Abstract
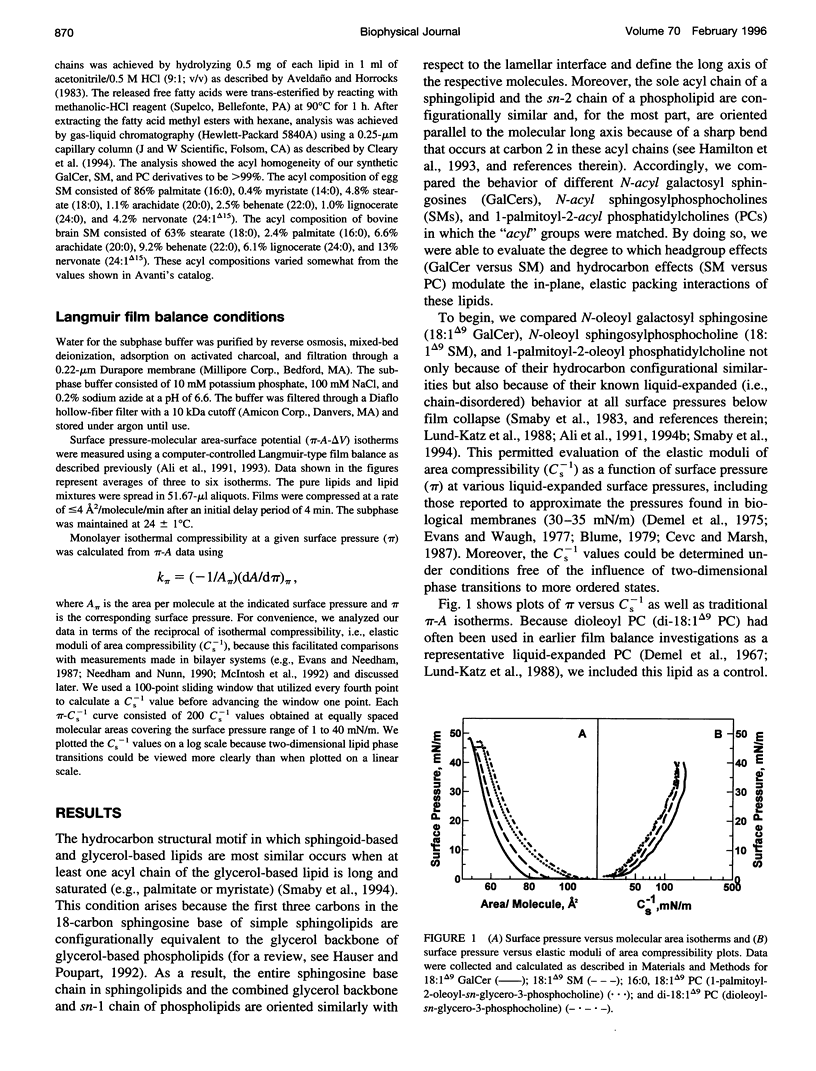
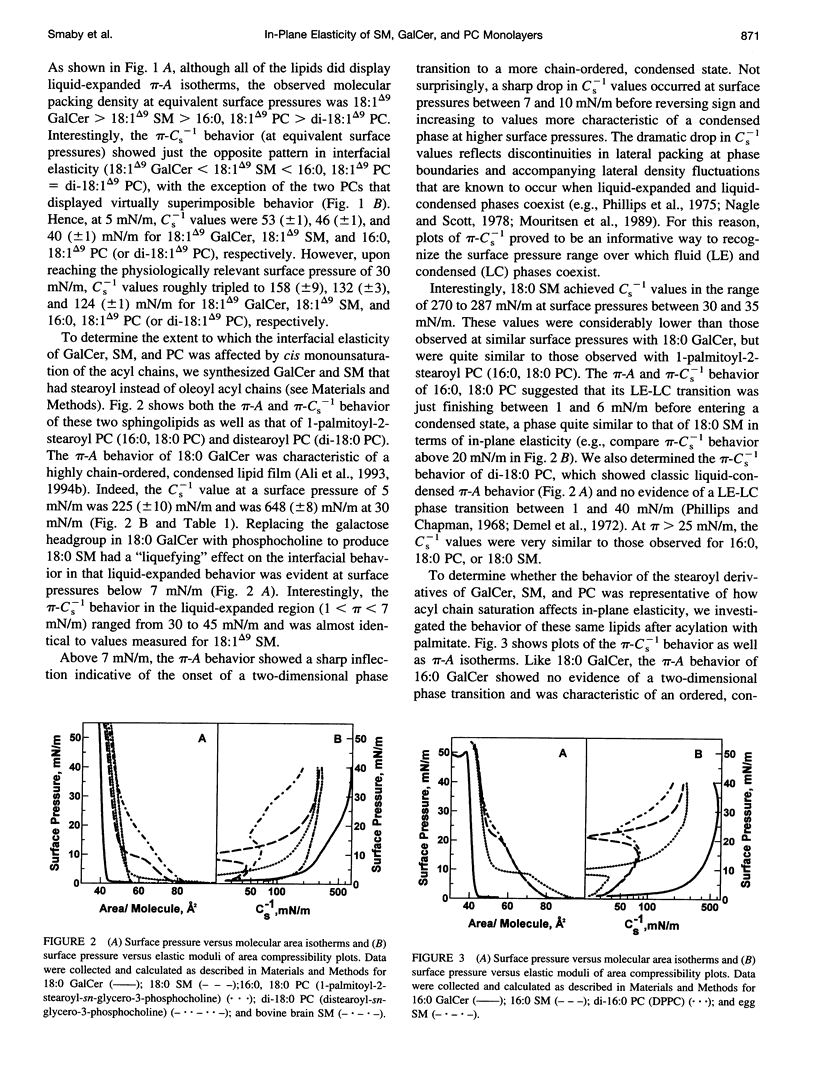
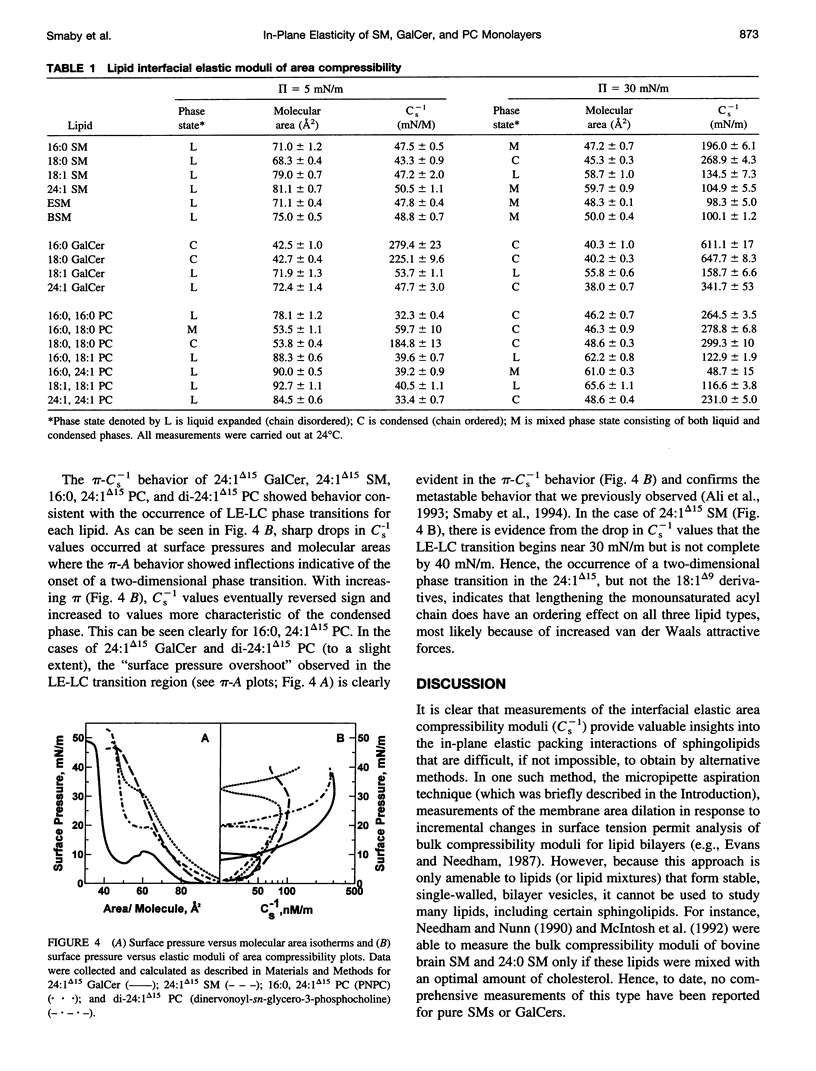
The interfacial elastic packing interactions of different galactosylceramides (GalCers), sphingomyelins (SMs), and phosphatidylcholines (PC) were compared by determining their elastic area compressibility moduli (Cs-1) as a function of lateral packing pressure (pi) in a Langmuir-type film balance. To assess the relative contributions of the lipid headgroups as well as those of the ceramide and diacylglycerol hydrocarbon regions, we synthesized various GalCer and SM species with identical, homogeneous acyl residues and compared their behavior to that of PCs possessing similar hydrocarbon structures. For PCs, this meant that the sn-1 acyl chain was long and saturated (e.g., palmitate) and the sn-2 chain composition was varied to match that of GalCer or SM. When at equivalent pi and in either the chain-disordered (liquid-expanded) or chain-ordered (liquid-condensed) state, GalCer films were less elastic than either SM or PC films. When lipid headgroups were identical (SM and PC), Cs-1 values (at equivalent pi) for chain-disordered SMs, but not chain-ordered SMs, were 25-30% higher than those of PCs. Typical values for fluid phase (liquid-expanded) GalCer at 30 mN/m and 24 degrees C were 158 (+/- 7) mN/m, whereas those of SM were 135 (+/- 7) mN/m and those of PC were 123 (+/- 2) mN/m. Pressure-induced transitions to chain-ordered states (liquid-condensed) resulted in significant increases (two- to fourfold) in the "in-plane" compressibility for all three lipid types. Typical Cs-1 values for chain-ordered GalCers at 30 mN/m and 24 degrees C were between 610 and 650 mN/m, whereas those of SM and of PC were very similar and were between 265 and 300 mN/m. Under fluid phase conditions, the pi-Cs-1 behavior for each lipid type was insensitive to whether the acyl chain was saturated or monounsaturated. Measurement of the Cs-1 values also provided an effective way to evaluate the two-dimensional phase transition region of SMs, GalCers, and PCs. Modest heterogeneity in the acyl composition led to transitional broadening. Our findings provide useful information regarding the in-plane elasticity of lipids that are difficult to investigate by alternative methods, i.e., micropipette aspiration technique. The results also provide insight into the stability of sphingolipid-enriched, membrane microdomains that are thought to play a role in the sorting and trafficking of proteins containing glycosylphosphatidylinositol anchors with cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Brockman H. L., Brown R. E. Structural determinants of miscibility in surface films of galactosylceramide and phosphatidylcholine: effect of unsaturation in the galactosylceramide acyl chain. Biochemistry. 1991 Nov 26;30(47):11198–11205. doi: 10.1021/bi00111a002. [DOI] [PubMed] [Google Scholar]
- Ali S., Smaby J. M., Brockman H. L., Brown R. E. Cholesterol's interfacial interactions with galactosylceramides. Biochemistry. 1994 Mar 15;33(10):2900–2906. doi: 10.1021/bi00176a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Smaby J. M., Brown R. E. Acyl structure regulates galactosylceramide's interfacial interactions. Biochemistry. 1993 Nov 2;32(43):11696–11703. doi: 10.1021/bi00094a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate K. R., Glomset J. A. Effect of acyl chain unsaturation on the packing of model diacylglycerols in simulated monolayers. J Lipid Res. 1991 Oct;32(10):1645–1655. [PubMed] [Google Scholar]
- Aveldaño M. I., Horrocks L. A. Quantitative release of fatty acids from lipids by a simple hydrolysis procedure. J Lipid Res. 1983 Aug;24(8):1101–1105. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Bittman R., Kasireddy C. R., Mattjus P., Slotte J. P. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 1994 Oct 4;33(39):11776–11781. doi: 10.1021/bi00205a013. [DOI] [PubMed] [Google Scholar]
- Blume A. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim Biophys Acta. 1979 Oct 19;557(1):32–44. doi: 10.1016/0005-2736(79)90087-7. [DOI] [PubMed] [Google Scholar]
- Brown D. A. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992 Nov;2(11):338–343. [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S. Conformation of the polar headgroup of sphingomyelin and its analogues. Biochim Biophys Acta. 1988 Apr 7;939(2):315–326. doi: 10.1016/0005-2736(88)90076-4. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Molecular conformations of cerebrosides in bilayers determined by Raman spectroscopy. Biophys J. 1980 Dec;32(3):1007–1021. doi: 10.1016/S0006-3495(80)85032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. P., Phillips F. C., Morton R. A. Liver, serum and adipose tissue fatty acid composition in suckling Zucker rats. Lipids. 1994 Nov;29(11):753–758. doi: 10.1007/BF02536696. [DOI] [PubMed] [Google Scholar]
- Cohen R., Barenholz Y., Gatt S., Dagan A. Preparation and characterization of well defined D-erythro sphingomyelins. Chem Phys Lipids. 1984 Oct;35(4):371–384. doi: 10.1016/0009-3084(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Kobayashi T., Kurzchalia T. V., Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993 Jun 29;32(25):6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Grönberg L., Ruan Z. S., Bittman R., Slotte J. P. Interaction of cholesterol with synthetic sphingomyelin derivatives in mixed monolayers. Biochemistry. 1991 Nov 5;30(44):10746–10754. doi: 10.1021/bi00108a020. [DOI] [PubMed] [Google Scholar]
- Hamilton K. S., Jarrell H. C., Brière K. M., Grant C. W. Glycosphingolipid backbone conformation and behavior in cholesterol-containing phospholipid bilayers. Biochemistry. 1993 Apr 20;32(15):4022–4028. doi: 10.1021/bi00066a024. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Holte L. L., Peter S. A., Sinnwell T. M., Gawrisch K. 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys J. 1995 Jun;68(6):2396–2403. doi: 10.1016/S0006-3495(95)80422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D. S., Chapman D. The properties of brain galactocerebroside monolayers. Biochim Biophys Acta. 1988 Jan 13;937(1):10–22. doi: 10.1016/0005-2736(88)90222-2. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot Y., Rappoport S., Wolman Y. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J Lipid Res. 1967 Mar;8(2):142–145. [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988 May 3;27(9):3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- Marcelja S. Chain ordering in liquid crystals. II. Structure of bilayer membranes. Biochim Biophys Acta. 1974 Oct 29;367(2):165–176. doi: 10.1016/0005-2736(74)90040-6. [DOI] [PubMed] [Google Scholar]
- Mason J. T., Broccoli A. V., Huang C. A method for the synthesis of isomerically pure saturated mixed-chain phosphatidylcholines. Anal Biochem. 1981 May 1;113(1):96–101. doi: 10.1016/0003-2697(81)90049-x. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Scott H. L., Jr Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta. 1978 Nov 2;513(2):236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- Needham D., McIntosh T. J., Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 1988 Jun 28;27(13):4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva J. L., Bron R., Corver J., Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994 Jun 15;13(12):2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Graham D. E., Hauser H. Lateral compressibility and penetration into phospholipid monolayers and bilayer membranes. Nature. 1975 Mar 13;254(5496):154–156. doi: 10.1038/254154a0. [DOI] [PubMed] [Google Scholar]
- Radin N. S. Preparation of psychosines (1-O-hexosyl sphingosine) from cerebrosides. Lipids. 1974 May;9(5):358–360. doi: 10.1007/BF02533114. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Sandhoff K. Lysogangliosides: synthesis and use in preparing labeled gangliosides. Methods Enzymol. 1987;138:319–341. doi: 10.1016/0076-6879(87)38028-0. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Lapidot Y. Synthesis of fatty acid anhydrides by reaction with dicyclohexylcarbodiimide. J Lipid Res. 1966 Jan;7(1):174–175. [PubMed] [Google Scholar]
- Slotte J. P., Ostman A. L., Kumar E. R., Bittman R. Cholesterol interacts with lactosyl and maltosyl cerebrosides but not with glucosyl or galactosyl cerebrosides in mixed monolayers. Biochemistry. 1993 Aug 10;32(31):7886–7892. doi: 10.1021/bi00082a008. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L., Brown R. E. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994 Aug 9;33(31):9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Yedgar S., Cohen R., Gatt S., Barenholz Y. Hydrolysis of monomolecular layers of synthetic sphingomyelins by sphingomyelinase of Staphylococcus aureus. Biochem J. 1982 Mar 1;201(3):597–603. doi: 10.1042/bj2010597. [DOI] [PMC free article] [PubMed] [Google Scholar]


