Abstract
More chemicals can be smelled than there are olfactory receptors for them, necessitating a combinatorial representation by somewhat broadly tuned receptors. To understand the perception of odor quality and concentration, it is essential to establish the nature of the receptor repertoires that are activated by particular odorants at particular concentrations. We have taken advantage of the one-to-one correspondence of glomeruli and olfactory receptor molecules in the mouse olfactory bulb to analyze the tuning properties of a major receptor population by high resolution calcium imaging of odor responses selectively in the presynaptic compartment of glomeruli. We show that eighty different olfactory receptors projecting to the dorsal olfactory bulb respond to high concentrations of aldehydes with limited specificity. Varying ensembles of about 10 to 20 receptors encode any particular aldehyde at low stimulus concentrations with high specificity. Even normalized odor response patterns are markedly concentration dependent, caused by pronounced differences in affinity within the aldehyde receptor repertoire.
Understanding odor perception has been furthered by the discovery of a large family of olfactory receptor (OR) genes (1) with approximately one thousand members in mouse. Olfactory receptor neurons (ORN) appear to express only a single OR gene each, and ORN expressing the same OR converge onto one to two glomeruli in the olfactory bulb (OB; refs. 2–4), i.e., there is a one-to-one correspondence of glomeruli and OR. In first approximation, glomerular positions appear invariant between individuals (3, 4); thus the two-dimensional glomerular layer in the OB (5) amounts to an invariant two-dimensional array of OR response properties. In other words, the coordinates of an odorant in “odor space”—the activation levels of all different OR it activates—could be visualized by optical imaging in the OB, if odor-induced presynaptic activity of ORN terminals in the glomeruli could be measured separately from postsynaptic signal components.
However, even those imaging methods that achieve glomerular resolution have not distinguished the activity of input neurons (ORN), output neurons (mitral cells), and interneurons (6–9). Hence, it is difficult to infer OR response properties from those results already available (6–12). Nonselective imaging studies have observed a chemotopic and interindividually rather invariant representation of classes of odorants (e.g., aldehydes or fatty acids) by groups of neighboring glomeruli (6–9). Within a class of odorants, the location of such response modules may shift with differences in chain length, and individual glomeruli may react differentially to different chain lengths. Patterns of activated glomeruli overlap considerably for odorants sharing a functional group, and some overlap is seen between classes of odorants as well. It is unclear to what extent these features reflect neuronal processing of odors in the OB, and to what extent they embody OR response properties. Odor responses of ORN have been reported as rather unspecific (13–15), whereas recombinant OR mostly have been reported as narrowly tuned (16–18). Particular unclear is the representation of odor concentration within the olfactory code. It is known from psychophysical tests that a few odorants and odorant mixtures change their perceived odor quality dramatically with increasing concentration, but most odorants do not (19–21). Optical imaging in the OB has shown recruitment of additional glomeruli with increasing stimulus concentration, but generally no change in normalized activation patterns (refs. 7, 8, and 22; but see refs. 9 and 11).
To realize the full potential of imaging methods for elucidating the properties of many OR simultaneously, we have introduced the activity indicator Calcium Green-dextran selectively into ORN terminals in the mouse OB to measure the presynaptic odor-induced activity in identified glomeruli. We show here that, at this level, the olfactory code is multidimensional and that, in addition to molecular features like the length of the carbon chain, the odorant concentration is encoded by pronounced differences in the receptor repertoire recruited to the response.
Materials and Methods
ORN Staining and Animal Preparation.
Twenty-one mice between 3 and 5 weeks, wild-type (129 or C57BL6/J) as well as two OR-IRES-tauGFP mouse strains (ref. 23; P. Mombaerts, unpublished results) were used for optical imaging. Results were undistinguishable between strains. For the determination of glomerular size and total number, OMP-GFP mice were used (23). For dye labeling of ORN, the animals were anesthetized with an i.p. injection of xylazine (0.45 mg/kg) and ketamine (96 mg/kg). A GELoader Tip (Eppendorf) was used to apply 8–9 μl of 6% Calcium Green-1-dextran (3 kDa; Molecular Probes) and 0.1% Triton X-100, dissolved in PBS. This Triton X-100 concentration has been shown to lead to a reversible loss of cilia, but not to neuronal death (24). SEM analysis of the nasal epithelium showed no signs of cell death, and functional recovery as measured by electroolfactogram (EOG) was complete 2–4 days after dye application (data not shown).
Five to seven days after dye injection, when the dye had distributed well into the terminals of the ORN, animals were anesthetized and immobilized with a headholder (25), and the skull overlaying the dorsal surface of the OB was first thinned with a dental drill and finally removed with a forceps. The OB was covered with artificial cerebrospinal fluid (ACSF) prebubbled with carbogen (95% O2, 5% CO2; ref. 26) and a cover glass. The preparation was viewed with an Axiovert S 100TV microscope (Zeiss), using a monochromator “Polychrome II” (TILL Photonics, Martinsried, Germany) and an FT 500, LP515 filter. To observe activity in the entire OB, a ×2.5 objective (NA 0.075; Zeiss) was used, which minimized out-of-focus effects. An experiment was continued until the responses to some repeatedly tested odorants declined noticeably in intensity, usually after 2 to 4 h.
Olfactometer and Odorants.
A simple olfactometer was built, by using manually operated glass valves, silicon tubings, and four glass syringes (10 ml) with shortened G18 injection needles as odor nozzle, placed ≈1 cm away from the nostril. The carrier air stream was charcoal filtered and water moisturized. Eighty microliters of odorant, odorant diluted in mineral oil, or mineral oil only (control) on Whatman paper were used to generate approximately vapor saturation in the syringes. The syringes and directly connected silicon tubings were cleaned with ethanol and water and were incubated for at least 12 h at 60°C before every experiment to remove trace odor contaminations.
Odorants (purity 95–99%) were purchased from Sigma-Aldrich. The essential oils rosemary and citronelli were a kind donation of the Muehlens GmbH (Cologne, Germany). Concentration was expressed as dilution factor. The final odor concentration in an odor stimulus is estimated to be 10 to 100 times below the vapor concentration in the syringe. Saturated vapor pressures of aldehydes were estimated from their boiling points by using the equation of Hass and Newton (27) to give values at 20°C and 1 atm (4.46 × 104 ppm, pentanal; 1.45 × 104 ppm, hexanal; 4.47 × 103 ppm, heptanal; 1.84 ×103 ppm, octanal; and 6.58 × 102 ppm, nonanal). The relative vapor pressure above odorant/mineral oil mixes is proportional to the dilution factor [0.12 for pentanal; 0.24, hexanal; 0.47, heptanal; 0.93, octanal; and 1.8, nonanal (9)].
Optical Imaging and Data Analysis.
Images (240 × 320 pixels after 2 × 2 binning) were acquired with a cooled charge-coupled device (CCD) camera (Imago S/N 381KL 0041, TILL Photonics) at 470 nm excitation with a frame rate of 2 Hz for 20 s per stimulus, with an interstimulus period of at least 2 min. Odor was applied for 5 frames. Odor responses were determined as ΔF/F. Five consecutive prestimulus frames were averaged to determine F, and five frames at the peak of the response were averaged and lowpass filtered (Gaussian filter, SD = 14.8 μm) to obtain ΔF. Three independent trials with the same odor were averaged, normalized to the maximal response amplitude, and false color coded. The color scale was customized to achieve approximately equal representation of each major color. In one case, a sigmoidal color scale was used to obtain a larger dynamic range. The color value for ΔF/F = 0 is identical in all representations. All calculations were done with the TILL vision software.
Statistical analyses were performed by using Microsoft Excel 2000. For determining detection thresholds, signals were considered significant when three times above noise level, defined as SD in control stimulations. To count the number of glomeruli specifically responding at high concentrations, thresholds were set just above the weak homogenous activation level (around 1 to 2% ΔF/F), which was observed exclusively at high concentrations. To determine the size of glomeruli and calcium signals, horizontal line scans of randomly chosen well isolated foci were performed, and diameters were determined as distances between adjacent minima. Estimates for KD values were obtained by standard sigmoidal fit [ΔF/F = ΔF/Fmax × dilution/(dilution + KD)] by using kaleidagraph 3.0 (Abelbeck Software, Reading, PA).
Results
Selective Imaging of Presynaptic Activity in the Olfactory Bulb.
We have labeled ORN terminals in the mouse OB by anterograde transport from the nose of the calcium indicator Calcium Green-1, coupled to dextran. The dextran moiety prevents compartmentalization and transsynaptic transfer of the dye (24, 28), so that calcium influx is measured selectively in ORN terminals. Staining intensity of individual glomeruli can differ (Fig. 1), potentially reflecting different innervation densities of glomeruli (cf. ref. 3). On the whole, the OB is stained rather homogeneously, with the posterior regions generally showing somewhat weaker fluorescence (Fig 2). Larger axonal distances to the posterior OB may result in somewhat smaller amounts of dye present in posterior terminals. However, differences in staining intensity should not strongly influence ΔF/F, and, indeed, we found that total fluorescence intensity appeared not to be correlated with signal strength (see e.g., Fig. 1A). We can observe about one tenth of the entire bulbar surface—the dorsal region, which corresponds to about 150 glomeruli and thus to approximately one sixth of the total OR repertoire of the mouse. The mouse OB has 1,800 glomeruli (29), which, because of the map duplication in rodents (3, 5), should correspond to about 900 receptors. Our observation window should contain mostly unduplicated map regions, because the plane of symmetry extends from slightly medial at the posterior OB toward ventromedial at the anterior OB (5).
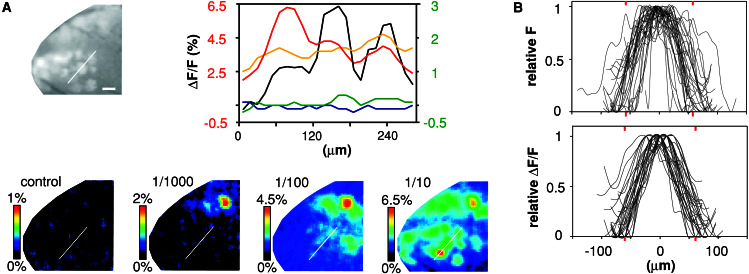
Figure 1.
Calcium signals originate in glomeruli. (A Upper Left) Glomeruli in the anterior dorsal OB are labeled with Calcium Green-1 dextran after uptake in receptor neuron somata and axonal transport from the nasal epithelium. View is from dorsal, anterior is left, medial is bottom. Several glomeruli are clearly visible; scale bar = 100 μm. The white line depicts the position of the line scan shown in the Upper Right panel (black line), which crosses three glomeruli. The four Lower panels show false color representation of calcium changes in the same sector, expressed as ΔF/F. From left to right, responses to mineral oil stimulus (control), and increasing concentrations of hexanal (dilutions 0.001, 0.01, and 0.1). Position of the line scan (white line) is the same as above. Line scans (Upper Right) are color coded as follows: blue, control; green, 0.001 dilution; yellow, 0.01 dilution; red, 0.1 dilution; and given as ΔF/F, red scale for the red line scan, green scale for all others. (B) Line scans of randomly chosen glomeruli are superimposed to show variability and mean diameter of glomeruli (red bars). For better visibility, line scans were smoothed, normalized to one, and aligned at their maximum. (Upper) Line scans of 33 glomeruli from three different animals, morphologically identified by enhanced green fluorescent protein (EGFP) expression in the OMP locus. (Lower) Line scans of calcium responses (ΔF/F) from 31 glomeruli from four different animals, labeled by Calcium Green-dextran.
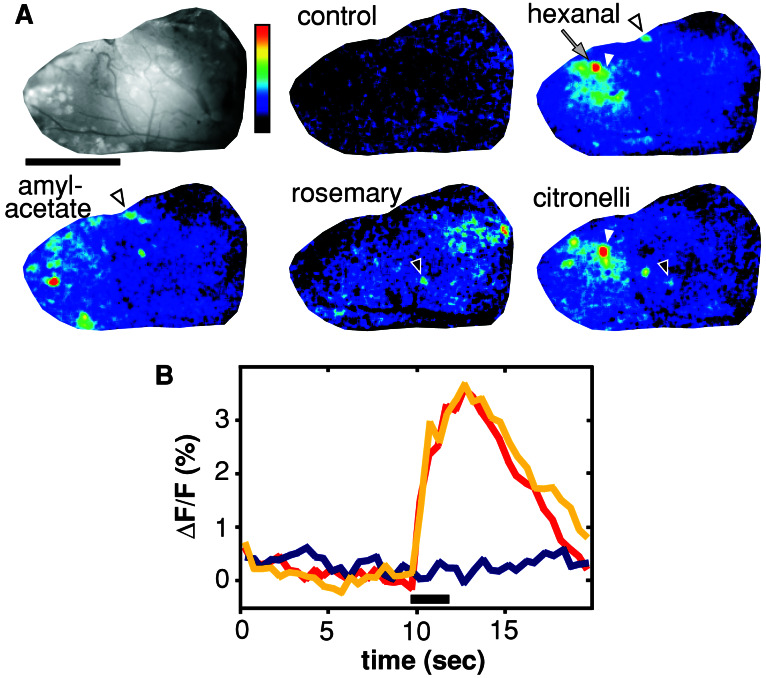
Figure 2.
Odor-induced calcium signals are specific and reproducible. (A Upper Left) Glomeruli in the dorsal OB are labeled with Calcium Green-1 dextran. Vessels appear dark, view is from dorsal, anterior is left, medial is bottom. Normalized false color representation of calcium changes, expressed as ΔF/F, by using the color scale shown: black, no change; red, for the odorants maximal change within that image (1.7–5.0%) and for control 1.5%. Dilution for hexanal is 10−2. Open arrowhead, a glomerulus responding to the aldehyde hexanal also is activated by the unrelated compound isoamylacetate. Filled arrowhead, response areas of the essential oils rosemary and citronelli are very different, but one glomerulus is shared. The arrow points to the glomerulus investigated in B. Scale bar = 1 mm. (B) Kinetics of the response; the bar indicates the stimulus pulse. Red and yellow lines, single trial hexanal responses, well separated in time by several other stimuli; blue line, no odorant added.
Odor stimuli typically elicit 6–7% change in fluorescence and maximally reach 13% change. Signals consist of many foci, which mostly exhibit a round shape and have a bell-shaped intensity distribution (Fig. 1B). The foci colocalize exactly with glomeruli visible by Calcium Green fluorescence (Fig. 1A). In addition, the mean focus diameter of calcium signals and morphologically identified glomeruli was found to be nearly identical (122 ± 4 vs. 116 ± 7, mean ± SEM; see also Fig. 1B). These diameters are within the upper range of glomerular diameters determined previously in sections (29). A seemingly smaller diameter of weakly activated glomeruli is presumably caused by the human perception of false color representations because the line scans show no difference in diameter (Fig. 1A). Individual glomeruli can be clearly resolved, even weakly activated glomeruli directly adjacent to strongly responding ones (Fig. 1A). Odor responses are highly reproducible for several hours, follow the stimulus closely, and despite partial overlap are characteristically different for different odors (Fig. 2). Most, but not all, morphologically discernible glomeruli were activated by at least 1 of the 70 different odorants (aldehydes, fatty acids, and some esters and ketones) and essential oils tested (data not shown), supporting the integrity of the dye-labeled neurons. Many odorants, including alcohols, some esters and ketones, and several complex mixtures (essential oils) do not elicit responses in the dorsal region (data not shown), confirming the specificity of the signals measured. We have chosen aliphatic aldehydes to analyze the ensemble codes for a group of systematically related odorants because they elicit robust calcium responses in the dorsal OB (Fig. 2) and thus are accessible for imaging odorant-induced presynaptic neuronal activity in vivo. The detection threshold as judged by calcium imaging is around 10−3 dilution for aldehydes (n = 6).
Aldehyde-Responsive Glomeruli Are Tuned Individually to Chain Length.
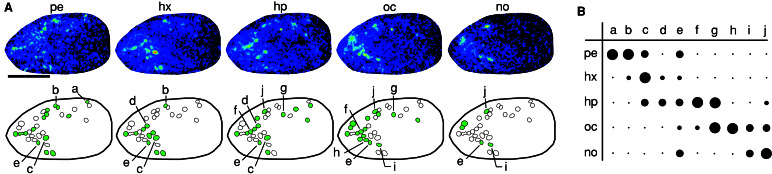
Natural odor stimuli are expected to generally occur at concentrations well below saturation of the detection system. We report that, at such low concentrations, aldehydes of different chain length elicit very distinctive and divergent patterns of glomerular activation within the aldehyde-responsive area (Fig. 3A). About 10 to 20 glomeruli respond to any particular aldehyde (n = 6 animals). Many glomeruli are tuned rather narrowly to a particular chain length, but glomeruli with intermediate and broad specificity are also present (Fig. 3B). Glomeruli sharing a response pattern were observed repeatedly; however, even these glomeruli might react differentially, if challenged with other variations on aldehyde structure. Thus, individual aldehydes are represented by a fingerprint of several distinct, differentially tuned aldehyde-responsive glomeruli.
Figure 3.
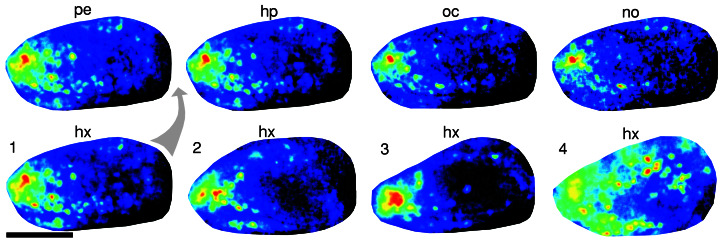
Ensemble coding of aliphatic aldehydes. (A) Responses to 10−2 dilutions of pentanal (pe), hexanal (hx), heptanal (hp), octanal (oc), and nonanal (no) are shown in normalized color scales (for pentanal, 150% of maximal change). (Lower) Schematic depiction of the active glomeruli; green-filled circles indicate clearly visible activity for that particular aldehyde. Scale bar = 1 mm. (B) Strength of response for glomeruli lettered in A. Small dots correspond to <50% of maximal activity, and small, medium, and large filled circles, linear scale between 50 and 100%.
Concentration Is Represented by Quantitative As Well As Qualitative Changes in the Glomerular Activation Pattern.
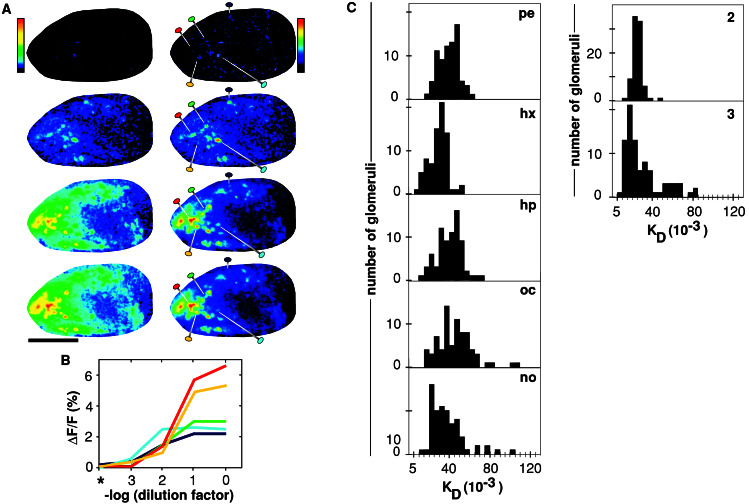
With increasing concentration of an odorant, new glomeruli are recruited into the response pattern and already active glomeruli respond more intensively. These effects were observed for all aldehydes tested (n = 6 animals) and are shown here for hexanal (Fig. 4A). By using an absolute scale, the response is dominated by massive increases in overall intensity with concentration. The normalized representations show that the relative weight of glomeruli within the response pattern varies with concentration. Although a decrease in absolute signal intensity is never observed with increasing concentration of odorant, in all animals tested, some glomeruli prominent at lower concentration become minor players at high concentration (Fig. 4 and data not shown). Thus, quantitative as well as qualitative changes lead to concentration-dependent odor response patterns.
Figure 4.
Concentration dependence and affinity estimates for aldehyde-responsive glomeruli. (A) Increasing concentrations of hexanal are tested, from Top to Bottom, 10−3, 10−2, 10−1, and undiluted, respectively. Left column, absolute responses shown with a sigmoidal color scale (black, no change; red, 6.5% ΔF/F). Right column, normalized responses shown with the standard color scale (black, no change; Top, red, 1.5-fold of maximal ΔF/F, 2.25%; all other panels, red, maximal ΔF/F, from Top to Bottom 1.5%, 2.5%, 5.7%, and 6.6%). The animal is the same as shown in Fig. 2; scale bar = 1 mm. (B) Dose-response curves for representative glomeruli, indicated in A by colored pins. Curve color matches pin color. Asterisk, no odorant added. Concentrations for half-maximal responses differ by one order of magnitude. (C) Distribution of KD estimates for the complete population of aldehyde-responsive glomeruli. Distributions are shown as histogram; KD estimates are obtained from sigmoidal fits of dose-response curves such as shown in B and expressed in units of dilution factor. Left column, from Top to Bottom: KD distributions for pentanal to nonanal in one animal (same animal as in A); Right column, KD distributions for pentanal in two other animals.
Dose-response curves have been generated for all aldehyde-responsive glomeruli and for all aliphatic aldehydes tested (Fig. 4B and data not shown). Often, but not always, these curves show saturation, as expected for a specific binding interaction. The rise phase generally comprises two log units of concentration, in good accordance with the expectation for a simple binding interaction (R + L ⇄ RL; ref. 30). Such an interaction can be approximated by a sigmoidal fit, which yields an estimate for the KD value. Regression analysis of sigmoidal curve fits gave correlation coefficients in the range of 0.95 to 0.999, many above 0.99, indicating the goodness of the fit. We found that affinities of different receptors for the same aldehyde vary about one order of magnitude (Fig. 4C). Note that this conclusion is valid independent of the units in which KD is expressed. The estimate for the affinity range is a lower limit because the nonsaturating binding curves found might result from lower affinity receptors. Higher affinities would have been visible as maximal responses throughout the concentration range studied. However, no such glomeruli were seen.
The selectivity of a glomerulus may be estimated by the range of its KD values toward aldehydes of different chain length. The KD range is expressed as the ratio between maximal KD and minimal KD. Some glomeruli are broadly tuned, with a minimal selectivity of 1.6-fold. The mean selectivity was 6-fold; the maximal value observed was 22-fold. Consistent with results obtained at one concentration (Fig. 3), all kinds of preferences were observed, pentanal-best to nonanal-best, with no clear prevalence of any one category.
Discriminatory Abilities of Glomeruli Decrease with Increasing Concentration.
Increases in numbers of active glomeruli were not noticeable between the two highest concentrations used (Fig. 4A), and only slight increases in intensity were observed, presumably because saturation has already been reached for many of the underlying receptors. As a consequence, glomeruli discriminate for chain length less well at these high concentrations, and each aldehyde activates nearly the same subset of glomeruli, i.e., nearly all glomeruli of the aldehyde-responsive domain (Fig. 5). Even at high concentrations, about half of the glomeruli in the dorsal OB do not respond specifically to aldehydes. A weak and spatially nearly homogenous activation level was observed exclusively at high concentrations. It is clearly distinguishable from the main signal, has the hallmarks of an unspecific interaction, and might be caused by the chemical reactivity of aldehydes and/or non-receptor-mediated activation of ORN (31). Thus, the olfactory representation or code at the level of the OR is sparse at low concentrations but dense at high concentrations.
Figure 5.
Decreased specificity and interanimal variability at high aldehyde concentrations. Upper row, from Left to Right, responses to aldehydes, undiluted, with increasing chain length. Normalized color scale; responses are rather similar. Absolute values decrease about 2-fold with increasing chain length, possibly related to partial obscurance of the more anterior response region for longer aldehydes (cf. ref. 8). Lower row, responses to hexanal, undiluted, four different animals, normalized color scale. 1, Same animal as above; 2, another common pattern (same animal as shown in Fig. 2); 3, response area more clustered than usual; 4, very disperse response pattern, a rare case.
Interindividual Variability of the Aldehyde Domain.
The aldehyde domain appears rather stereotyped between animals, as a V-shaped response area with an anterior-dorsal center and several glomeruli neighboring the center. However, the degree of dispersal is variable; for typical and rare cases, see Fig. 5. Accordingly, a stringent interanimal identification of individual glomeruli by response pattern has only rarely been possible (data not shown). This finding is consistent with a significant amount of variability in the position of genetically marked glomeruli (4). On the other hand, the total number of aldehyde-responsive glomeruli was rather constant (79 ± 3, mean ± SE, n = 5), which is to be expected for a genetically fixed receptor repertoire.
Discussion
In an attempt to categorize odorants, the concept of a multidimensional odor space was developed a long time ago. Traditionally, dimensions of this odor space have been defined according to psychophysical criteria or the physicochemical nature of odorants (32–34) or by measuring odorant responses of many individual ORN (13–15, 35–38)—no deduction of responses to individual OR has been possible here, however. More recently, glomerular response patterns obtained by intrinsic imaging have been taken to represent odor space coordinates (8, 9). However, it appears most appealing to link the concept of odor space as directly as possible to the ligand binding properties of the OR, because it is the binding interaction that defines a chemical as an odorant. Here, each OR is thought of as a dimension of odor space, and its degree of activation by any particular odorant amounts to the coordinates of that odorant for that dimension. All coordinates taken together constitute the—multidimensional, but finite—response vector of that odorant, its unique representation. The optical imaging of the receptor repertoire at the resolution of individual glomeruli enables us to experimentally visualize the receptor-based odor space.
We have measured odorant-elicited calcium increases in presynaptic nerve terminals of ORN. How much does that tell us about OR properties? No exception to the one glomerulus—one receptor hypothesis has been found in the mammalian main olfactory system so far. A glomerulus integrates the signals from many receptor neurons and, measuring at the glomerular level, thus averages over the individual responses of hundreds of ORN. Thus, the presynaptic signal from one glomerulus should be qualitatively identical to that of the individual ORN converging onto this glomerulus. Centrifugal innervation of the OB terminates mainly on interneurons and projection neurons and thus is not expected to notably distort the receptor neuron signal (39, 40). Within the ORN a signal transduction cascade comprising several steps lies between the receptor/odorant interaction and the presynaptic calcium influx measured here. It is not known whether any steps in this cascade saturate earlier than the receptor/ligand interaction and whether the cascade is identical in all different types of ORN (but see refs. 41 and 42). However, for each glomerulus, all but the highest responses observed with any odorant are obviously below saturation of the signal transduction apparatus. Especially when restricting oneself to comparisons of relative affinity, it should then be possible to draw conclusions about receptor properties from a study of presynaptic glomerular response patterns.
An estimate for the complete aldehyde receptor repertoire may be derived assuming that glomeruli responding to high concentrations of aliphatic saturated unbranched aldehydes in the dorsal OB should encompass receptors optimally tuned for many different kinds of aldehydes. Thus the total number of aldehyde-responsive glomeruli, and therefore, receptors, should equal at least 3 times 80, because at least two additional aldehyde-responsive regions exist (6). It follows that a very considerable proportion of the total OR repertoire is involved in recognition of aldehydes. Aldehyde receptors should belong to several different subfamilies and even families of OR genes, because already the few sequences that are known in mouse belong to different families (cf. refs. 16, 18, and 43) and because the maximal family size appears to be much smaller than 240 (44). Consistent with results obtained by other imaging techniques (8, 9), but in contrast to properties of a family of amino acid-responsive fish OR (45), we find chemically unrelated substances activating some of the aldehyde-responsive glomeruli.
Affinities of different receptors for the same aldehyde vary at least one order of magnitude. Thus, despite the high number of different aldehyde receptors, the aldehyde/receptor binding interaction appears to exhibit a limited variation width. When comparing apparent affinities of the same glomerulus for different aldehydes, a similar width of variation was seen, but larger affinity differences might have been detected with aldehydes structurally more diverse that the aliphatic, unbranched panel examined here. From pentanal-best to nonanal-best receptors, all preferences were observed. However, saturated vapor pressure, as well as strength of interaction with solvent compared with interaction with self, differs between aldehydes and may influence relative affinities. By using published values for both parameters (9, 27), we find a strong shift to the nonanal-best category. Receptors preferring shorter chain lengths still occur, but constitute a clear minority of the population. The maximal chain length-best response category is predominant whenever liquid odorant stimuli are used, for which accurate determination of concentration is possible (17, 45). It is conceivable that the predominance of bell-shaped dependence on chain length reported for gas phase stimuli (8, 9, 18) may result because such correction factors have not been considered. Selectivities of individual receptors (expressed as maximal KD/minimal KD) also change. However, the distribution of selectivities in the receptor population is not influenced much (mean value derived from corrected affinities is 9-fold instead of 6-fold, by using uncorrected affinities).
We observe concentration-dependent quantitative and qualitative differences in the pattern of activated glomeruli, which may be useful for discrimination of concentration (19). Because shifts in relative weight often occur between neighboring glomeruli, lateral inhibition of adjacent glomeruli would accentuate these pattern differences and thus facilitate discrimination of concentration. This finding may indicate another role for lateral inhibition, besides enhancing odorant discrimination (46) or synchronizing coactive mitral cells (47). However, concentration-dependent patterns do pose a problem for the constancy of odor quality perception, which is observed for many odorants (19, 21). The pungent note characteristic of many odors at high concentration is believed to result from activation of the less sensitive trigeminal nerve (48). Concentration-invariant recognition may occur at a more central level, after the receptor neuron input to the OB, and presumably requires major processing of the input signal.
Coarse location of intrinsic signals is consistent with the results obtained in this study. Intrinsic signals generally are of a somewhat more diffuse nature and seem to consist of fewer activated glomeruli than those obtained by calcium imaging, possibly caused by lower signal/noise ratios because of the small size of intrinsic signals (cf. ref. 9). Odorant tuning properties of glomeruli as deduced from intrinsic signals appear similar to tuning properties measured in this study. Unfortunately, one cannot derive an estimate of the postsynaptic signal in the glomeruli from a comparison of presynaptic signals with intrinsic signals, because the proportion of pre- vs. postsynaptic signal is not known for intrinsic signals. To shed light on the calculations performed in the OB, it will be necessary to develop imaging methods selectively addressing the mitral cell population for direct comparison of input and output signal of glomeruli. Furthermore, labeling of individual glomeruli by gene targeting has already been achieved (e.g., ref. 3) and, in combination with the calcium imaging technique described here, should now allow to analyze the ligand requirements of identified OR in vivo, a further step toward elucidation of the olfactory code.
Acknowledgments
We thank Walter Nadler for critical review of the manuscript and Peter Mombaerts for the kind gift of transgenic mouse strains. This work was supported by the Human Frontiers Science Program.
Abbreviations
- OR
olfactory receptor(s)
- ORN
olfactory receptor neuron(s)
- OB
olfactory bulb
References
- 1.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Ressler K J, Sullivan S L, Buck L B. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 4.Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. J Neurosci. 2000;20:6927–6938. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagao H, Yoshihara Y, Mitsui S, Fujisawa H, Mori K. Neuroreport. 2000;11:3023–3027. doi: 10.1097/00001756-200009110-00039. [DOI] [PubMed] [Google Scholar]
- 6.Johnson B A, Woo C C, Hingco E E, Pham K L, Leon M. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- 7.Rubin B D, Katz L C. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 8.Uchida N, Takahashi Y K, Tanifuji M, Mori K. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- 9.Meister M, Bonhoeffer T. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthrie K M, Gall C M. Chem Senses. 1995;20:271–282. doi: 10.1093/chemse/20.2.271. [DOI] [PubMed] [Google Scholar]
- 11.Johnson B A, Leon M. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Rubin B D, Katz L C. Nat Neurosci. 2001;4:355–356. doi: 10.1038/85997. [DOI] [PubMed] [Google Scholar]
- 13.Sicard G, Holley A. Brain Res. 1984;292:283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- 14.Duchamp-Viret P, Chaput M A, Duchamp A. Science. 1999;284:2171–2174. doi: 10.1126/science.284.5423.2171. [DOI] [PubMed] [Google Scholar]
- 15.Ma M H, Shepherd G M. Proc Natl Acad Sci USA. 2000;97:12869–12874. doi: 10.1073/pnas.220301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touhara K, Sengoku S, Inaki K, Tsuboi A, Hirono J, Sato T, Sakano H, Haga T. Proc Natl Acad Sci USA. 1999;96:4040–4045. doi: 10.1073/pnas.96.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malnic B, Hirono J, Sato T, Buck L B. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 18.Araneda R C, Kini A D, Firestein S. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 19.Gross-Isseroff R, Lancet D. Chem Senses. 1988;13:191–204. [Google Scholar]
- 20.Ohloff G. Riechstoffe und Geruchssinn: Die Molekulare Welt der Düfte. Berlin: Springer; 1990. p. 61. [Google Scholar]
- 21.Pelz C, Gerber B, Menzel R. J Exp Biol. 1997;200:837–847. doi: 10.1242/jeb.200.4.837. [DOI] [PubMed] [Google Scholar]
- 22.Joerges J, Kuttner A, Galizia C G, Menzel R. Nature (London) 1997;387:285–288. [Google Scholar]
- 23.Potter S M, Zheng C, Koos D S, Feinstein P, Fraser S E, Mombaerts P. J Neurosci. 2001;21:9713–9723. doi: 10.1523/JNEUROSCI.21-24-09713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrich R W, Korsching S I. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 25.Fried H-U, Linnig H-D, Korsching S I. Physiol Behav. 2001;74:253–255. doi: 10.1016/s0031-9384(01)00481-4. [DOI] [PubMed] [Google Scholar]
- 26.Czéh G, Obih J C, Somjen G G. Brain Res. 1988;446:50–60. doi: 10.1016/0006-8993(88)91295-4. [DOI] [PubMed] [Google Scholar]
- 27.Hass H B, Newton R F. In: Handbook of Chemistry and Physics. 55th Ed. Weast R C, editor. Boca Raton, FL: CRC; 1974. pp. D155–D156. [Google Scholar]
- 28.Dudkin E A, Myers P Z, Ramirez-Latorre J A, Gruberg E R. Neurosci Lett. 1998;258:124–126. doi: 10.1016/s0304-3940(98)00870-2. [DOI] [PubMed] [Google Scholar]
- 29.Royet J P, Souchier C, Jourdan F, Ploye H. J Comp Neurol. 1988;270:559–568. doi: 10.1002/cne.902700409. [DOI] [PubMed] [Google Scholar]
- 30.Sveinsson T, Hara T J. Comp Biochem Physiol. 2000;97:279–287. [Google Scholar]
- 31.Lerner M R, Reagan J, Gyorgyi T, Roby A. Proc Natl Acad Sci USA. 1988;85:261–264. doi: 10.1073/pnas.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffman S S. Science. 1974;185:112–117. doi: 10.1126/science.185.4146.112. [DOI] [PubMed] [Google Scholar]
- 33.Chastrette M. Chem Senses. 1981;6:157–163. [Google Scholar]
- 34.Ohloff G, Giersch W, Thommen W, Willhalm B. Helv Chim Acta. 1983;66:1343–1354. [Google Scholar]
- 35.Holley A, Duchamp A, Revial M-F, Juge A. Ann N Y Acad Sci. 1974;237:102–114. doi: 10.1111/j.1749-6632.1974.tb49847.x. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Hirono J, Tonoike M, Takebayashi M. J Neurophysiol. 1994;72:2980–2989. doi: 10.1152/jn.1994.72.6.2980. [DOI] [PubMed] [Google Scholar]
- 37.Bozza T C, Kauer J S. J Neurosci. 1998;18:4560–4569. doi: 10.1523/JNEUROSCI.18-12-04560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanhueza M, Schmachtenberg O, Bacigalupo J. Am J Physiol Cell Physiol. 2000;279:C31–C39. doi: 10.1152/ajpcell.2000.279.1.C31. [DOI] [PubMed] [Google Scholar]
- 39.Hildebrand J G, Shepherd G M. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- 40.Aroniadou-Anderjaska V, Zhou F M, Priest C A, Ennis M, Shipley M T. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- 41.Belluscio L, Gold G H, Nemes A, Axel R. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 42.Brunet L J, Gold G H, Ngai J. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 43.Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K. J Neurosci. 2001;21:6018–6025. doi: 10.1523/JNEUROSCI.21-16-06018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glusman G, Yanai I, Rubin I, Lancet D. Genome Res. 2001;11:685–702. doi: 10.1101/gr.171001. [DOI] [PubMed] [Google Scholar]
- 45.Fuss S H, Korsching S I. J Neurosci. 2001;21:8396–8407. doi: 10.1523/JNEUROSCI.21-21-08396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoi M, Mori K, Nakanishi S. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurent G. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 48.Hummel T. Int J Psychophysiol. 2000;36:147–155. doi: 10.1016/s0167-8760(99)00108-7. [DOI] [PubMed] [Google Scholar]