Abstract
The symptoms of schizophrenia imply disruption to brain systems supporting higher-order cognitive activity, but whether these systems are impacted differentially against a background of diffuse cortical gray-matter deficit remains ambiguous. Some unaffected first-degree relatives of schizophrenics also manifest cortical gray-matter deficits, but it is unclear whether these changes are isomorphic with those in patients, and the answer is critical to understanding the neurobiological conditions necessary for disease expression given a predisposing genotype. Here we report three-dimensional cortical surface maps (probabilistic atlases matching subjects' anatomy point by point throughout cortex) in monozygotic (MZ) and dizygotic (DZ) twins discordant for chronic schizophrenia along with demographically matched control twins. A map encoding the average differences between schizophrenia patients and their unaffected MZ co-twins revealed deficits primarily in dorsolateral prefrontal cortex, superior temporal gyrus, and superior parietal lobule. A map encoding variation associated with genetic proximity to a patient (MZ co-twins > DZ co-twins > control twins) isolated deficits primarily in polar and dorsolateral prefrontal cortex. In each case, the statistical significance was confirmed through analysis of 10,000 Monte Carlo permutations, and the remaining cortex was shown to be significantly less affected by contrast analysis. The disease-related deficits in gray matter were correlated with measures of symptom severity and cognitive dysfunction but not with duration of illness or antipsychotic drug treatment. Genetic and disease-specific influences thus affect gray matter in partially nonoverlapping areas of predominantly heteromodal association cortex, changes that may act synergistically in producing overt behavioral features of the disorder.
The pathophysiology of schizophrenia remains substantially a mystery despite intensive investigation (1, 2). Recent postmortem evidence indicating a deficit in dendritic arborization and synaptic contacts on cortical pyramidal neurons without loss of cell bodies (3) provides a potential unifying framework linking in vivo findings of cortical gray-matter deficits (4, 5), decreased density of cortical D1 receptors (6), and altered cortical physiologic activation during cognitive challenge tasks (7, 8). If neuropil deficits play a role in the pathophysiology of schizophrenia, given the nature and complexity of the syndrome's primary behavioral features the deficits should be selective or differentially severe in the most recently evolved brain systems that support integrated higher-order cognitive activity [i.e., the “heteromodal” association cortices (9, 10)]. Yet, it has proven surprisingly difficult to detect a differential impact on these systems against a background of diffuse cortical deficit (1, 2, 4, 5). Postmortem investigation of neuropil volume in schizophrenia has been limited thus far to two areas, dorsolateral prefrontal cortex [Brodmann's areas (BAs) 9 and 46] and primary visual cortex (BA 17); neuropil deficits were noted in both regions (11–13), but given the small sample sizes and small number of neurons examined, it is unclear whether the deficits are of significantly greater magnitude in prefrontal cortex. Further, abnormal physiologic activity has been observed in some heteromodal regions including dorsolateral prefrontal cortex and superior temporal gyrus by using behavioral paradigms designed to activate these areas such as working and episodic memory tasks (7, 8, 14, 15). However, it is not clear whether these alterations are derived from disturbed cellular interactions within these regions or their afferents.
In vivo radiological studies of cortical anatomy have the potential to resolve the topography of cortical gray-matter deficits in schizophrenia. Several such studies have found that frontal and temporal lobe regions may be particularly affected (4, 5, 16, 17). Whether this regional pattern reflects differential involvement of specific cytoarchitectonic areas remains to be determined but potentially could be resolved by voxel-based morphometric analysis (18, 19). A critical issue in this regard is how to match up the locations and shapes of the major sulci and gyri across subjects. Sulcal/gyral anatomy shows marked variation between individuals and is dissimilar even within pairs of healthy identical or monozygotic (MZ) co-twins (20, 21). Unless explicitly modeled, this variation in gyral patterning and shape could obscure regional differences in cortical gray-matter volume associated with illness (22).
A further complication is that some clinically unaffected first-degree relatives of schizophrenic patients also show evidence of cortical gray-matter deficits (17, 23). In prior studies of twins discordant for schizophrenia, both the patients and their unaffected MZ co-twins manifested lower than normal whole-brain, hemispheric, and/or frontal lobe gray-matter volumes, suggesting that genetic factors contribute to cortical gray-matter deficits in schizophrenia (23–25). However, because these studies either did not evaluate regional cortical variation or did not account for individual differences in gyral/sulcal patterning and shape, it is unclear to what extent the cortical gray-matter deficits in patients are isomorphic with those in their identical co-twins. The answer to this question is critical to understanding the neurobiological conditions necessary for overt symptom expression given a predisposing genotype, because any deficit shared equally by affected and unaffected gene carriers could participate in symptom expression only indirectly.
Here we used a recently created algorithm for cortical pattern matching (22) to develop a series of probabilistic 3-D atlases (18) encoding the topography of cortical gray-matter deficits in schizophrenic patients and their unaffected MZ and dizygotic (DZ) co-twins. The pattern matching procedure ensured that regional variation in gray-matter volume was assessed in the same anatomical reference locations across subjects after modeling and adjusting for individual differences in the cortical folding pattern as well as in overall brain size. Because MZ twins share 100% of their genes, comparing patients to their own unaffected MZ co-twins allowed us to isolate the topography of nongenetic, disease-specific differences in cortical gray-matter volume. Conversely, the topography of genetically encoded deficits should be revealed by elucidating regions in which volume varies inversely with increasing genetic proximity to an affected individual. Finally, areas of cortex uniquely or more severely affected in the patients compared with their unaffected MZ co-twins should be correlated differentially with behavioral indicators of disease severity.
Methods
Subjects and Clinical Evaluation.
Subjects were drawn from a cohort of same-sex twins born in Finland from 1940 to 1957 (26–28). Because the onset of schizophrenia after age 40 is exceedingly rare (29), new cases are unlikely to occur in this population. Ten discordant MZ pairs were recruited randomly and matched with 10 discordant DZ pairs and 10 sets of control pairs of each zygosity. As shown in Table 1, the four groups were balanced on age, gender, handedness, duration of cohabitation, parental social class, Cluster A personality disorder diagnoses, and history of substance abuse. Each co-twin was given a structured diagnostic interview by a different examiner who was blind to zygosity and diagnostic status of the other co-twin. Diagnostic reliability was 0.96 ± 0.02. The patients had a mean ± SD age at onset of 26 ± 6 years and had been treated with (primarily traditional) antipsychotic drugs for an average of 14 ± 10 years. Average symptom severity at the time of scanning was in the moderate range in terms of negative symptoms [average scores of 1.9 ± 0.9 on scale for the assessment of negative symptoms global items (30)] and in the moderate-to-severe range in terms of positive symptoms [average scores of 2.4 ± 0.8 on scale for the assessment of positive symptoms global items (31)].
Table 1.
Demographic characteristics of the discordant and control twins
| Discordant pairs | Control pairs | |||
|---|---|---|---|---|
| Characteristic* | MZ (n = 20) | DZ (n = 20) | MZ (n = 20) | DZ (n = 20) |
| Female, n(%) | 10 (50.0) | 10 (50.0) | 10 (50.0) | 10 (50.0) |
| Left-handed, n(%) | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Substance abuse, n(%) | 1 (5.0) | 1 (5.0) | 0 (0.0) | 1 (5.0) |
| Cluster A disorder, n(%) | 2 (10.0) | 0 (0.0) | 0 (0.0) | 2 (10.0) |
| Age, mean (SD) | 48.3 (2.9) | 49 (3.9) | 48.3 (3.8) | 47.9 (4.2) |
| Parental social class, mean (SD) | 4.6 (0.8) | 4.2 (1.7) | 4.0 (1.0) | 4.4 (0.8) |
| Years cohabitation, mean (SD) | 20.7 (3.4) | 19.9 (3.6) | 22.7 (5.7) | 19.8 (2.9) |
Discordant and control pairs do not differ significantly from each other on any of these characteristics.
A comprehensive neuropsychological test battery was administered to each co-twin by a different examiner who was blind to zygosity and diagnostic status of the other co-twin. Because measures of verbal (32) and visuospatial (33) episodic memory were unique in discriminating patients from their MZ co-twins (28), these measures were evaluated in relation to regional gray-matter density in the patients. We also evaluated a summary measure representing the linear combination of performance indices uniquely reflective of disease-related variation (composed primarily of measures of verbal and spatial episodic memory, spatial working memory, divided attention, and motor speed) as described (28). All groups were within 1 SD of normal in terms of average age-scaled standard scores on the vocabulary, similarities, block design, and digit symbol subtests of the revised Wechsler adult intelligence test (34). Patients (mean ± SD, 7.9 ± 2.5) performed significantly worse on this measure than the MZ co-twins (9.9 ± 2.8), DZ co-twins (10.2 ± 3.3), and control twins (11.3 ± 1.5); MZ co-twins performed significantly worse than control twins.
Discordant pairs in which the proband had a diagnosis of schizoaffective disorder (affective type) or in which the co-twin had a psychotic disorder diagnosis currently or on a lifetime basis were excluded. Control pairs were excluded if there was a history of psychosis-related treatment in their first-degree relatives or if either co-twin was judged to have a psychotic disorder. Studied probands were comparable to the remainder of the discordant twin proband population in terms of year of birth, sex, age at first hospital admission, number of hospital admissions, and eligibility for disability pension (28). Studied MZ probands were equivalent to studied DZ probands in terms of age at evaluation, age at onset, positive-symptom severity, and negative-symptom severity (28). There were no differences in demographics, illness history, or symptom severity between probands from discordant and concordant pairs (28). We have found previously that the heritability of schizophrenia is 0.83 in the twin population in Finland (26). This estimate is comparable to those from the United Kingdom (35). The genetic variation in Finland is expected to be the same as in other populations for common diseases such as schizophrenia (36).
Because there were no differences in parental social class or duration of cohabitation between discordant and control pairs or between discordant MZ and discordant DZ pairs (Table 1), differences in the similarity of shared postnatal environmental experiences would not seem to compete with a genetic explanation of any liability-related gray-matter deficits. Factors associated with the shared intrauterine environment could play a role, but to compete with a genetic explanation, obstetric complications would have to be more frequent in the histories of discordant MZ pairs than discordant DZ pairs, a pattern that was not observed in relation to prenatal or perinatal complications coded blindly from the original obstetric records from about half of the studied twin pairs (28). Among cases in which complications were noted, the events were not recorded as having affected one co-twin preferentially. Our subject-matching procedure eliminated schizophrenia-related personality disorders, handedness, and substance abuse as potential explanations for any liability-related differences in gray-matter volume (Table 1). Subjects positive for these factors had regional gray-matter volumes within 1 SD of the mean of their respective group distributions. Sample sizes were too small for meaningful statistical evaluation of group differences by gender, but it is doubtful that gender differences in these effects are large (37). Finally, because the studied probands from discordant MZ and DZ pairs did not differ from each other or from the remainder of the discordant twin proband population in demographic or clinical characteristics, any disease- or liability-related differences in gray-matter density are not likely to reflect an unusual clinical or demographic profile in the probands.
For all pairs, zygosity was determined by DNA analysis using the following markers: D1S80 (20 alleles), D17S30 (13 alleles), apoB (20 alleles), COL2A1 (10 alleles), vWA (9 alleles), and HUMTH01 (6 alleles). Assuming an average heterozygosity rate of 70% per marker, we estimate that this procedure will classify a DZ pair as MZ falsely in ≈1 of 482 cases.
Image Acquisition and Analysis.
T1-weighted MPRAGE MRI volumes were acquired on a 1.5-Tesla scanner (Siemens, Iselin, NJ) in the Department of Radiology at Helsinki University Central Hospital (128 contiguous 1.2-mm slices in the sagittal plane, repetition time = 11.4, echo time = 4.4, 256 × 256 matrix). A radio-frequency bias field-correction algorithm eliminated intensity drifts caused by scanner field inhomogeneity. Scans were histogram-matched and a supervised, nearest-neighbor tissue classifier generated detailed maps of gray matter, white matter, and cerebrospinal fluid. Briefly, 120 samples of each tissue class were tagged interactively to compute the parameters of a Gaussian mixture distribution that reflects statistical variability in the intensity of each tissue type (38).
Three-dimensional (3D) Cortical Maps.
A high-resolution surface model of cortex was extracted automatically from the segmented image for each subject (39). Medial prefrontal, cingulate, and medial temporal regions were excluded from the analysis, because models of these regions were not obtainable by using the surface extraction procedure. A single rater blind to zygosity, diagnosis, and all demographic information drew 38 gyral and sulcal boundaries, representing the primary gyral pattern, as 3D curves on each of the digitized models by using a detailed anatomical protocol (www.loni.ucla.edu/∼esowell/new_sulcvar.html). This protocol was compiled by neuroanatomists with reference to a sulcal atlas (40) as described (41, 42). Raters are trained on a set of six brains until they can trace landmarks with an inter- and intrarater 3D rms error no greater than 2 mm everywhere and <1 mm on average.
3D location information was retained through color coding as each cortical image was flattened and projected onto a sphere. Cortical models were used to compute a 3D vector deformation field, which then is used to reconfigure each subject's anatomy to the average configuration of the normal twins, matching landmark points, surfaces, and curved anatomic interfaces (2). A local measurement termed gray-matter density was made in each subject (22, 43), whereby the proportion of gray matter is measured in a small sphere of fixed radius (15 mm) around each cortical point. This proportion thus reflects the density of voxels segmenting as gray matter in the sphere (i.e., relative to the total, which also may include voxels segmenting as white matter, cerebrospinal fluid, or background). Maps representing the variability in gray-matter density across cortex then can be generated for any within-group or between-subject contrast.
Statistical Analysis.
Maps of gray-matter differences associated with schizophrenia were generated for each discordant MZ pair and were realigned elastically for averaging across the 10 pairs. Maps of genetically encoded differences were generated by assessing the correlation between gray-matter density and genetic liability, modeled as a continuum of genetic proximity to a proband, in which MZ co-twins of patients (n = 10) were highest, DZ co-twins of patients (n = 10) were intermediate, and control twins (n = 20, one twin selected at random from each pair) were the lowest. Statistics evaluating these associations were computed pointwise across the cortex, and their robustness after controlling for multiple comparisons was assessed by analysis of 10,000 Monte Carlo permutations for each map (22). In the case of within-pair differences, the permutations were formed by random pairings of unrelated subjects of the same gender and within 5 years of age but constrained to result in samples balanced on gender. In the case of liability-related differences, permutations were formed by randomly reassigning subjects to liability classes but constrained to result in samples balanced on age and gender distribution. We preferred this approach to using an analytical null distribution to avoid assuming that the smoothness tensor of the residuals of the statistical model were stationary across the cortical surface (18). Regional specificity was assessed by testing whether each voxel had the same or greater degree of association with the predictor (diagnosis or liability class) as the average voxel in frontal cortex. Frontal, temporal, parietal, and occipital regions were drawn on the atlas representing the aggregate of all subjects; these regions of interest then were back-propagated onto each subject's 3D cortical model (i.e., elastic realignment in reverse to recover the original cortical shape for the regions of interest in the individual subject) to permit analysis of variation in regional gray-matter density in relation to individual differences in clinical and neurocognitive variables.
Maps of within-pair resemblance in gray matter in discordant MZ and DZ co-twins were created by using the intraclass correlation coefficient and compared statistically by Student's t test. The corresponding maps for control pairs have been reported (42) but are reprinted here to facilitate comparison with the maps in discordant pairs. Heritability estimates (44) have wide confidence intervals at these sample sizes and thus are not presented here.
Results
Nongenetic Influences.
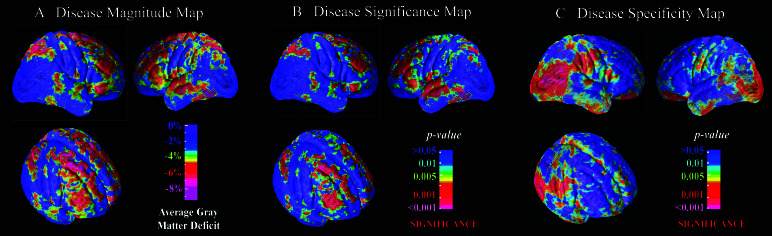
Fig. 1A gives the map plotting the average within-pair differences in gray-matter density between patients and their MZ co-twins. This map detected deficits in gray matter in the range of 5–8% in dorsolateral prefrontal cortex (approximate cytoarchitectonic reference: BA 9/46), Broca's area (BA 44/45), premotor cortex and frontal eye fields (BA 6/8), superior parietal lobule (BA 7/40), superior temporal gyrus including a small portion of Heschl's gyrus (BA 41/42), and middle temporal gyrus (BA 21). The remaining cortex was remarkably free of within-pair differences. Deficits in this range were statistically significant by paired Student's t test (Fig. 1B). Permutation analysis confirmed the significance of these differences in all the above regions bilaterally (P values < 0.05) except for superior temporal gyrus in the right hemisphere, indicating that the differences are statistically robust after accounting for the number of comparisons conducted.
Figure 1.
Cortical maps showing the average magnitude (A), significance (B), and regional specificity (C) of gray-matter deficits in schizophrenic twins relative to their healthy MZ co-twins (n = 10 pairs) viewed from the right, left, and right-oblique perspectives. These maps isolate nongenetic disease-specific deficits by eliminating genetic differences between cases and controls. Disease-specific deficits are observed primarily in dorsolateral prefrontal, superior temporal, and superior parietal association areas. The significance of deficits survived correction for multiple comparisons in each of these regions bilaterally except for superior temporal gyrus in the right hemisphere. Most of the remaining cortex is significantly less affected than the average deficit in frontal cortex (C).
As shown in Fig. 1C, the hypothesis that the disease-related deficit in gray matter is as large as that in the frontal lobe could be rejected statistically in the entirety of the occipital cortex, a broad band of sensorimotor cortex, and in large portions of inferior parietal, temporal, and frontal cortex. Overall, the regions that showed statistical evidence of relative sparing (Fig. 1C) were the inverse of those that showed statistical evidence of disease-related change (Fig. 1B).
As shown in Table 2, these disease-specific deficits in cortical gray matter were correlated with symptom severity, particularly with respect to negative symptoms (e.g., flat affect, anhedonia, and asociality), and with neurocognitive deficits, particularly in the domain of verbal learning and memory, but not with duration of illness or years of antipsychotic drug treatment. There also were no significant correlations between duration of illness or antipsychotic drug treatment and the differences in regional gray-matter volumes between patients and their MZ co-twins (all P values > 0.50).
Table 2.
Correlations* between regional cortical gray matter and clinical variables among twins with schizophrenia (n = 20)
| Region of cortex | ||||
|---|---|---|---|---|
| Variable | Frontal | Temporal | Parietal | Occipital |
| Duration of illness | −0.09 | −0.05 | 0.15 | 0.26 |
| Years ofneuroleptic treatment | −0.01 | 0.04 | 0.09 | 0.01 |
| Negative symptoms (30) | −0.44† | −0.43† | −0.38 | −0.23 |
| Positive symptoms (31) | −0.34 | −0.29 | −0.37 | 0.03 |
| Disease-related cognitive variate (28) | 0.50† | 0.52† | 0.60† | 0.10 |
| Verbal memory (32) | 0.59† | 0.42† | 0.42† | 0.30 |
| Visual memory (33) | 0.32 | 0.49† | 0.50† | 0.20 |
Partial correlations controlling for overall brain volume.
†, P < 0.05.
Genetic Influences.
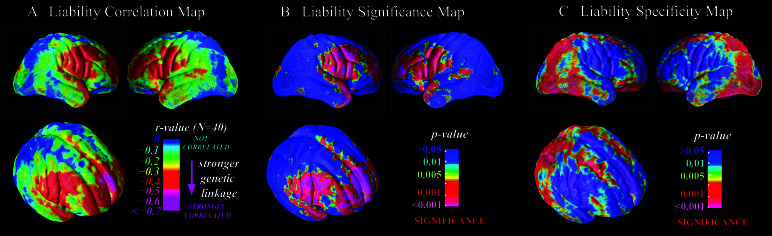
Fig. 2A gives the map modeling variation in gray matter as a function of a continuum of genetic affinity with a patient. This map detected inverse correlations on the order of −0.40 to −0.70 between decreasing gray-matter volume and increasing genetic risk for schizophrenia in dorsolateral (BA 9/46) and polar (BA 10) prefrontal cortex, Broca's area (BA 44/45), supplementary motor areas (BA 6), inferior sensorimotor strip (BA 4), Wernicke's area (BA 22), and temporal pole (BA 38). Correlations in this range were statistically significantly different from 0 (Fig. 2B). Permutation analysis confirmed the significance of these associations in each of the above regions bilaterally after accounting for the number of comparisons conducted (all P values < 0.05).
Figure 2.
Cortical maps showing the magnitude (A), significance (B), and regional specificity (C) of liability-related gray-matter deficits viewed from the right, left, and right-oblique perspectives. “R-value” refers to the correlation between gray-matter density and genetic liability to schizophrenia, modeled as highest among unaffected MZ co-twins of probands (n = 10), intermediate among unaffected DZ co-twins of probands (n = 10), and lowest among normal control twins (n = 20, where one co-twin was chosen at random from each pair). The map reveals liability-related deficits primarily in polar and dorsolateral prefrontal regions, the significance of which survived correction for multiple comparisons. Most of the remaining cortex is significantly less affected than the average deficit in frontal cortex (C).
As shown in Fig. 2C, the hypothesis that the liability-related deficit in gray matter is as large as that in the frontal lobe could be rejected statistically in nearly all of occipital and parietal cortex, a broad band of superior frontal gyrus (but excluding BA 10), and large portions of inferior temporal and frontal cortex. Overall, the regions that showed statistical evidence of relative sparing (Fig. 2C) were the inverse of those that showed statistical evidence of liability-related change (Fig. 2B).
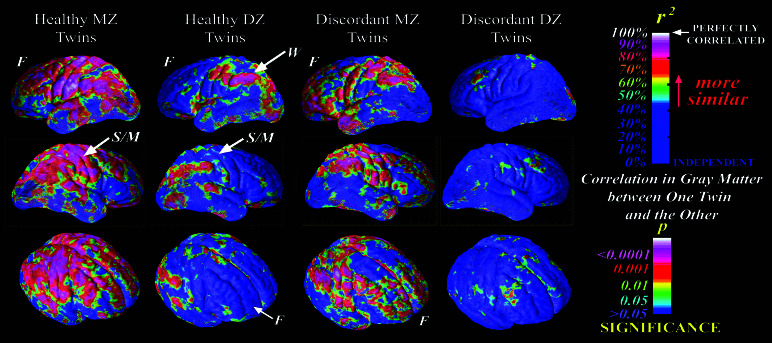
Fig. 3 shows the maps of the intraclass correlation coefficients in gray matter in healthy and discordant twins. Among the discordant pairs, the DZ correlations were uniformly low and nonsignificant throughout cortex, whereas the MZ correlations were highly significant (and significantly greater than the DZ correlations) in frontal and temporal pole, a large band of sensorimotor strip, and Wernicke's area. Correlations were lower in discordant than in healthy MZ pairs in dorsolateral prefrontal cortex and superior temporal gyrus, which parallels the mapping of disease-specific influences to these regions. Correlations were higher among discordant compared with normal MZ pairs in frontal pole, inferior frontal gyrus, and temporal pole, which are areas with the most prominent liability-related deficits. The intraclass correlation coefficients (± SE) for overall gray-matter volume were 0.83 ± 0.10 and 0.32 ± 0.29 for healthy MZ and DZ pairs, respectively [t (10) = 2.7, P = 0.01)], and 0.73 ± 15 and 0.05 ± 0.32 for discordant MZ and DZ pairs, respectively [t (10) = 2.9, P = 0.01].
Figure 3.
Cortical maps showing the intraclass correlations in gray matter in healthy MZ, healthy DZ, discordant MZ, and discordant DZ twin pairs (n = 10 pairs in each group). The maps for healthy twins are reproduced with permission from ref. 42 (Copyright 2001, Nature Publishing Group) to facilitate comparison. Among the discordant pairs, the DZ correlations were uniformly low and nonsignificant throughout cortex, whereas the MZ correlations were highly significant (and significantly greater than the DZ correlations) in frontal and temporal pole, a large band of sensorimotor strip, and Wernicke's area. These results are consistent with the liability maps presented in Fig. 2 in demonstrating differential heritable influences on gray matter in these regions among twins discordant for schizophrenia. F, frontal lobe; W, Wernicke's area; S/M, sensorimotor cortex.
Discussion
Consistent with its primary behavioral features, schizophrenia manifests with a specific topographic distribution of cortical gray-matter deficits. The majority of affected cortex is in heteromodal convergence areas, with relatively smaller regions of primary auditory, somatosensory, and motor cortex also affected. This regional specificity was revealed only after accounting for intersubject variation in gyral patterning and shape. Both genetic and disease-specific factors influence gray-matter deficits in these regions, with primarily nonoverlapping areas affected by these two sets of influences.
These findings help to specify the neurobiological conditions promoting phenotypic expression of schizophrenia given a predisposing genotype. Disease-specific effects were present in a relatively small fraction of the total surface area affected by genetic influences, corresponding primarily to middle frontal gyrus (dorsolateral prefrontal cortex) but contributed uniquely to gray-matter deficits in prefrontal regions involved in eye movements and motor planning and in temporal and parietal areas that support multimodal sensory and perceptual integration, auditory perception, and episodic memory. Genetic influences, which account for ≈80% of liability to schizophrenia (26), would be expected to influence some of the anatomical systems involved in disease causation, but such genetically encoded changes could participate in phenotypic expression only indirectly, because they are equally severe in the affected and unaffected members of discordant MZ twin pairs. Consistent with prior studies of relatives of schizophrenics (17, 23), here genetic influences were isolated primarily to polar, inferior, and dorsolateral prefrontal brain areas that support the highest degree of integrated cognitive activity such as abstract reasoning, sequencing, working memory, selection, categorization, and context processing, functions that rely on integrated perceptual input from heteromodal regions in the temporal and parietal lobes (45). There was greater resemblance of MZ compared with DZ discordant co-twins in the frontal region, a pattern that also appears in healthy twins (42, 46). The neocortical contribution to overt schizophrenic symptoms thus may reflect an interaction of disrupted perceptual, memory, and motor planning systems, induced primarily by nongenetic influences, with disturbed executive processes deriving primarily from an inherited genotype. This contribution may also depend on interactions with medial and midline cortical and subcortical structures, including the hippocampus, parahippocampal gyrus, anterior cingulate cortex, and thalamus, regions that are known to be affected in schizophrenia (47, 48) but are not amenable to analysis using the present algorithm for cortex modeling.
No specific nongenetic etiological factor was measured in this study, thus the precise mechanisms underlying the disease-specific differences in cortical anatomy remain to be identified. A number of candidate mechanisms exist including gestational and perinatal complications (49) as well as postnatal factors such as head injuries and stressful life events (50). Any of these factors, to the extent that they specifically or preferentially impact one co-twin, may explain clinical as well as neurobiological phenotypic discordance, although each of their influences may depend on specific genetic diatheses. Further, because no specific gene for schizophrenia has been identified (51), there is not a specific candidate genetic mechanism to account for the liability-related deficits in gray-matter density. However, these findings to some extent are constraining of the mechanism(s), because they demonstrate that the impact of schizophrenia genes on cortical anatomy is fairly well localized to anterior and superior prefrontal cortex. Our results also may be useful in quantitative trait loci identification, because they indicate that deficits in prefrontal gray-matter covary with the degree of genetic loading for schizophrenia regardless of clinical phenotypic expression.
Postmortem investigation of the cellular and molecular basis of cortical gray-matter deficits in schizophrenia indicates a deficit in neuropil volume (10–12). Thus, candidates for genetic and nongenetic mechanisms affecting cortical anatomy in schizophrenia include those affecting dendritic arborization and synaptic density. These findings do not comment on whether gray-matter deficits in schizophrenia are atrophic or neurodevelopmental in origin. A recent longitudinal study applied the same cortical mapping methods to childhood-onset schizophrenic patients and controls, observing a dynamic wave of gray-matter reduction beginning in posterior regions and later extending to anterior regions (52). Whether gray-matter deficits are progressive in chronic patients with adult-onset schizophrenia is controversial (53, 54), but it is clear that deficits in cortical gray matter are present at illness debut before treatment with antipsychotic drugs (55).
Acknowledgments
We thank Ulla Mustonen, Antti Tanksanen, Tiia Pirkola, and Annamari Tuulio-Henriksson for their contributions to subject recruitment and assessment and the subjects, whose participation made this project possible. This work was supported by a research grant from the National Institute of Mental Health (to T.D.C.) and a resource grant from the National Center for Research Resources (to P.M.T. and A.W.T.). Additional support for algorithm development was provided by the National Library of Medicine, National Institute of Neurologic Disease and Stroke, the National Science Foundation, and a Human Brain Project grant to the International Consortium for Brain Mapping, funded jointly by the National Institute of Mental Health and the National Institute of Drug Abuse.
Abbreviations
- BA
Brodmann's areas
- MZ
monozygotic
- 3D
three-dimensional
- DZ
dizygotic
References
- 1.Harrison P J. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 2.Pearlson G D. Ann Neurol. 2000;48:556–566. [PubMed] [Google Scholar]
- 3.Lewis D A, Gonzalez-Burgos G. Brain Res Bull. 2000;52:309–317. doi: 10.1016/s0361-9230(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 4.Pearlson G D, Marsh L. Biol Psychiatry. 1999;46:627–649. doi: 10.1016/s0006-3223(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 5.McCarley R W, Wible C G, Frumin M, Hirayasu Y, Levitt J J, Fischer I A, Shenton M E. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, et al. Nature (London) 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 7.Berman K F, Torrey E F, Daniel D G, Weinberger D R. Arch Gen Psychiatry. 1992;49:927–934. doi: 10.1001/archpsyc.1992.01820120015004. [DOI] [PubMed] [Google Scholar]
- 8.Wood F, Flowers D L. Schizophr Bull. 1990;16:413–424. doi: 10.1093/schbul/16.3.413. [DOI] [PubMed] [Google Scholar]
- 9.Ross C A, Pearlson G D. Trends Neurosci. 1996;19:171–176. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- 10.Selemon L D, Goldman-Rakic P S. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 11.Selemon L D, Rajkowska G, Goldman-Rakic P S. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 12.Glantz L A, Lewis D A. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Selemon L D, Rajkowska G, Goldman-Rakic P S. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 14.Callicott J H, Bertolino A, Mattay V S, Langheim F J, Duyn J, Coppola R, Goldberg T E, Weinberger D R. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 15.Manoach D S, Gollub R L, Benson E S, Searl M M, Goff D C, Halpern E, Saper C B, Rauch S L. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan E V, Lim K O, Mathalon D, Marsh L, Beal D M, Harris D, Hoff A L, Faustman W O, Pfefferbaum A. Cereb Cortex. 1998;8:117–124. doi: 10.1093/cercor/8.2.117. [DOI] [PubMed] [Google Scholar]
- 17.Cannon T D, van Erp T G, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen V P, Standertskjold-Nordenstam C G, Gur R E, Yan M. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 18.Thompson P M, Woods R P, Mega M S, Toga A W. Hum Brain Mapp. 2000;9:81–92. doi: 10.1002/(SICI)1097-0193(200002)9:2<81::AID-HBM3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston K J. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 20.Bartley A J, Jones D W, Weinberger D R. Brain. 1997;120:257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann G, von Cramon D Y, Steinmetz H. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- 22.Thompson P M, Mega M S, Woods R P, Zoumalan C I, Lindshield C J, Blanton R E, Moussai J, Holmes C J, Cummings J L, Toga A W. Cereb Cortex. 2001;11:1–16. doi: 10.1093/cercor/11.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Baare W F, van Oel C J, Hulshoff Pol H E, Schnack H G, Durston S, Sitskoorn M M, Kahn R S. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Reveley M A, Reveley A M, Baldy R. Arch Gen Psychiatry. 1987;44:625–632. doi: 10.1001/archpsyc.1987.01800190045008. [DOI] [PubMed] [Google Scholar]
- 25.Suddath R L, Christison G W, Torrey E F, Casanova M F, Weinberger D R. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 26.Cannon T D, Kaprio J, Loennqvist J, Huttunen M, Koskenvuo M. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Kaprio J, Koskenvuo M, Rose R J. Acta Genet Med Gemellol (Roma) 1990;39:427–439. doi: 10.1017/s0001566000003652. [DOI] [PubMed] [Google Scholar]
- 28.Cannon T D, Huttunen M O, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper J E, Day R, Bertelsen A. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen N C. Scale for the Assessment of Negative Symptoms. Iowa City, IA: Univ. of Iowa College of Medicine; 1983. [Google Scholar]
- 31.Andreasen N C. Scale for the Assessment of Positive Symptoms. Iowa City, IA: Univ. of Iowa College of Medicine; 1983. [Google Scholar]
- 32.Delis D, Kramer J H, Kaplan E, Ober B A. California Verbal Learning Test: Research Edition. Cleveland: The Psychological Corporation; 1983. [Google Scholar]
- 33.Russell E W. J Consult Clin Psychol. 1975;43:800–809. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scale: Revised Manual. Cleveland: The Psychological Corporation; 1981. [Google Scholar]
- 35.Farmer A E, McGuffin P, Gottesman II. Arch Gen Psychiatry. 1987;44:634–641. doi: 10.1001/archpsyc.1987.01800190054009. [DOI] [PubMed] [Google Scholar]
- 36.Terwilliger J D, Weiss K M. Curr Opin Biotechnol. 1998;9:578–594. doi: 10.1016/s0958-1669(98)80135-3. [DOI] [PubMed] [Google Scholar]
- 37.Wright I C, Rabe-Hesketh S, Woodruff P W, David A S, Murray R M, Bullmore E T. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 38.Zijdenbos A P, Dawant B M. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]
- 39.MacDonald D, Avids D, Evans A C. In: Proceedings of the Society of Photo-optical Instrumentation Engineers Conference on Visualization in Biomedical Computing. Robb R A, editor. Vol. 2359. MN: Rochester; 1994. pp. 160–169. [Google Scholar]
- 40.Ono M, Kubik S, C.D. A. Atlas of the Cerebral Sulci. New York: Thieme; 1990. [Google Scholar]
- 41.Thompson P M, Toga A W. Med Image Anal. 1997;1:271–294. doi: 10.1016/s1361-8415(97)85002-5. [DOI] [PubMed] [Google Scholar]
- 42.Thompson P M, Cannon T D, Narr K L, van Erp T, Poutanen V P, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam C G, Kaprio J, Khaledy M, et al. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 43.Sowell E R, Thompson P M, Holmes C J, Jernigan T L, Toga A W. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 44.Falconer D S. Introduction to Quantitative Genetics. Essex, U.K.: Longman; 1989. [Google Scholar]
- 45.Kolb B, Whishaw I Q. Fundamentals of Human Neuropsychology. New York: Freeman; 1990. [Google Scholar]
- 46.Baare W F, Pol H E, Boomsma D I, Posthuma D, de Geus E J, Schnack H G, van Haren N E, van Oel C J, Kahn R S. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- 47.Benes F M. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 48.Andreasen N C, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O'Leary D, Ehrhardt J C, Yuh W T. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 49.McNeil T F, Cantor-Graae E, Weinberger D R. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 50.Walker E F, Neumann C C, Baum K, Davis D M, Diforio D, Bergman A. Dev Psychopathol. 1996;8:547–665. [Google Scholar]
- 51.Pulver A E. Biol Psychiatry. 2000;47:221–230. doi: 10.1016/s0006-3223(99)00281-4. [DOI] [PubMed] [Google Scholar]
- 52.Thompson P M, Vidal C, Giedd J N, Gochman P, Blumenthal J, Nicolson R, Toga A W, Rapoport J L. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gur R E, Cowell P, Turetsky B I, Gallacher F, Cannon T, Bilker W, Gur R C. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 54.Mathalon D H, Sullivan E V, Lim K O, Pfefferbaum A. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 55.Nopoulos P, Torres I, Flaum M, Andreasen N C, Ehrhardt J C, Yuh W T. Am J Psychiatry. 1995;152:1721–1723. doi: 10.1176/ajp.152.12.1721. [DOI] [PubMed] [Google Scholar]