Abstract
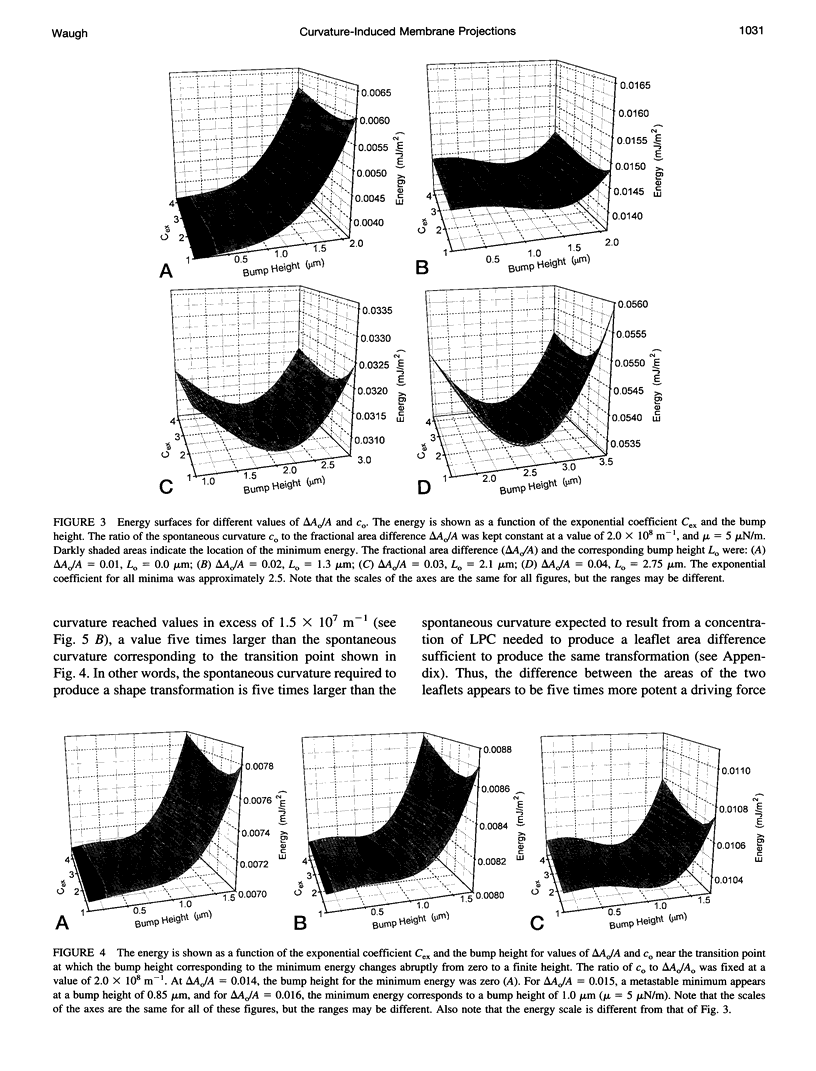
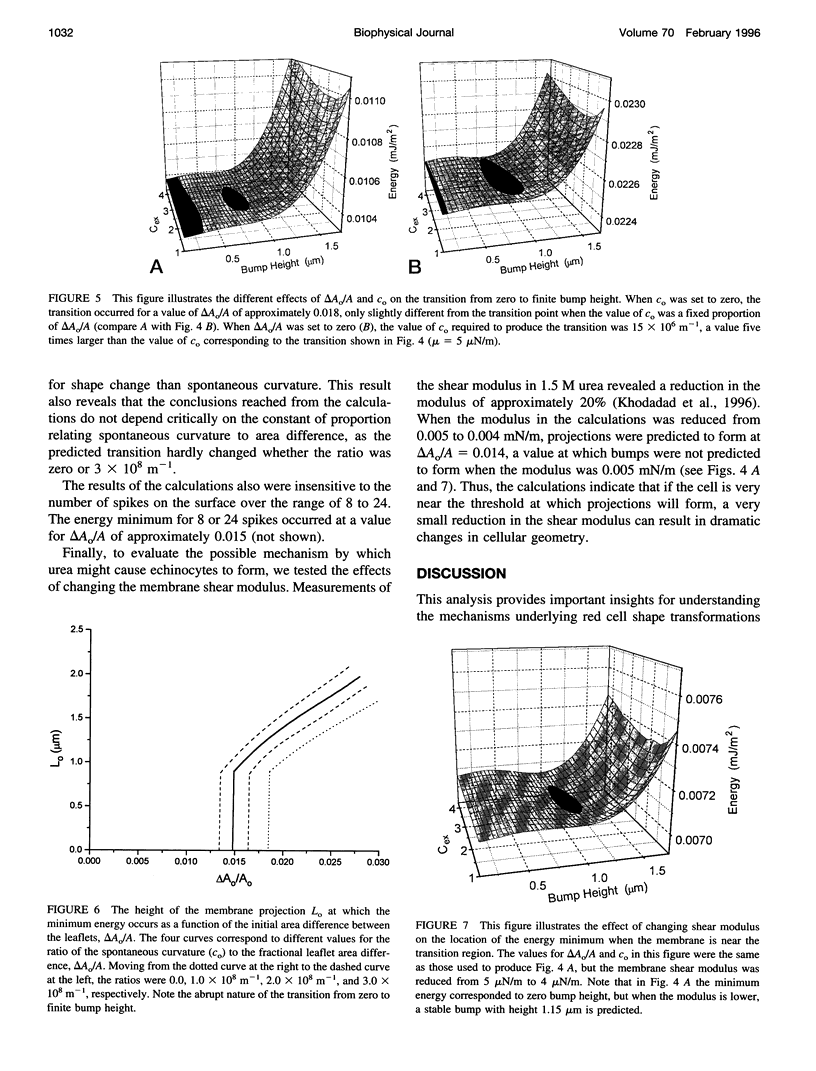
Model calculations were performed to explore quantitative aspects of the discocyte-echinocyte shape transformation in red blood cells. The shape transformation was assumed to be driven by changes in the preferred curvature of the membrane bilayer and opposed by the elastic shear rigidity of the membrane skeleton. The energy required for echinocyte bump formation was calculated for a range of bump shapes for different preferred curvatures. Energy minima corresponding to nonzero bump heights were found when the stress-free area difference between the membrane leaflets or the spontaneous curvature of the membrane became sufficiently large, but the calculations predict that the membrane can tolerate significant differences in the resting areas of the inner and outer leaflets or significant spontaneous curvature without visible changes in shape. Thus, if the cell is near the threshold for bump formation, the calculations predict that small changes in membrane properties would produce large changes in cellular geometry. These results provide a rational framework for interpreting observations of shape transformations in red cells and for understanding the mechanism by which small changes in membrane elastic properties might lead to significant changes in geometry.
Full text
PDF
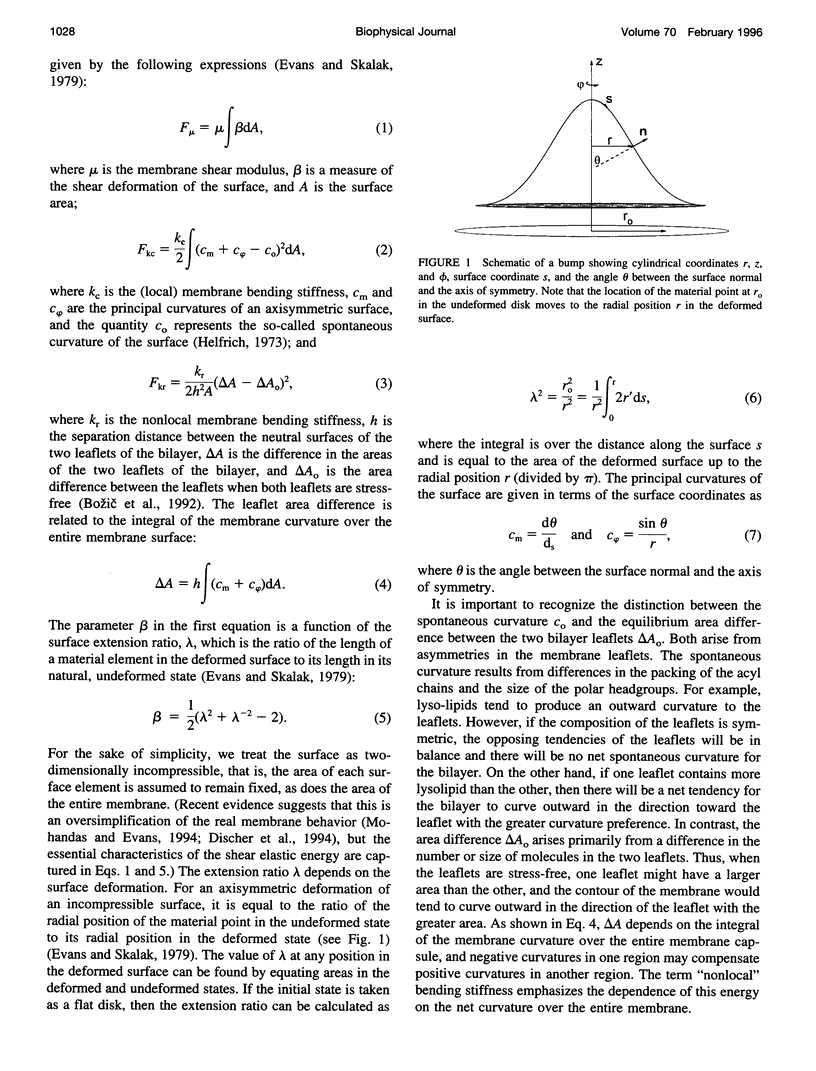
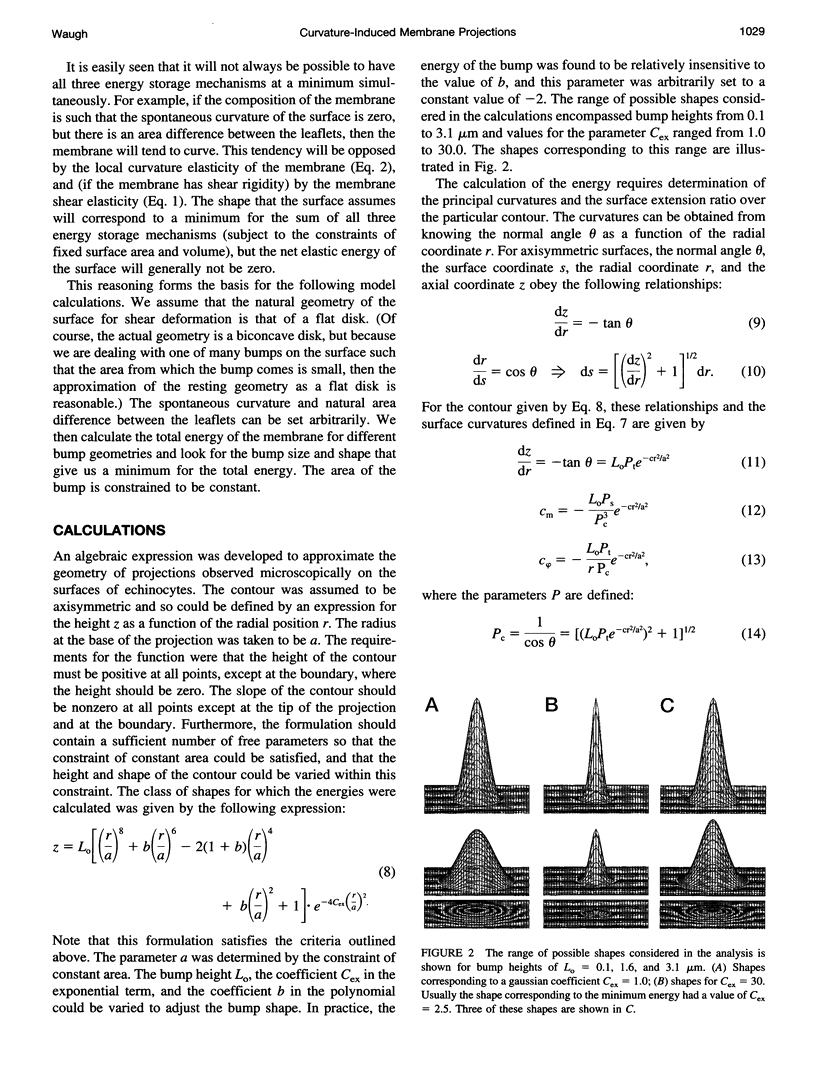




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozic B., Svetina S., Zeks B., Waugh R. E. Role of lamellar membrane structure in tether formation from bilayer vesicles. Biophys J. 1992 Apr;61(4):963–973. doi: 10.1016/S0006-3495(92)81903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E., Mohandas N., Evans E. A. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science. 1994 Nov 11;266(5187):1032–1035. doi: 10.1126/science.7973655. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983 Jul;43(1):27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Bending resistance and chemically induced moments in membrane bilayers. Biophys J. 1974 Dec;14(12):923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Skalak R. Mechanics and thermodynamics of biomembranes: part 2. CRC Crit Rev Bioeng. 1979 Nov;3(4):331–418. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry. 1985 Jun 4;24(12):2849–2857. doi: 10.1021/bi00333a006. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Lange Y., Slayton J. M. Interaction of cholesterol and lysophosphatidylcholine in determining red cell shape. J Lipid Res. 1982 Nov;23(8):1121–1127. [PubMed] [Google Scholar]
- Mohandas N., Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Greenquist A. C., Shohet S. B. Bilayer balance and regulation of red cell shape changes. J Supramol Struct. 1978;9(3):453–458. doi: 10.1002/jss.400090315. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Waugh R. E. Bending rigidity of SOPC membranes containing cholesterol. Biophys J. 1993 Jun;64(6):1967–1970. doi: 10.1016/S0006-3495(93)81566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. The human erythrocyte membrane skeleton may be an ionic gel. II. Numerical analyses of cell shapes and shape transformations. Eur Biophys J. 1986;13(4):219–233. doi: 10.1007/BF00260369. [DOI] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. The human erythrocyte membrane skeleton may be an ionic gel. III. Micropipette aspiration of unswollen erythrocytes. J Theor Biol. 1986 Nov 21;123(2):205–211. doi: 10.1016/s0022-5193(86)80154-0. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Bauserman R. G. Physical measurements of bilayer-skeletal separation forces. Ann Biomed Eng. 1995 May-Jun;23(3):308–321. doi: 10.1007/BF02584431. [DOI] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]