Abstract
We have used a transgenic approach to visualize functionally activated neurons and their projections. The transgenic mice contain a tau-lacZ fusion gene regulated by the promoter for c-fos, an immediate early gene that is rapidly induced in neurons after functional stimulation. Constitutive expression of β-galactosidase (β-gal), the lacZ product, was low and in accord with previous reports of c-fos expression. However, expression of β-gal in positive neurons was clearly in cell bodies, axons, and dendrites. Treatment of the mice with kainic acid, a strong inducer of c-fos expression, resulted in high induction of β-gal. β-gal was induced in the same defined populations of neurons in the brain as those that express c-fos after kainic acid induction. Furthermore, the pattern of β-gal expression within the neurons changed over time after kainic acid treatment. Early after kainate treatment, β-gal was found mainly in cell bodies; at later times, expression extended further along the neuronal processes. This expression pattern is consistent with induction and anterograde transport of the Fos-Tau-β-gal protein in the neurons. To test whether a functionally activated pathway could be visualized, transgenic mice were deprived of water, which activates nuclei involved in body fluid homeostasis. β-gal induction was traced in neurons and their processes in the lamina terminalis, in magnocellular neurons of the supraoptic and paraventricular nuclei, and in their projections to the posterior pituitary gland. This strategy allowed the mapping of an activated osmoregulatory pathway. This transgenic approach may have general application in the mapping of functionally activated circuitry in the brain.
In neurobiology, a central aim is to determine the components and circuitry that are responsible for a given brain function. An approach to determine which parts of the brain are involved in a particular function is to look for markers of neuronal activation. One group of markers is the immediate early genes, which are rapidly transcribed after neuronal stimulation. The immediate early genes include c-fos and c-jun and related proteins Krox-20 (Egr-2) and Krox-24 (NGFI-A, Egr-1, Zif268, 1–3). Of these, c-fos is the most studied and generally induced by different stimuli (1–4).
The different types of stimuli that activate c-fos range from brain injury, including mechanical injury and ischemia, seizures and kindling, stress, osmotic stimulation, learning and memory, circadian rhythms and differences in the sleep–wake cycle, sensory stimulation, endocrine hormones, and a large range of pharmacological agents (1–4). In animals that have received no stimulus, levels of Fos are generally reported to be low, although not absent, in the brain.
However, c-fos is expressed exclusively in the cell nucleus, and what are identified in these studies are only those nuclei within activated neurons. For this reason, such markers give no idea of connectivity or the morphology of activated neurons within the nervous system. Callahan and Thomas (5) described a method of specifically labeling neuronal processes, which utilizes the enzyme reporter molecule Escherichia coli β-galactosidase (β-gal) and the microtubule-binding protein Tau. They constructed an axon-targeted β-gal reporter by fusing the cDNA encoding Tau to lacZ, the E. coli gene encoding β-gal. This reporter labels cell bodies and axons when expressed by developing and adult Drosophila neurons. The tau-lacZ fusion gene has also been expressed in mice after targeted insertion into one of the genes for an olfactory receptor (P2), which permitted the derivation of an olfactory sensory map (6). In addition to β-gal, other reporter-based tracers, including green fluorescent protein, have been generated to look at different connectivities within the brain (7).
We have used a related genetic approach as a first step to directly visualize functionally activated circuitry in the brain. We have generated transgenic mice in which the tau-lacZ fusion gene is under the regulation of the promoter for the c-fos gene. In these fos-tau-lacZ (FTL) transgenic mice, neurons that express Fos also express Tau-β-gal in their axons and dendrites, permitting direct visualization of their projections in the brain. This transgenic model will aid in the visualization and mapping of functionally activated circuitry in the brain, in which c-fos is activated.
Materials and Methods
Construction of FTL Fusion Genes and Generation of Transgenic Mice.
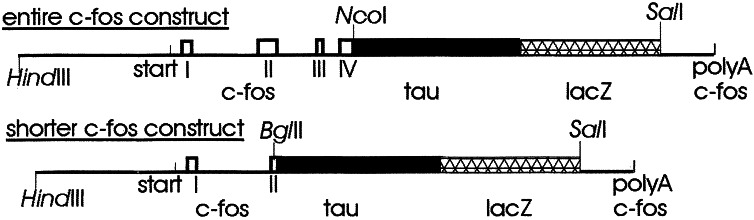
Two different FTL fusion constructs were generated from different parts of the c-fos gene in combination with full-length bovine tau and E. coli lacZ genes. One construct contained essentially the entire c-fos gene, including upstream coding sequences, introns, and downstream elements, which may be involved in regulation of c-fos transcription and translation (refs. 8 and 9; Fig. 1). The second construct contained a shorter region of c-fos, which was deleted from the beginning of exon 2 through to the end of the coding sequence (Fig. 1), and thus coded for only the first 18 aa of c-fos. This region of c-fos shows the same transcriptional activation as the entire c-fos gene in cultured cells (8, 9). To generate these different sequences, plasmid pc-fos (mouse)-3 (10), obtained from the American Type Culture Collection plasmid library, was digested with HindIII/BamHI, and the resulting 5-kb fragment was cloned into pGEM-3Zf.
Figure 1.
Constructs used for the generation of transgenic mice. Clear boxes represent exons of c-fos retained in the constructs, black indicates tau sequence, and crosshatched box indicates lacZ sequence. 5′ and 3′ sequences are derived from genomic c-fos (pc-fos). The transcription start site is indicated (start), and sequence 5′ to this contains identified transcriptional regulatory sequences of c-fos (8, 9).
We obtained a construct with the tau gene and the lacZ gene combined as an in-frame fusion gene (5). This tau-lacZ fusion gene was modified before making a triple fusion construct with the c-fos gene. The 3′ end was modified by removing a small BsiWI/XbaI fragment and replacing it with a fragment generated by PCR, which contains a unique SalI site directly 3′ to the stop codon. The 5′ end of tau-lacZ was modified by replacing a 600-bp HindIII/StyI fragment at the start of the tau gene, with a 500-bp PCR-generated fragment that contains either a NcoI site or a BglII site at the start codon. From this modified tau-lacZ construct, a 4.5-kb NcoI/SalI or BglII/SalI fragment, containing the entire tau-lacZ fusion gene, was purified.
c-fos in plasmid pGEM-3Zf was digested with either NcoI or BglII, and SalI, to release the two different fragments of c-fos. This left the rest of the plasmid, which was purified and ligated to the corresponding tau-lacZ fusion fragment. The resultant genes thus contain tau and lacZ in frame inserted into two different c-fos genes deleted for different regions as shown in Fig. 1.
Transfection of PC12 Cells with FTL Constructs.
Both constructs were tested before the generation of transgenic mice, by transfection into the pheochromocytoma cell line, PC12, according to Chu et al. (11), with the following modifications: 80 μg of DNA was electroporated into 2 × 107 cells in a volume of 500 μl, at 240 V and 960 μF. Transiently transfected cells were plated into a 12-well tissue culture plate, coated with fibronectin (50 μg/well, GIBCO) in DMEM containing 10% FBS and 5% horse serum, and allowed to adhere overnight at 37°C in an atmosphere of 10% CO2/90% air. The following day, the wells were washed three times in DMEM and incubated in DMEM containing nerve growth factor (NGF; 50 ng/ml) for 48 h to induce differentiation. To induce Fos in the differentiated cells, NGF-containing medium was removed and replaced with DMEM alone; the cultures were then incubated for 4 h, and then NGF (50 ng/ml) and phorbol 12-tetradecanoate 13-acetate (0.3 μg/ml) were added, and incubation was continued for 1 h. Cells were then fixed in 0.05% glutaraldehyde for 5 min and processed for β-gal activity as described below.
Generation of Transgenic Mice.
The shorter construct was excised from pGEM-3Zf, purified by agarose gel electrophoresis and chromatography on an Elutip minicolumn (Schleicher and Schüll, Dassel, Germany), and injected into the pronuclei of mouse oocytes. The injected oocytes were then transferred into pseudopregnant C57Bl6/BM1 mice for the generation of transgenic animals. The injections, embryo transfers, and subsequent generation of offspring were undertaken by the Transgenic Embryo Service of the Walter and Eliza Hall Institute of Medical Research (Parkville, VIC, Australia). Five primary transgenic lines were generated, of which three were found to express the FTL transgene.
Genotyping.
Genomic DNA was solubilized from mouse tail biopsies by digestion with proteinase K (200 μg/ml; Worthington) in 100 mM Tris⋅HCl/5 mM EDTA/0.2% SDS/200 mM NaCl, pH 8.5, overnight at 55°C, and precipitated with an equal volume of isopropyl alcohol. The DNA was genotyped by PCR within the lacZ gene by using primers (forward: 5′-GCATCGAGCTGGGTAATAAGCG-3′; reverse: 5′-GACACCAGACCAACTGGTAATGGTAGCG-3′) (12). The reaction, in 20 μl, was 1 cycle at 95°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. Taq DNA polymerase (Bioscientific, Sydney) was used in the presence of 1.5 mM MgCl2. The amplified fragment was 800 base pairs in length.
Housing of the Mice Under Basal Conditions and Analysis of β-gal Expression.
Mice were housed in a constant-temperature environment (22°C) in same-sex groups of up to six per cage, with a 12-h day/night cycle. Food and water were supplied ad libitum. Cages were cleaned once a week and litter was replaced. For analysis of β-gal expression under basal conditions, mice were taken from their home cages and immediately injected i.p. with an overdose of sodium pentobarbital (200 μl of Nembutal, Rhone-Merieux, Pinkenba, Australia). After the animals were deeply anesthetized, they were perfused transcardially with 30 ml of 0.9% normal saline followed by the same volume of 4% paraformaldehyde/2 mM MgCl2/2 mM EGTA in PBS. Brains were removed and postfixed in fresh perfusion solution for 1 h at 4°C, transferred to 10% sucrose in PBS containing 2 mM MgCl2 and 2 mM EGTA, and equilibrated for 48 h. Brains were then frozen in Tissue Tek, and cryostat sections were cut (40 μm) and collected in wells of 24-well tissue culture plates (two or three sections per well) in wash buffer (2 mM MgCl2/2 mM EGTA/0.01% sodium deoxycholate/0.05% Igepal CA-630). To obtain sections in which the pituitary gland was attached to the brain, the brain was first decalcified by immersion in 20% EDTA in 0.1 M phosphate buffer, pH 7.4, for 2 weeks at 4°C, with daily changes of buffer.
For staining using β-gal enzyme activity and histochemistry, sections were rinsed for 30 min in wash buffer and then stained for β-gal by incubation in wash buffer containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal; Astral, Sydney) for 24 h at 37°C. After staining, sections were rinsed in PBS, placed into 0.5% gelatin in H2O, and mounted onto slides coated with 1% gelatin. Sections were allowed to dry overnight, then dehydrated through graded alcohols to Histolene, rehydrated through graded alcohols, rinsed in water, and counterstained in 0.05% nuclear fast red for 15 min. Sections were rinsed in H2O, dehydrated again, and mounted in Safety mount (Histolabs, Australia). Sections were analyzed microscopically, and areas that were β-gal-positive were identified by comparison with an atlas of the mouse brain (13).
For β-gal immunohistochemistry, cryostat sections, either free-floating or mounted, were incubated overnight at 4°C in rabbit anti-β-gal antisera (Cappel) diluted 1:10,000 in CAS-Block (Zymed). After washing in PBS, sections were incubated for 2 h in Alexa fluor 488-labeled goat anti-rabbit IgG (Molecular Probes) diluted 1:400 in PBS. They were then washed in PBS, and the free-floating sections were mounted onto gelatinized slides, dried. and coverslipped in fluorescent mounting medium (Dako).
Fluorescence photographs were obtained by using an ImagePoint cooled charge-coupled device camera (Photometrics, Tucson, AZ) and V for Windows imaging software (Digital Optics, Auckland, New Zealand). Images were processed with Corel PHOTOPAINT and Corel DRAW software (Corel, Dublin). For confocal microscopy, sections were imaged with a Zeiss Axioplan 2 microscope fitted with a Bio-Rad 1024 confocal illuminating system by using a ×100 objective and the appropriate fluorescence filter. For construction of neurons in three dimensions, 30 images through a Z-series were taken at 1-μm intervals through the section, and the images were reconstructed by using LASERSHARP software.
Treatment with Kainic Acid and Water Deprivation Studies.
Kainic acid was injected i.p. into the mice, and after specified times; the mice were killed and processed as described above. The dose of kainic acid we used (10 mg/kg) induces mild distress in the mice (all derived from C57/BL6), and they show slight muscle spasms that begin approximately 15 min after injection and last for approximately 30 min. The animals then seem to fully recover. Animals used in water deprivation studies were housed singly for at least 5 days, and then water was removed for 48 h, after which time the animals were killed as described above. Water-replete animals had access to water for the 48 h.
Results
Generation of FTL Transgenic Mice.
To generate mice that express a tau-lacZ fusion gene under the control of c-fos promoter elements, it was necessary to construct a transgene containing all known c-fos promoter elements. Elements 5′, 3′, and within c-fos are required for its correct transcriptional regulation (8, 9). We made two constructs (see Fig. 1), which contain different regions of c-fos fused to the tau-lacZ fusion construct (5). The entire construct contained essentially the same regions of c-fos previously used in the generation of a fos-lacZ mouse (14) and contained a region of the c-fos from 500 bp 5′ from the transcription start site to near the end of exon 4 in addition to a region of c-fos consisting of the 3′ untranslated region, including the A+U-rich region and the polyadenylation signal region. The shorter construct was similar except that the 5′ region of the c-fos gene extended only to the beginning of exon 2, which deletes most of the coding sequence for c-fos.
Both constructs were transfected into PC12 cells to determine their ability to appropriately express β-gal in processes and in an inducible manner. Both constructs were expressed in the PC12 cells, and both showed predominant expression in cell bodies and processes of differentiated PC-12 cells (data not shown). In addition, treatment of the cells with phorbol esters and NGF, which up-regulates c-fos expression, resulted in an increase in the percentage of cells that expressed β-gal (data not shown). However, the shorter construct gave higher transfection efficiencies and was more inducible with NGF, compared with the longer construct. We thus used this construct for the generation of transgenic mice. Five transgenic lines were generated and three expressed β-gal in the brain. We have focused on one of these lines of mice, termed FTL, whose expression of β-gal closely resembles endogenous c-fos expression, as described below.
Expression of β-gal in FTL Mouse Brains.
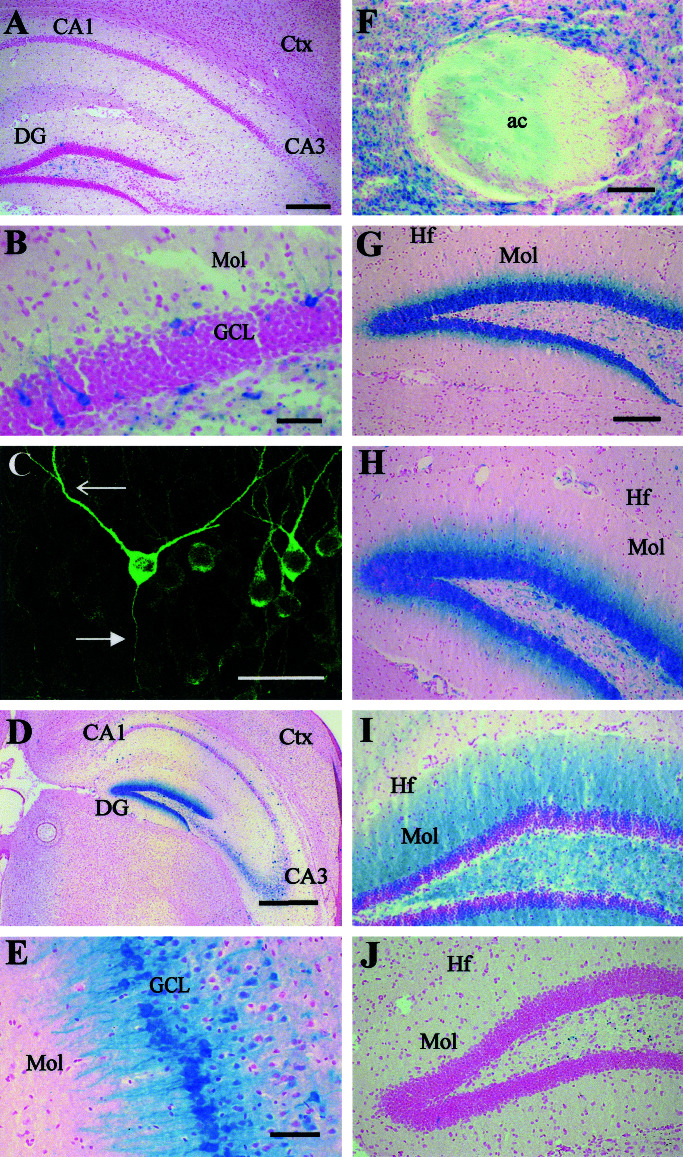
The FTL mice were initially examined for basal expression of β-gal by using enzyme activity and histochemistry. Expression was generally low or absent throughout the brain (Fig. 2A) and completely in accord with that reported for endogenous c-fos under basal conditions (1–3, 15). In the hippocampus, there was low but significant expression in the dentate gyrus and CA3 regions. In the dentate gyrus, a small number of cells in the granule cell layer were β-gal-positive. Staining was evident in both cell bodies and processes extending into the molecular layer (Fig. 2B). Some brain sections were stained with anti-β-gal antibodies and immunofluorescence. High-power confocal imaging of the neurons in the dentate gyrus from these sections showed complete labeling of cell bodies, dendrites, and axons in the β-gal-positive neurons (Fig. 2C). The nuclei of the cells were essentially negative for β-gal (Fig. 2 B and C). There was also staining in cell bodies and processes in the polymorphic layer. There was weak staining of cell bodies in the cerebral cortex (not shown) and in the Edinger–Westphal nucleus, where β-gal staining was present throughout in cell bodies, but at low levels (data not shown). Expression throughout the rest of the brain was mostly scattered, with cells in some brain nuclei staining positively for β-gal. These nuclei included the paraventricular thalamic nucleus, posterior hypothalamus, lateral geniculate nuclei, raphe nucleus, and periaqueductal gray.
Figure 2.
Expression of β-gal in FTL mice under basal conditions and after treatment with kainic acid. FTL mice were taken directly from their home cages (A–C) and killed, and coronal sections of brains were processed for β-gal. (A) Low-power view of brain, including hippocampus and cortex. (B) Dentate gyrus, showing β-gal-stained cell bodies with processes extending into the molecular layer. (C) High-power confocal image of dentate gyrus showing immunostained granule cells with labeled dendrites (open arrow) and axons (closed arrow). (D–F) Mice were treated with kainate and processed for β-gal expression after 4 h. (D) Hippocampus, most cells are positive in dentate gyrus. (E) High-power view of CA3 region. (F) Anterior commissure. (G–J) Dentate gyrus of animals treated with kainate for 2 h (G), 4 h (H), 24 h (I), and 48 h (J); note staining in molecular layer. ac, Anterior commissure; Ctx, cortex; DG, dentate gyrus; GCL, granule cell layer; Hf, hippocampal fissure; Mol, molecular layer. [Scale bar: 500 μm (A), 50 μm (B and C), 250 μm (D), 60 μm (E), and 125 μm (F–J).]
β-gal was also expressed in skin, hair follicles, and bone of the transgenic mice (data not shown), as would be expected for a c-fos regulatable transgene. Expression of β-gal in all of these areas has also been reported for fos-lacZ transgenic mice (14).
Induction of β-gal in FTL Mice with Kainic Acid.
Many stimuli can induce c-fos expression, including kainic acid, a convulsant and glutamate agonist (1, 3, 15). The areas of the brain that express c-fos in response to kainic acid have been accurately mapped (1, 3, 15), and thus it is possible to identify kainic acid-inducible expression of β-gal in our transgenic mice and compare it with that already known. We injected kainic acid i.p. into FTL transgenic mice and, after 2 h, killed the mice and mapped the expression of β-gal throughout the brain. Many areas of the brain showed medium to strong expression of β-gal. β-gal was expressed in both cell bodies and processes after kainate treatment. Strong expression was found within the hippocampus and particularly the dentate gyrus, where a high proportion of cells were positive (Fig. 2 D and G). The CA3 region was also quite strongly labeled (Fig. 2 D and E). In the CA1 region, there was also significant β-gal expression (Fig. 2D), but this occurred more prominently in temporal regions of the hippocampus (data not shown).
In other regions of the brain, the strongest expression was found in the amygdala and in particular in lateral and basolateral regions, where a high proportion of the cells were strongly labeled with β-gal (data not shown). Other regions also showed significant β-gal expression but not as strong as the amygdala. In the cortex, there was strong expression in a variety of different areas (data not shown). In summary, these expression patterns are completely consistent with endogenous Fos expression and on β-gal expression in fos-lacZ mice after treatment with kainic acid (1, 3, 15). There was also clear expression of β-gal within the anterior commissure, present as fine blue staining through this tract (Fig. 2F).
β-gal Expression Extends Along Neuronal Processes over Time After Kainate Treatment.
The induced FTL transgene product must be first synthesized in the cell body and then transported into neuronal processes. To look for a shift in expression from cell body to process, we looked at the effect of kainic acid at different time points after treatment and analyzed the hippocampal areas of their brains for β-gal expression. After 1 h, most of the β-gal staining was restricted to the cell bodies in the hippocampus (data not shown). However, by 2 h after kainate injection, not only was the β-gal staining found in the cell bodies, it also extended into the processes of the neurons in the inner molecular layer of the dentate gyrus (Fig. 2G). By 4 h after kainic acid injection, more of the dendritic field of the dentate gyrus stained positively for β-gal (Fig. 2H). The staining was uniform across the dendritic field, although fine, positively stained dendrites could be distinguished throughout this region. By 24 h, β-gal was still observed in the dendritic field (Fig. 2I) but was absent from the cell bodies. Other tracts became positive at this time, including the alveus and the fimbria-fornix (not shown). By 48 h, β-gal had returned to basal levels in the dentate gyrus (Fig. 2J) and throughout the brain.
Water Deprivation Stimulates β-gal Expression in the Lamina Terminalis and Hypothalamo-Neurohypophysial Pathway.
The above experiments demonstrate that β-gal in the FTL mice is expressed in the same cells as endogenous c-fos under both basal and kainate-stimulated conditions. To test whether the circuitry of a physiologically regulated system could be visualized in the FTL mice, the mice were deprived of water for 48 h to activate a specific and well-defined osmoregulatory pathway, which is involved in body fluid homeostasis. This path begins with the stimulation of osmotically sensitive neurons in the organum vasculosum of the lamina terminalis, subfornical organ, and median preoptic nucleus that have efferent neural connections to the magnocellular neurons of the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) (Fig. 3A). These magnocellular neurons in the SON and PVN project their axons to and along the base of the third ventricle, in the internal zone of the median eminence, to the posterior pituitary, at which site the neurosecretion of vasopressin and oxytocin occurs (16). Neurons at all levels of this pathway express c-fos in response to water deprivation (17–19).
Figure 3.
Expression of β-gal in FTL mice after water deprivation. (A) Schematic diagram of the osmoregulatory pathway investigated. Neurons (filled red circles) in the lamina terminalis (consisting of the organum vasculosum of the lamina terminalis, median preoptic nucleus, and subfornical organ) that are responsive to plasma hypertonicity send efferent axonal projections (red lines) to magnocellular neurons (filled blue circles) in the supraoptic nucleus and hypothalamic paraventricular nucleus. The processes (blue lines) of these magnocellular neurons form the hypothalamo-neurohypophysial pathway that courses in the median eminence to reach the posterior pituitary, where neurosecretion of vasopressin and oxytocin into the general circulation occurs as the result of this pathway being activated in response to dehydration. Mice were either water replete (C, E, G, and I) or deprived (B, D, F, H, and J) for 48 h and analyzed for β-gal expression by histochemistry (B–H) or immunofluorescence (I and J). (B) High-power view of region of the organum vasculosum of the lamina terminalis indicated with open arrow in D. (C and D) Sagittal sections of midline of ventral brain around third ventricle. (E and F) Hypothalamus, showing the SON and PVN. (G and H) Sections through the median eminence. (I and J) Sections through the pituitary. Ten mice from control and water-deprived animals were analyzed, and figures are representative of findings from each of the animals. 3V, third ventricle; ∗, anterior commissure; AL, IL, and PL, anterior, intermediate, and posterior lobe of pituitary, respectively; iME, internal median eminence; MnPO, median preoptic nucleus; oc, optic chiasm; ot, optic tract; OVLT, organum vasculosum of the lamina terminalis; Pit, pituitary; PVN, hypothalamic paraventricular nucleus. [Scale bar: 50 μm (B), 500 μm (C and D), 200 μm (E and F), and 100 μm (G–J).]
We did not detect β-gal expression in the organum vasculosum of the lamina terminalis, subfornical organ, median preoptic nucleus, SON, PVN, median eminence, or posterior pituitary in water-replete animals (Fig. 3 C, E, G, and I) by using either β-gal histochemistry or immunofluorescence. However, in the water-deprived animals, expression was clearly and strongly detectable by using β-gal histochemistry or immunofluorescence in these sites. Expression of β-gal in the water-deprived animals could be detected in neuronal perikarya and processes throughout the lamina terminalis (Fig. 3 B and D) and magnocellular neuronal cell bodies in both SON and PVN (Fig. 3F). Distinct label was also present in the hypothalamo-neurohypophysial tract extending from the SON and PVN through the internal zone of the median eminence (Fig. 3H) to the posterior pituitary (Fig. 3J). In the posterior pituitary, β-gal expression formed a loose semicircle at the perimeter of the posterior lobe of the pituitary, corresponding to the tract projecting from the median eminence (Fig. 3J). We have thus tracked this functionally activated pathway from sensors (osmoreceptors) in the lamina terminalis to hypothalamic magnocellular neurons in the SON and PVN and their axons in the median eminence, and finally to their neurosecretory terminals in the posterior pituitary.
Other parts of the brain showed increased expression of β-gal after water deprivation, including nuclei in the thalamus and the anterior pituitary (Fig. 3D, compare with Fig. 3C). We have not determined what caused this up-regulation; however, it may have been associated with induction of stress responses in the water-deprived animals.
Discussion
We have developed an approach to visualize functionally activated neurons and their projections. This approach should aid accurate mapping of functionally activated circuits in the brain. Current approaches to do this employ techniques ranging from functional imaging studies (20) to the use of tracers (7, 21–23). However, these techniques have associated problems—e.g., functional imaging does not show circuits, and no tracers are specific for functionally activated pathways.
We made two constructs for the generation of the transgenic mice, which differed in the region of the c-fos gene encoding exons 2 to 4. Both constructs contain all known regulatory elements of the c-fos promoter (8, 9). The deletion of most of exons 2 to 4 in the shorter construct results in this construct encoding only the first 18 aa of c-fos. This probably resulted in deletion of regions of Fos concerned with its nuclear localization. Chida and coworkers (24) have indirect evidence that Fos and Jun interact by means of their leucine zippers before entry to the nucleus and translocate to the nucleus by using the strong nuclear localization signal of Jun. For this reason, we used the shorter construct for the generation of transgenic mice. In addition, transfection of this construct into PC12 cells gave increased levels of expression compared with the longer construct.
Basal Expression of β-gal in FTL Transgenic Mice.
Previous reports of c-fos expression, or of β-gal expression in fos-lacZ mice, show generally low or no expression in most parts of the brains under basal conditions (1, 15). In our FTL mice, there was generally low to negligible expression of β-gal in the brain. In the hippocampus, a small percentage of the cells were positive in the mice. Expression in the hippocampus was mainly restricted to the more temporal areas, although low but significant β-gal positivity was found in the polymorphic layer of the dentate gyrus in anterior through to posterior regions. A range of other structures showed a small percent of β-gal-positive cells, including thalamic and hypothalamic nuclei, both of which are reported to express low levels of c-fos constitutively (25). Within the brainstem, the Edinger–Westphal nucleus shows low but consistent expression. This is in agreement with reported expression of c-fos in this nucleus under basal conditions (26). The pattern of expression of β-gal in the FTL mice under basal conditions is entirely consistent with reported constitutive c-fos expression (1, 15).
Expression of β-gal in the FTL mice was in both cell bodies and processes. This was very clear in the hippocampus, where there was clear staining of individual cells from cell body to process. The nuclei of the cells were not labeled. This expression pattern demonstrates successful targeting of β-gal expression into cell bodies and processes in the FTL mice in vivo.
Induction of β-gal in Transgenic Mice.
We looked at the induction of β-gal in the transgenic mice to determine whether β-gal expression was inducible in a similar manner to c-fos. Expression of c-fos correlates with functional activation in many different systems in the brain. In many cases, c-fos expression reflects deoxyglucose uptake studies, which implicate a given brain region being activated in response to a particular stimulus (1). The induction of c-fos may represent a monitor of intracellular second-messenger levels (1). Our view is that c-fos expression in neurons is indicative of nonbasal protein synthesis, which occurs after different stimuli. We also wished to determine whether the FTL fusion protein was anterogradely transported after induction of synthesis.
Our experiments indicated that after injection of kainate, the transgenic mice showed expression of β-gal in the same regions as previously reported for c-fos for both rats and mice (15, 27), and for β-gal in transgenic fos-lacZ mice (14). These regions are the same as those involved in the generation of paroxysmal activity produced by kainate (28). In addition, these regions previously showed significant changes in 2-deoxyglucose consumption after kainate administration (29). Within the hippocampus, the dentate gyrus showed the strongest expression of β-gal. There was also a strong expression of β-gal expression throughout the CA3 region.
Of the other structures that showed strong expression of β-gal in response to kainic acid, the amygdala showed the greatest expression, and in particular the lateral regions of the amygdala, where the majority of cells expressed β-gal. In the cortex, strong expression was found particularly in piriform, orbital, prelimbic, and cingulate cortices. These patterns of expression are consistent with the patterns of inducible expression of c-fos in these brain regions after kainic acid injection (14, 15, 27). In addition, we found inducible expression of β-gal in the anterior commissure, which demonstrates transport of the transgene product in long axon tracts.
We followed the progressive increase in staining in the dendritic layers after kainic acid treatment. By 6 h, the entire dendritic field of the dentate gyrus was stained for β-gal. The pattern of expression persisted for at least 24 h, wherein expression of β-gal was evident only in the dendritic layer. Thus, by this time synthesis of FTL fusion protein ceased and all remaining fusion protein was in the neuronal processes. This finding is consistent with the time course of endogenous Fos after kainic acid treatment. Fos is present for up to 12 h but then diminishes; by 24 h, there is very little expression (15). These experiments also demonstrate that the transgene product persists for at least 24 h after a stimulus and remains in the neuronal processes for significant periods after synthesis is finished. In this, it is quite different from endogenous Fos, which is more rapidly degraded after synthesis (15).
Visualization of a Functionally Activated Circuit.
The hormone vasopressin, which is synthesized in magnocellular neurons of the hypothalamic SON and PVN, then secreted from their axon terminals in the posterior pituitary gland, plays a crucial role in regulating the volume and concentration of urine excreted by the kidneys (30, 31). In dehydrated animals, both the hypertonicity and hypovolemia of body fluids provide stimuli that activate neurons in the SON and PVN, as well as sensor sites in the lamina terminalis projecting to these two nuclei (18). Electrophysiological investigations and studies of expression of c-fos in the SON and PVN show that both vasopressin- and oxytocin-containing neurons in these nuclei, as well as neurons in the lamina terminalis, are activated by hypertonicity and dehydration in the rat (17, 32–34). The vasopressin and oxytocin-containing neurons send efferent neural connections via the base of the hypothalamus and median eminence to the posterior pituitary gland, where oxytocin and vasopressin are secreted into the circulation (16). In the present experiment, water deprivation resulted in β-gal expression in the lamina terminalis and magnocellular neurons of the SON and PVN and their projections through the median eminence to the posterior pituitary. This result indicates that a physiological stimulus, known to cause c-fos expression in the lamina terminalis, SON, and PVN, also results in the production of FTL fusion protein under the control of the c-fos promoter in these transgenic mice. As a result, the fusion protein is transported throughout the neural processes of the magnocellular neurons, enabling visualization of the entire neurosecretory pathway from hypothalamus to neurohypophysis. The visualization of osmoregulatory neurons in the lamina terminalis and their processes is also made possible.
These data together indicate that the FTL mice may prove useful in the mapping of functionally activated parts of the brain, in which c-fos is involved, and in the localization and morphology of neurons, neuronal processes, and pathways that are associated with this functional activation.
Acknowledgments
We thank Drs. Gary Sedman, Tony Shafton, Sandra Rees, and Colin Anderson for intellectual input, Dr. Ann Turnley for assistance with molecular biology, Leonie Malcolm at the Walter and Eliza Hall Institute for pronuclear injections, and Drs. John Thomas and Chris Callahan for the tau-lacZ plasmid. This research was supported by the National Health and Medical Research Council of Australia.
Abbreviations
- β-gal
β-galactosidase
- FTL
fos-tau-lacZ
- PVN
paraventricular nucleus
- SON
supraoptic nucleus
- NGF
nerve growth factor
References
- 1.Morgan J I, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 2.Herdegen T, Leah J D. Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 3.Herrera D G, Robertson H A. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A. NeuroReport. 1997;8:v–ix. [PubMed] [Google Scholar]
- 5.Callahan C A, Thomas J B. Proc Natl Acad Sci USA. 1994;91:5972–5976. doi: 10.1073/pnas.91.13.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 7.Callahan C A, Yoshikawa S, Thomas J B. Curr Opin Neurobiol. 1998;8:582–586. doi: 10.1016/s0959-4388(98)80084-6. [DOI] [PubMed] [Google Scholar]
- 8.Treisman R. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson L M, Kerppola T K, Vendrell M, Luk D, Smeyne R J, Bocchiaro C, Morgan J I, Curran T. Neuron. 1995;14:241–252. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 10.Curran T, MacConnell W P, van Straaten F, Verma I M. Mol Cell Biol. 1983;3:914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu G, Hayakawa H, Berg P. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L, Panthier J J, Arnheiter H. Development (Cambridge, UK) 2000;127:5379–5389. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Franklin K B J. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 2001. [Google Scholar]
- 14.Smeyne R J, Schilling K, Robertson L, Luk D, Oberdick J, Curran T, Morgan J I. Neuron. 1992;8:13–23. doi: 10.1016/0896-6273(92)90105-m. [DOI] [PubMed] [Google Scholar]
- 15.Popovici T, Represa A, Crepel V, Barbin G, Beaudoin M, Ben-Ari Y. Brain Res. 1990;536:183–194. doi: 10.1016/0006-8993(90)90024-6. [DOI] [PubMed] [Google Scholar]
- 16.Sofroniew M V. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, editors. Vol. 4. Amsterdam: Elsevier; 1985. pp. 93–165. [Google Scholar]
- 17.Sharp F R, Sagar S M, Hicks K, Lowenstein D, Hisanaga K. J Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinley M J, Hards D K, Oldfield B J. Brain Res. 1994;653:305–314. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 19.Morien A, Garrard L, Rowland N E. Brain Res. 1999;816:1. doi: 10.1016/s0006-8993(98)00828-2. [DOI] [PubMed] [Google Scholar]
- 20.Berns G S. Life Sci. 1999;65:2531–2540. doi: 10.1016/s0024-3205(99)00297-0. [DOI] [PubMed] [Google Scholar]
- 21.Aston-Jones G, Card J P. J Neurosci Methods. 2000;103:51–61. doi: 10.1016/s0165-0270(00)00295-8. [DOI] [PubMed] [Google Scholar]
- 22.Kobbert C, Apps R, Bechmann I, Lanciego J L, Mey J, Thanos S. Prog Neurobiol. 2000;62:327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 23.Reiner A, Veenman C L, Medina L, Jiao Y, Del Mar N, Honig M G. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 24.Chida K, Nagamori S, Kuroki T. Cell Mol Life Sci. 1999;55:297–302. doi: 10.1007/s000180050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chastrette N, Pfaff D W, Gibbs R B. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Martinez V, Vale W, Tache Y. Brain Res. 2000;855:47–57. doi: 10.1016/s0006-8993(99)02200-3. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg J L, Mitchelmore C, Macgregor-Leon P F, Hempstead J, Morgan J I, Curran T. J Neurosci Res. 1989;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Ari Y, Riche D, Tremblay E, Charton G. Eur Neurol. 1981;20:173–175. doi: 10.1159/000115228. [DOI] [PubMed] [Google Scholar]
- 30.Verney E B. Proc R Soc London Ser B. 1947;135:25–106. [PubMed] [Google Scholar]
- 31.Robertson G L. Kidney Int. 1987;32:S20–S26. [PubMed] [Google Scholar]
- 32.Bourque C W, Oliet S H, Richard D. Front Neuroendocrinol. 1994;15:231–274. doi: 10.1006/frne.1994.1010. [DOI] [PubMed] [Google Scholar]
- 33.Brimble M J, Dyball R E J. J Physiol (London) 1977;271:253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovanelli L, Shiromani P J, Jirikowski G F, Bloom F E. Brain Res. 1990;531:299–303. doi: 10.1016/0006-8993(90)90789-e. [DOI] [PubMed] [Google Scholar]