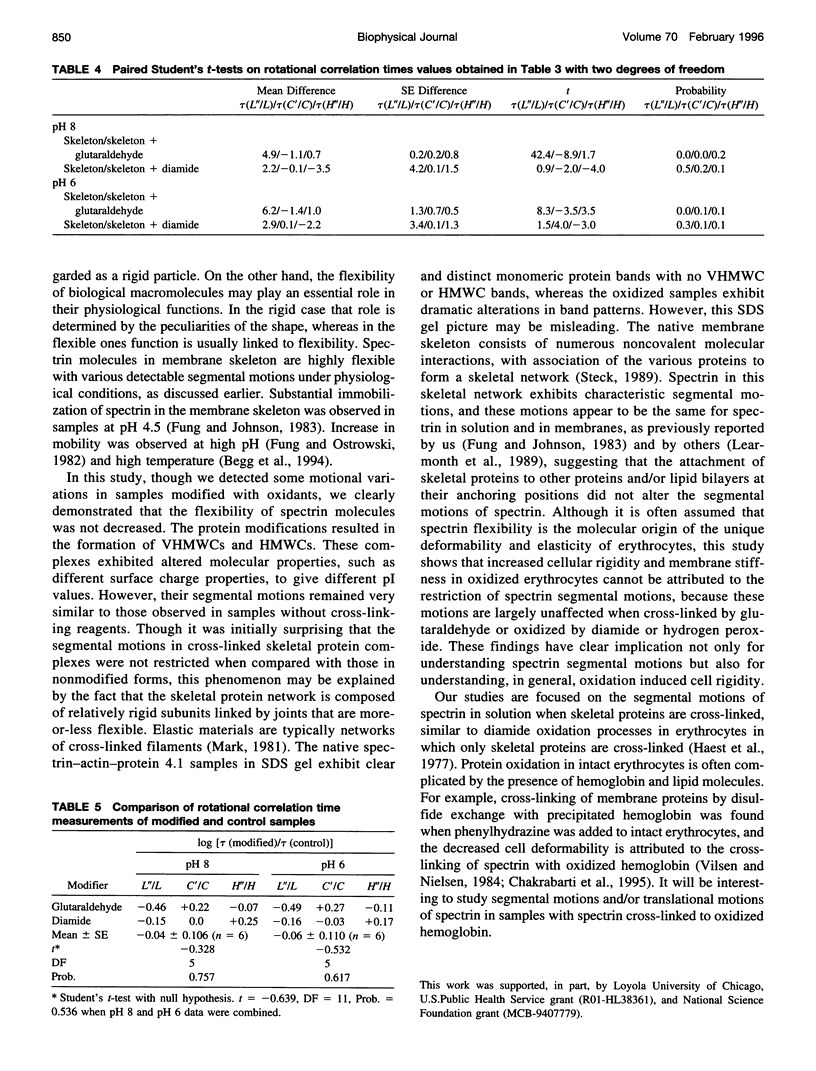
The segmental motions of cross-linked erythrocyte skeletal protein (spectrin-actin-protein 4.1) samples, labeled with nitroxide spin labels, were monitored by conventional first-harmonic and saturation transfer second-harmonic electron paramagnetic resonance methods. Skeletal proteins were extracted from human red blood cells and treated with three oxidative reagents (diamide, hydrogen peroxide, and phenylhydrazine) to cross-link sulfhydryl groups and with one fixative reagent (glutaraldehyde) to cross-link lysine residues. The treatments provided extensive cross-linking between spectrin-actin-protein 4.1 molecules, as determined by gel electrophoresis, and surface charge modification, as determined by pl measurements. However, segmental motions of the cross-linked skeletal proteins remained generally similar to those in normal skeletal proteins. Both the weakly immobilized and the strongly immobilized motions were similar in cross-linked and control samples. Small differences in some motional components were detected. In some cases, faster mobilities were observed, with approximately 5% of the strongly immobilized motions converted to the weakly immobilized motions in the cross-linked samples. It is often believed that the consequence of membrane protein oxidation is restricted protein dynamics, giving membrane rigidity. However, our studies provide needed experimental evidence to indicate that segmental motions are maintained with very little modification even in the presence of extensive cross-linking. Thus cross-linking does not restrict the internal molecular flexibility that gives rise to segmental motions.
Full text
PDF


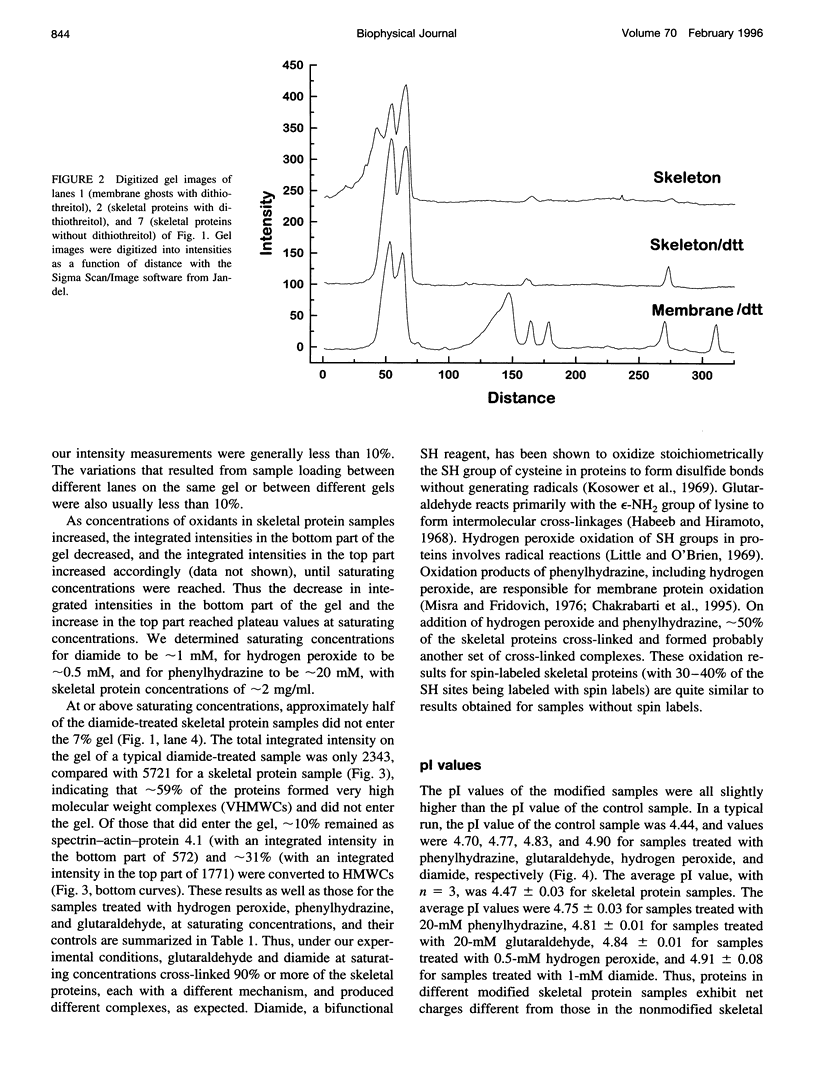
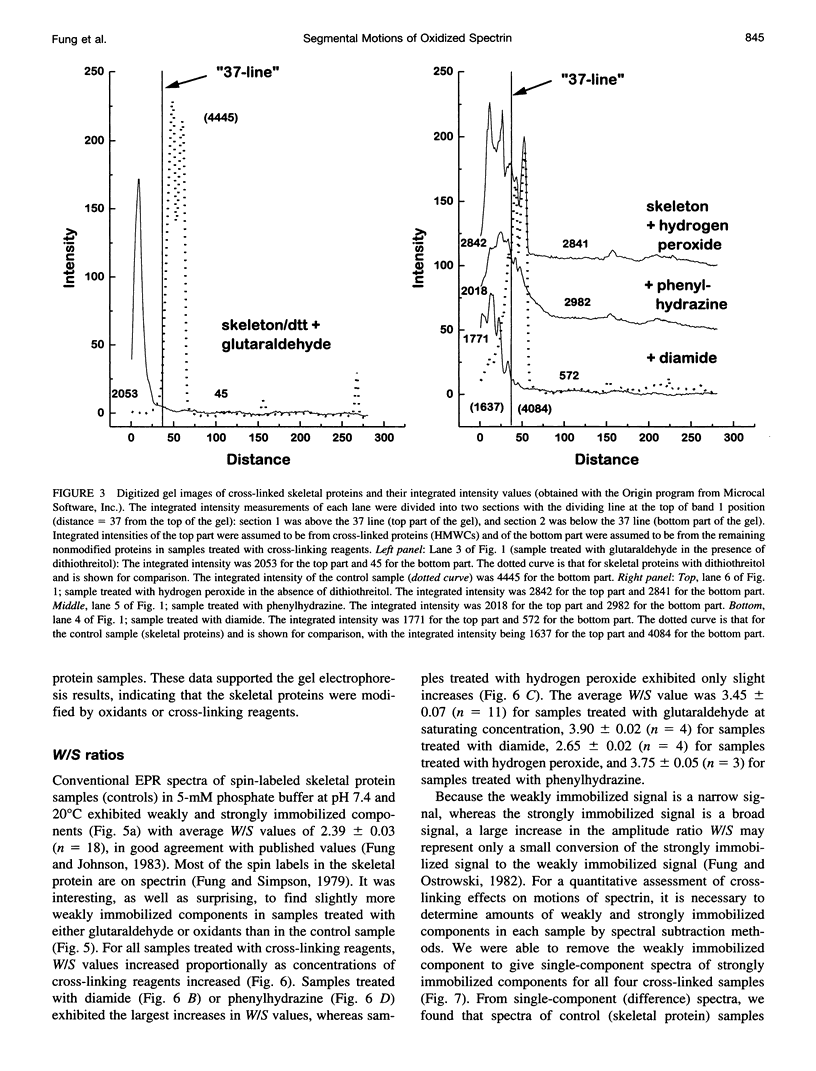
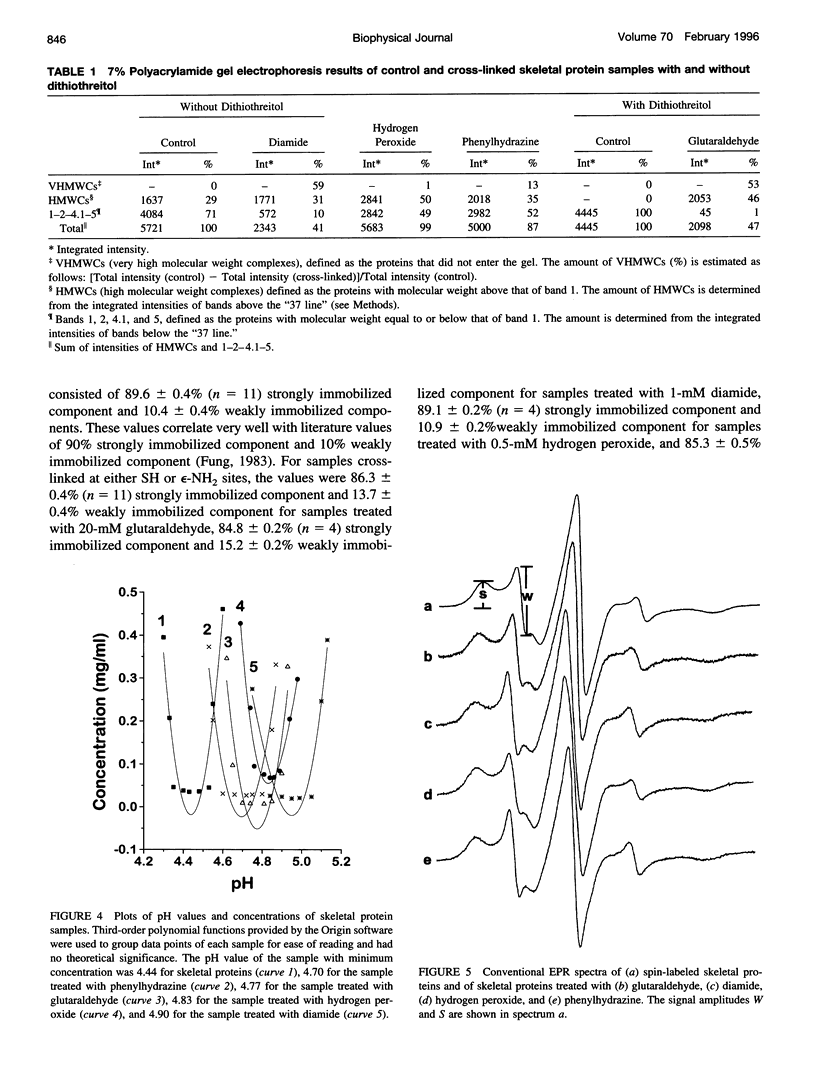

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arduini A., Chen Z., Stern A. Phenylhydrazine-induced changes in erythrocyte membrane surface lipid packing. Biochim Biophys Acta. 1986 Nov 6;862(1):65–71. doi: 10.1016/0005-2736(86)90469-4. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Cohen C. M., Lux S. E. The effect of mild diamide oxidation on the structure and function of human erythrocyte spectrin. J Biol Chem. 1986 Apr 5;261(10):4620–4628. [PubMed] [Google Scholar]
- Begg G. E., Ralston G. B., Morris M. B. A proton nuclear magnetic resonance study of the mobile regions of human erythroid spectrin. Biophys Chem. 1994 Sep;52(1):63–73. doi: 10.1016/0301-4622(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Budzynski D. M., Benight A. S., LaBrake C. C., Fung L. W. Dynamic light scattering investigations of human erythrocyte spectrin. Biochemistry. 1992 Apr 14;31(14):3653–3660. doi: 10.1021/bi00129a014. [DOI] [PubMed] [Google Scholar]
- Calvert R., Ungewickell E., Gratzer W. A conformational study of human spectrin. Eur J Biochem. 1980 Jun;107(2):363–367. doi: 10.1111/j.1432-1033.1980.tb06037.x. [DOI] [PubMed] [Google Scholar]
- Chabanel A., Sung K. L., Rapiejko J., Prchal J. T., Palek J., Liu S. C., Chien S. Viscoelastic properties of red cell membrane in hereditary elliptocytosis. Blood. 1989 Feb;73(2):592–595. [PubMed] [Google Scholar]
- Chakrabarti S., Sonaye B., Naik A. A., Nadkarni P. P. Erythrocyte membrane protein damage by oxidation products of phenylhydrazine. Biochem Mol Biol Int. 1995 Feb;35(2):255–263. [PubMed] [Google Scholar]
- Clague M. J., Harrison J. P., Morrison I. E., Wyatt K., Cherry R. J. Transient dichroism studies of spectrin rotational diffusion in solution and bound to erythrocyte membranes. Biochemistry. 1990 Apr 24;29(16):3898–3904. doi: 10.1021/bi00468a016. [DOI] [PubMed] [Google Scholar]
- Dubreuil Y. L., Cassoly R. A dynamical study on the interactions between the cytoskeleton components in the human erythrocyte as detected by saturation transfer electron paramagnetic resonance of spin-labeled spectrin, ankyrin, and protein 4.1. Arch Biochem Biophys. 1983 Jun;223(2):495–502. doi: 10.1016/0003-9861(83)90614-8. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Stokke B. T., Mikkelsen A., Branton D. The molecular basis of erythrocyte shape. Science. 1986 Dec 5;234(4781):1217–1223. doi: 10.1126/science.3775380. [DOI] [PubMed] [Google Scholar]
- Fung L. W. Analysis of spin-labeled erythrocyte membranes. Ann N Y Acad Sci. 1983;414:162–168. doi: 10.1111/j.1749-6632.1983.tb31682.x. [DOI] [PubMed] [Google Scholar]
- Fung L. W., Lu H. Z., Hjelm R. P., Jr, Johnson M. E. Quantitative detection of rapid motions in spectrin by NMR. Life Sci. 1989;44(11):735–740. doi: 10.1016/0024-3205(89)90385-8. [DOI] [PubMed] [Google Scholar]
- Fung L. W., Lu H. Z., Hjelm R. P., Jr, Johnson M. E. Selective detection of rapid motions in spectrin by NMR. FEBS Lett. 1986 Mar 3;197(1-2):234–238. doi: 10.1016/0014-5793(86)80333-7. [DOI] [PubMed] [Google Scholar]
- Fung L. W., Ostrowski M. S. Spin label electron paramagnetic resonance (EPR) studies of Huntington disease erythrocyte membranes. Am J Hum Genet. 1982 May;34(3):469–480. [PMC free article] [PubMed] [Google Scholar]
- Fung L. W., Simpson M. J. Topology of a protein spin label in erythrocyte membranes. FEBS Lett. 1979 Dec 1;108(1):269–273. doi: 10.1016/0014-5793(79)81226-0. [DOI] [PubMed] [Google Scholar]
- Fung L. W. Spin-label detection of hemoglobin-membrane interaction at physiological pH. Biochemistry. 1981 Dec 8;20(25):7162–7166. doi: 10.1021/bi00528a017. [DOI] [PubMed] [Google Scholar]
- Habeeb A. J., Hiramoto R. Reaction of proteins with glutaraldehyde. Arch Biochem Biophys. 1968 Jul;126(1):16–26. doi: 10.1016/0003-9861(68)90554-7. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Plasa G., Deuticke B. Intra- and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim Biophys Acta. 1977 Sep 5;469(2):226–230. doi: 10.1016/0005-2736(77)90186-9. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Leung A., Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990 Sep 1;76(5):1015–1020. [PubMed] [Google Scholar]
- Hochstein P., Jain S. K. Association of lipid peroxidation and polymerization of membrane proteins with erythrocyte aging. Fed Proc. 1981 Feb;40(2):183–188. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Zipser Y., Faltin Z., Shomrat R. Dynamic changes of red cell membrane thiol groups followed by bimane fluorescent labeling. Biochim Biophys Acta. 1981 Feb 6;640(3):748–759. doi: 10.1016/0005-2736(81)90105-x. [DOI] [PubMed] [Google Scholar]
- Kurantsin-Mills J., Lessin L. S. Aggregation of intramembrane particles in erythrocyte membranes treated with diamide. Biochim Biophys Acta. 1981 Feb 20;641(1):129–137. doi: 10.1016/0005-2736(81)90576-9. [DOI] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Learmonth R. P., Woodhouse A. G., Sawyer W. H. Rotational dynamics of erythrocyte spectrin. Biochim Biophys Acta. 1989 Dec 11;987(1):124–128. doi: 10.1016/0005-2736(89)90463-x. [DOI] [PubMed] [Google Scholar]
- Little C., O'Brien P. J. Mechanism of peroxide-inactivation of the sulphydryl enzyme glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1969 Oct;10(3):533–538. doi: 10.1111/j.1432-1033.1969.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kon K., Imaizumi K., Sekiya M., Shiga T. Alteration of rheological properties of human erythrocytes by crosslinking of membrane proteins. Biochim Biophys Acta. 1983 Oct 26;735(1):104–112. doi: 10.1016/0005-2736(83)90265-1. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry. 1976 Feb 10;15(3):681–687. doi: 10.1021/bi00648a036. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- Mohandas N. Molecular basis for red cell membrane viscoelastic properties. Biochem Soc Trans. 1992 Nov;20(4):776–782. doi: 10.1042/bst0200776. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Gratzer W. B. Structural determinants of the rigidity of the red cell membrane. Biorheology. 1993 Sep-Dec;30(5-6):397–407. doi: 10.3233/bir-1993-305-611. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C., Hochstein P. Alterations in erythrocyte membrane fluidity by phenylhydrazine-induced peroxidation of lipids. Biochem Biophys Res Commun. 1981 Jun;100(4):1537–1542. doi: 10.1016/0006-291x(81)90693-8. [DOI] [PubMed] [Google Scholar]
- Sahr K. E., Laurila P., Kotula L., Scarpa A. L., Coupal E., Leto T. L., Linnenbach A. J., Winkelmann J. C., Speicher D. W., Marchesi V. T. The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem. 1990 Mar 15;265(8):4434–4443. [PubMed] [Google Scholar]
- Snyder L. M., Fortier N. L., Trainor J., Jacobs J., Leb L., Lubin B., Chiu D., Shohet S., Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985 Nov;76(5):1971–1977. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Vertessy B. G., Steck T. L. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys J. 1989 Feb;55(2):255–262. doi: 10.1016/S0006-3495(89)82800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsen B., Nielsen H. Reaction of phenylhydrazine with erythrocytes. Cross-linking of spectrin by disulfide exchange with oxidized hemoglobin. Biochem Pharmacol. 1984 Sep 1;33(17):2739–2748. doi: 10.1016/0006-2952(84)90690-7. [DOI] [PubMed] [Google Scholar]
- Winkelmann J. C., Costa F. F., Linzie B. L., Forget B. G. Beta spectrin in human skeletal muscle. Tissue-specific differential processing of 3' beta spectrin pre-mRNA generates a beta spectrin isoform with a unique carboxyl terminus. J Biol Chem. 1990 Nov 25;265(33):20449–20454. [PubMed] [Google Scholar]
- de la Torre J. G. Hydrodynamics of segmentally flexible macromolecules. Eur Biophys J. 1994;23(5):307–322. doi: 10.1007/BF00188655. [DOI] [PubMed] [Google Scholar]