Abstract
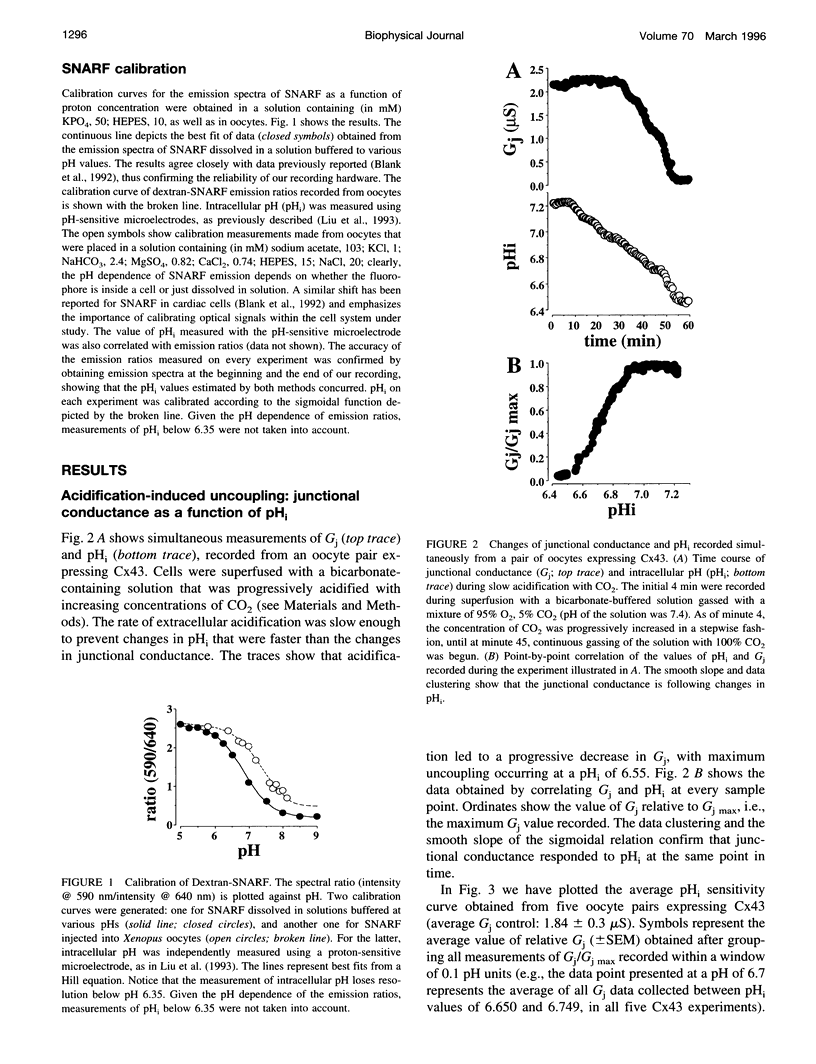
We have previously proposed that acidification-induced regulation of the cardiac gap junction protein connexin43 (Cx43) may be modeled as a particle-receptor interaction between two separate domains of Cx43: the carboxyl terminal (acting as a particle), and a region including histidine 95 (acting as a receptor). Accordingly, intracellular acidification would lead to particle-receptor binding, thus closing the channel. A premise of the model is that the particle can bind its receptor, even if the particle is not covalently bound to the rest of the protein. The latter hypothesis was tested in antisense-injected Xenopus oocyte pairs coexpressing mRNA for a pH-insensitive Cx43 mutant truncated at amino acid 257 (i.e., M257) and mRNA coding for the carboxyl terminal region (residues 259-382). Intracellular pH (pHo) was recorded using the dextran form of the proton-sensitive dye seminaphthorhodafluor (SNARF). Junctional conductance (Gj) was measured with the dual voltage clamp technique. Wild-type Cx43 channels showed their characteristic pH sensitivity. M257 channels were not pH sensitive (pHo tested: 7.2 to 6.4). However, pH sensitivity was restored when the pH-insensitive channel (M257) was coexpressed with mRNA coding for the carboxyl terminal. Furthermore, coexpression of the carboxyl terminal of Cx43 enhanced the pH sensitivity of an otherwise less pH-sensitive connexin (Cx32). These data are consistent with a model of intramolecular interactions in which the carboxyl terminal acts as an independent domain that, under the appropriate conditions, binds to a separate region of the protein and closes the channel. These interactions may be direct (as in the ball-and-chain mechanism of voltage-dependent gating of potassium channels) or mediated through an intermediary molecule. The data further suggest that the region of Cx43 that acts as a receptor for the particle is conserved among connexins. A similar molecular mechanism may mediate chemical regulation of other channel proteins.
Full text
PDF

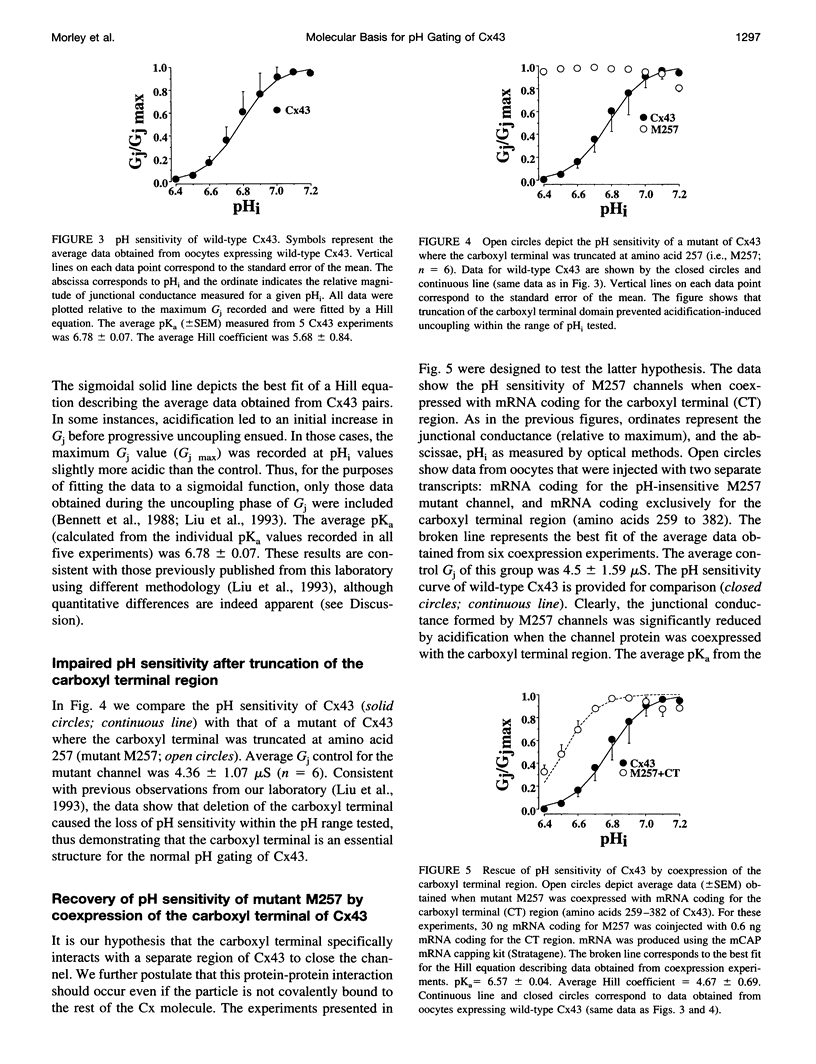





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arellano R. O., Ramón F., Rivera A., Zampighi G. A. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J Membr Biol. 1988;101(2):119–131. doi: 10.1007/BF01872827. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Verselis V. K. Biophysics of gap junctions. Semin Cell Biol. 1992 Feb;3(1):29–47. doi: 10.1016/s1043-4682(10)80006-6. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank P. S., Silverman H. S., Chung O. Y., Hogue B. A., Stern M. D., Hansford R. G., Lakatta E. G., Capogrossi M. C. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. Am J Physiol. 1992 Jul;263(1 Pt 2):H276–H284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham S. H., Shah M. M., Zuppan C. W., Fletcher W. H. Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med. 1995 May 18;332(20):1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Paul D. L. Expression of chimeric connexins reveals new properties of the formation and gating behavior of gap junction channels. J Cell Sci. 1994 Apr;107(Pt 4):955–967. doi: 10.1242/jcs.107.4.955. [DOI] [PubMed] [Google Scholar]
- Delmar M., Jalife J., Michaels D. C. Effects of changes in excitability and intercellular coupling on synchronization in the rabbit sino-atrial node. J Physiol. 1986 Jan;370:127–150. doi: 10.1113/jphysiol.1986.sp015926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar M., Michaels D. C., Johnson T., Jalife J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ Res. 1987 May;60(5):780–785. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dunham B., Liu S., Taffet S., Trabka-Janik E., Delmar M., Petryshyn R., Zheng S., Perzova R., Vallano M. L. Immunolocalization and expression of functional and nonfunctional cell-to-cell channels from wild-type and mutant rat heart connexin43 cDNA. Circ Res. 1992 Jun;70(6):1233–1243. doi: 10.1161/01.res.70.6.1233. [DOI] [PubMed] [Google Scholar]
- Ek J. F., Delmar M., Perzova R., Taffet S. M. Role of histidine 95 on pH gating of the cardiac gap junction protein connexin43. Circ Res. 1994 Jun;74(6):1058–1064. doi: 10.1161/01.res.74.6.1058. [DOI] [PubMed] [Google Scholar]
- Esinduy C. B., Chang C. C., Trosko J. E., Ruch R. J. In vitro growth inhibition of neoplastically transformed cells by non-transformed cells: requirement for gap junctional intercellular communication. Carcinogenesis. 1995 Apr;16(4):915–921. doi: 10.1093/carcin/16.4.915. [DOI] [PubMed] [Google Scholar]
- Fishman G. I., Moreno A. P., Spray D. C., Leinwand L. A. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot I., Dekel N. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994 Dec 2;269(48):30502–30509. [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Koval M., Geist S. T., Westphale E. M., Kemendy A. E., Civitelli R., Beyer E. C., Steinberg T. H. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol. 1995 Aug;130(4):987–995. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Goulding E., Siegelbaum S. A. Potassium channel inactivation peptide blocks cyclic nucleotide-gated channels by binding to the conserved pore domain. Neuron. 1994 Mar;12(3):655–662. doi: 10.1016/0896-6273(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Lang L. M., Beyer E. C., Schwartz A. L., Gitlin J. D. Molecular cloning of a rat uterine gap junction protein and analysis of gene expression during gestation. Am J Physiol. 1991 May;260(5 Pt 1):E787–E793. doi: 10.1152/ajpendo.1991.260.5.E787. [DOI] [PubMed] [Google Scholar]
- Lazrak A., Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J. 1993 Nov;65(5):2002–2012. doi: 10.1016/S0006-3495(93)81242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Taffet S., Stoner L., Delmar M., Vallano M. L., Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: the carboxyl tail length. Biophys J. 1993 May;64(5):1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo L. W., Berestecky J. M., Kanemitsu M. Y., Lau A. F. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J Biol Chem. 1995 May 26;270(21):12751–12761. doi: 10.1074/jbc.270.21.12751. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Sáez J. C., Fishman G. I., Spray D. C. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994 Jun;74(6):1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987 Jan;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S. C., Kidder G. M., Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995 Mar 24;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Risek B., Guthrie S., Kumar N., Gilula N. B. Modulation of gap junction transcript and protein expression during pregnancy in the rat. J Cell Biol. 1990 Feb;110(2):269–282. doi: 10.1083/jcb.110.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz J. E., Davis L. M., Darrow B. J., Kanter H. L., Laing J. G., Beyer E. C. The molecular basis of anisotropy: role of gap junctions. J Cardiovasc Electrophysiol. 1995 Jun;6(6):498–510. doi: 10.1111/j.1540-8167.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Bennett M. V. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Burt J. M. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990 Feb;258(2 Pt 1):C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Ginzberg R. D., Morales E. A., Gatmaitan Z., Arias I. M. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes. J Cell Biol. 1986 Jul;103(1):135–144. doi: 10.1083/jcb.103.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer K. A. The gap junction proteins beta 1-connexin (connexin-32) and beta 2-connexin (connexin-26) can form heteromeric hemichannels. J Biol Chem. 1995 Mar 24;270(12):6768–6772. [PubMed] [Google Scholar]
- Stauffer K. A., Unwin N. Structure of gap junction channels. Semin Cell Biol. 1992 Feb;3(1):17–20. doi: 10.1016/s1043-4682(10)80004-2. [DOI] [PubMed] [Google Scholar]
- Suchyna T. M., Xu L. X., Gao F., Fourtner C. R., Nicholson B. J. Identification of a proline residue as a transduction element involved in voltage gating of gap junctions. Nature. 1993 Oct 28;365(6449):847–849. doi: 10.1038/365847a0. [DOI] [PubMed] [Google Scholar]
- Tabb T., Thilander G., Grover A., Hertzberg E., Garfield R. An immunochemical and immunocytologic study of the increase in myometrial gap junctions (and connexin 43) in rats and humans during pregnancy. Am J Obstet Gynecol. 1992 Aug;167(2):559–567. doi: 10.1016/s0002-9378(11)91453-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L., Ottolia M., Stefani E., Latorre R. Structural determinants in the interaction of Shaker inactivating peptide and a Ca(2+)-activated K+ channel. Biochemistry. 1994 Jun 14;33(23):7220–7228. doi: 10.1021/bi00189a026. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Two configurations of a channel-forming membrane protein. Nature. 1984 Feb 16;307(5952):609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Gating properties of connexin32 cell-cell channels and their mutants expressed in Xenopus oocytes. Proc Biol Sci. 1991 Jan 22;243(1306):5–11. doi: 10.1098/rspb.1991.0002. [DOI] [PubMed] [Google Scholar]
- White T. W., Bruzzone R., Wolfram S., Paul D. L., Goodenough D. A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994 May;125(4):879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. W., Paul D. L., Goodenough D. A., Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995 Apr;6(4):459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Ochalski A., Hertzberg E. L., Nagy J. I. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J Comp Neurol. 1990 Dec 22;302(4):853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. The topological structure of connexin 26 and its distribution compared to connexin 32 in hepatic gap junctions. J Membr Biol. 1994 Apr;139(1):15–29. doi: 10.1007/BF00232671. [DOI] [PubMed] [Google Scholar]


