Abstract
Remyelination in the adult central nervous system has been demonstrated in different experimental models of demyelinating diseases. However, there is no clear evidence that remyelination occurs in multiple sclerosis, the most diffuse demyelinating disease. In this article, we explore the possibility of promoting myelination in experimental allergic encephalomyelitis, a widely used experimental model of multiple sclerosis, by recruiting progenitors and channeling them into oligodendroglial lineage through administration of thyroid hormone (T4). A large number of proliferating cells (BrdUrd uptake and Ki67-IR) and the expression of markers for undifferentiated precursors (nestin) increased in the subventricular zone and spinal cord of experimental allergic encephalomyelitis animals. T4 administration reduces proliferation and nestin-immunoreactivity and up-regulates expression of markers for oligodendrocyte progenitors [polysialylated-neural cell adhesion molecule (PSA-NCAM), O4, A2B5] and mature oligodendrocytes (myelin basic protein) in the spinal cord, olfactory bulb, and subventricular zone.
Multiple sclerosis is an inflammatory-autoimmune disease with multiple foci of demyelination in the central nervous system (CNS) in the chronic stage of the disease (1). Although there is evidence of remyelination in different experimental conditions in the adult CNS (2), remyelination attempts observed in early plaques in multiple sclerosis are not followed by repair of the lesion (3). The reason for this is still unknown. A significant number of oligodendrocyte precursor cells were found in early lesions in multiple sclerosis tissue (4, 5), although they were in a relatively quiescent state in chronic lesions (6).
One crucial question to be answered in the evaluation of repair potentiality in this demyelinating disease regards the capability of a substantial number of oligodendrocyte precursors to differentiate and remyelinate within the brain and spinal cord. Oligodendrocyte precursors are disseminated within the white and gray matter of the adult CNS, but they also can be generated from stem cells present in different areas of the CNS (2, 7). Consequently, a potentially unlimited number of myelinating cells could be recruited in the adult CNS. Multipotential precursors have been demonstrated in the subventricular zone (SVZ) of the lateral ventricle, the dentate gyrus of the hippocampus (8–10), and the spinal cord (11, 12), where ependymal cells are able to differentiate in vitro into adult cells (13).
Therefore, the possibility of recruiting populations of precursor cells to replace degenerating neurons or glial cells through an in vitro or in vivo approach has become very attractive. Our previous results demonstrated that the proliferative rate and expression of nestin, a marker for neuroepithelial cells, is up-regulated in the SVZ (14) during experimental allergic encephalomyelitis (EAE). Moreover, exogenous administration of thyroid hormone in adult rats modifies proliferation and expression of stem cell markers in the SVZ (15). We therefore explored the possibility of stimulating endogenous repair potential during EAE by activating progenitors in the SVZ and spinal cord through exogenous administration of molecules known to be key signals for oligodendrocyte development. We focused on brief exposure to thyroid hormone (thyroxine, T4) during acute phase of EAE. T4 is known to induce more oligodendrocytes to form from multipotent neural stem cells (16) and to influence several stages of oligodendrocyte development (17, 18). We analyzed proliferation (Ki67-immunoreactivity and BrdUrd uptake) and expression of markers for neuroectodermal cells (nestin), oligodendroglial-committed precursor [nestin and polysialylated-neural cell adhesion molecule (PSA-NCAM)], oligodendrocyte progenitors at different stages of differentiation (A2B5 and platelet-derived growth factor receptor α (PDGFRα) as progenitor and preoligodendrocyte; O4 as preoligodendrocyte and immature oligodendrocyte; and O4 and myelin basic protein (MBP) as nonmyelinating and myelinating mature oligodendrocyte (see ref. 7) in spinal cord, SVZ, and olfactory bulb. We found that this treatment reduces the number of proliferating cells in SVZ and spinal cord and favors precursor olgodendrocyte differentiation in EAE rats. The expression of markers for undifferentiated precursors (nestin) actually decreases in EAE animals treated with T4. T4 treatment induces the onset of O4-positive cells and up-regulation of A2B5-immunoreactivity. Also mRNA for PDGFRα is up-regulated by T4 treatment in EAE animals. PDGF is a powerful inductor of oligodendrocyte lineage from stem cells. More importantly, MBP, which decreases in EAE animals, is significantly up-regulated in T4-treated EAE animals.
Materials and Methods
Animals and Treatment.
Female, pathogen-free Lewis rats (Charles River Breeding Laboratories) were used. A group of rats was sensitized with a medium containing 0.15 g/ml guinea pig spinal cord tissue in complete Freund's adjuvant (CFA, Sigma) (50% vol/vol) to which 5 mg/ml of heat-inactivated Mycobacterium (Difco H37Ra) was added. Uninjected and CFA-injected rats were used as controls. When animals displayed severe signs of force deficit (12–14 days after immunization), some of the animals were treated with T4. Each animal received 0.2 mg T4 s.c. on days 1, 3, and 5 and was killed on day 6. All animal protocols described here were carried out according to the European Community Council Directives of November 24, 1986 (86/609/EEC) and approved by our intramural committee and Ministero Istruzione, Universitá, Ricerca, in compliance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Proliferation Rate.
For proliferation studies, five animals/group were analyzed. Proliferation rate was investigated by BrdUrd uptake. A single bolus of BrdUrd (50 mg/kg) was administered i.p. Animals were then killed 24 h after injection, and sections (20 μm thick) were treated as follows (12): 50% formamide in 2×SSC at 65°C for 2 h; 2×SSC at room temperature for 15 min; 2 M HCl at 37°C for 30 min; 0.1 M borate buffer, pH 8.5 at room temperature for 10 min; 0.1 M TBS, pH 7.5 at room temperature, six changes, 5 min each. Sections were then incubated overnight at 4°C in the presence of monoclonal anti-BrdUrd (Roche Molecular Biochemicals) diluted 1:100 in 0.1 M TBS, pH 7.5. Sections were then washed in TBS, a FITC-conjugated anti-mouse antibody was applied, sections were washed again, and mounted or processed for double-staining immunohistochemistry.
Immunohistochemistry.
For immunocytochemistry studies, 3–5 animals/group were analyzed. Brains and spinal cord were fixed (4% paraformaldehyde) and indirect immunofluorescence and avidine-biotine (ABC) procedures were used to visualize the following antigens: nestin (monoclonal, PharMingen), PSA-NCAM (PharMingen), glial fibrillary acidic protein (monoclonal, Chemicon; polyclonal, Eurodiagnostica, Bologna, Italy), OX42 (monoclonal, Serotec), O4 (monoclonal MAB345, Chemicon), A2B5 (monoclonal MAB312R, Chemicon), and Ki67 (rabbit polyclonal, NovoCastra, Newcastle, U.K.). Staining specificity was assessed by in vitro overnight preincubation of the antiserum with the respective antigen (100 mg/ml). This treatment prevented staining. Sections were collected from the lumbar tract of the spinal cord, the olfactory bulb (6.7 mm from the bregma, according to the Paxinos and Watson stereotaxic atlas of the brain, ref. 19), and SVZ samples at level 1.7 from the bregma. For the ABC technique, vibratome free-floating sections were used and diaminobenzidine (0.5 mg/ml in 0.1 M Tris⋅HCl, pH 7.5 + 0.03% H2O2) was added to detect the immunocomplex. For double-labeling experiments, 4-chloro-1-naphthol was used to detect the second antigen. Alternatively, cryostat sections were processed for indirect immunofluorescence. For double experiments with BrdUrd, the immunocytochemical procedure was applied after BrdUrd visualization. Confocal laser scan microscopy [Olympus FV500, Ar/HeNe (G) lasers and appropriate filters for green and red fluorescence] was used to sample nestin-immunoreactivity in the SVZ. Briefly, optical sectioning was performed every 0.375 μm, and a final image merging 10 sections was generated. These images then were analyzed by using the image proplus software for color images to assess the area covered by nestin-immunoreactivity. For this purpose, an automatic thresholding procedure in the range of the red signal was established and a standard area (see Fig. 4B) was analyzed to determine % area covered by the immunoreactive signal. In each animal, three sections were bilaterally analyzed, and the mean value then was used for statistical analysis.
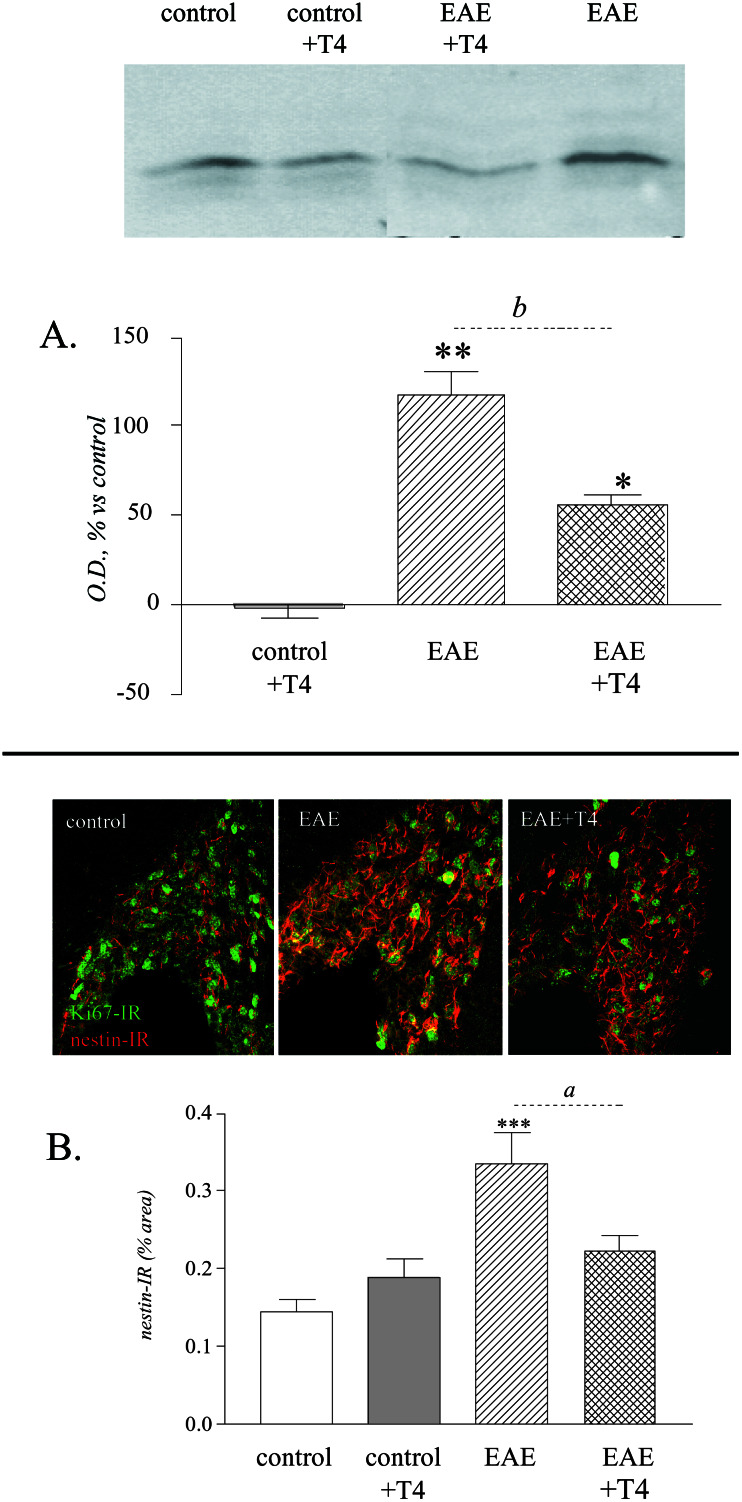
Figure 4.
T4 treatment reduces proliferation and expression of precursor-associated markers in the SVZ of EAE animals. (A) Western blot and quantitative analysis of Ki67 protein in the SVZ. T4 treatment reduces the strong up-regulation of Ki67 protein in the SVZ of EAE-affected rats. (B) Confocal images of codistribution of Ki67-IR and nestin-immunoreactivity in the SVZ of control, EAE, and EAE T4-treated rats. Semiquantitative analysis has been performed on images merging 10 sections, 0.375 μm thick. Images and quantitative analysis illustrates up-regulation of nestin expression in EAE, and its reduction by T4 treatment. (Magnification: ×250.) Statistical analysis: Anova and Dunnett test, *, P < 0.05, **, P < 0.01; Student's t test for T4 effect, a, P < 0.05, b, P < 0.01.
NADPH-Diaphorase Histochemistry.
NADPH-diaphorase activity was rendered visible by incubating cryostat sections in 0.1 M phosphate buffer, pH 8.0, containing 1 mM NADPH (Sigma), 2 mM nitro blue tetrazolium (Sigma), and 0.3% Triton X-100 at 37°C.
Western Blotting Procedure.
For Western blot experiments, five animals/group were used. To study SVZ, a block of forebrain tissue was obtained by using the brain matrix device (World Precision Instruments, Sarasota, FL) between levels +2.2 and +1.2 from bregma, according to the Paxinos and Watson stereotaxic atlas (19). Tissue around the lateral ventricle was then bilaterally dissected out by a microknife under stereomicroscopy. Tissue homogenates from the lumbar tract of the spinal cord and SVZ were prepared by using a 10 mM Hepes/1 mM DTT, pH 7.5 lysing buffer, containing a protease inhibitor mixture (Sigma). Equal amounts of protein were separated in 15% SDS-polyacrylamide gels and electroblotted to nitrocellulose membranes. To block nonspecific protein binding sites, filters were incubated with blocking solution (Pierce) for 2 h at room temperature, and primary antibodies were then incubated overnight at 4°C. After washing for 1 h with TTBS (TBS/0.05% Tween-20), filters were incubated with secondary antibodies for 30 min at room temperature and washed again for another hour. The antibodies used were: rabbit polyclonal anti-MBP 1:2,000 (ref. 20, Dako), rabbit polyclonal anti-Ki67 1:5,000 (NovoCastra), and anti-rabbit antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology). Finally, proteins were detected by using an ECL chemioluminescent kit (Pierce) and exposed to radiographic film. Densitometric analysis was performed by using the AIS Imaging System (Ontario, Canada), and results are shown as mean ± SEM of five different blots per group.
Reverse Transcription–PCR.
For reverse transcription–PCR experiments, five animals/group were analyzed. mRNA was prepared by using an mRNA isolation kit (Roche Molecular Biochemicals). mRNA (0.25 μg) was reverse-transcripted (200 units of Moloney murine leukemia virus reverse transcriptase, GIBCO/BRL) in the presence of 50 μM p(dN)6 random primer (Roche Molecular Biochemicals) for 50 min at 37°C. The first-stand cDNA obtained was used to perform the PCR assays to determine the levels of mRNA encoding the PDGFRα. PCR analysis of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a control for cDNA quantities used as templates for PCR assays. Oligonucleotides used as specific primers were: PDGFRα sense, 5′-AGATAGCTTCATGAGCCGAC-3′; PDGFRα antisense, 5′-GGAACAGGGTCAATGTCTGG-3′ (21); GAPDH sense, 5′-TCCATGACAACTTTGGCATCGTGG-3′; and GAPDH antisense, 5′-GTTGCTGTTGAAGTCACAGGAGAC-3′ (22). Amplifications were performed by using 0.05 units/μl of TaqDNA polymerase (Sigma), and the cycling parameters of amplification were as follows: 95°C 1 min, 60°C 1 min 30 s, 72°C 1 min 30 s, 30 cycles for PDGFRα, obtaining a 1,016-bp fragment; and 95°C 1 min, 60°C 30 s, 72°C 30 s, 25 cycles for GAPDH, obtaining a 376-bp fragment. PCR products were electrophoresed on agarose gels, stained with ethidium bromide, and visualized under UV light.
Nerve Growth Factor (NGF) Assays.
For NGF measurement, five rats/group were killed by decapitation under ketamine anesthesia. NGF levels were measured by using a two-site immunoenzymatic assay, according to Weskamp and Otten (23), and modified (24, 25). Specificity for NGF assay was also assessed by using recombinant human brain-derived neurotrophic factor (Genentech, San Francisco). Data were represented as pg/g wet weight and all assays were performed in duplicate.
Statistical Analysis.
Descriptive analysis data were expressed as mean ± SE. Statistical analysis was carried out by using one-way ANOVA and Dunnett's test to compare the different experimental groups. Student's t test (GraphPad prism software, San Diego) was also used when appropriate. Probability level was set at 5% (two-tailed).
Results
Markers for Cell Proliferation and Stem Cells in Spinal Cord During EAE.
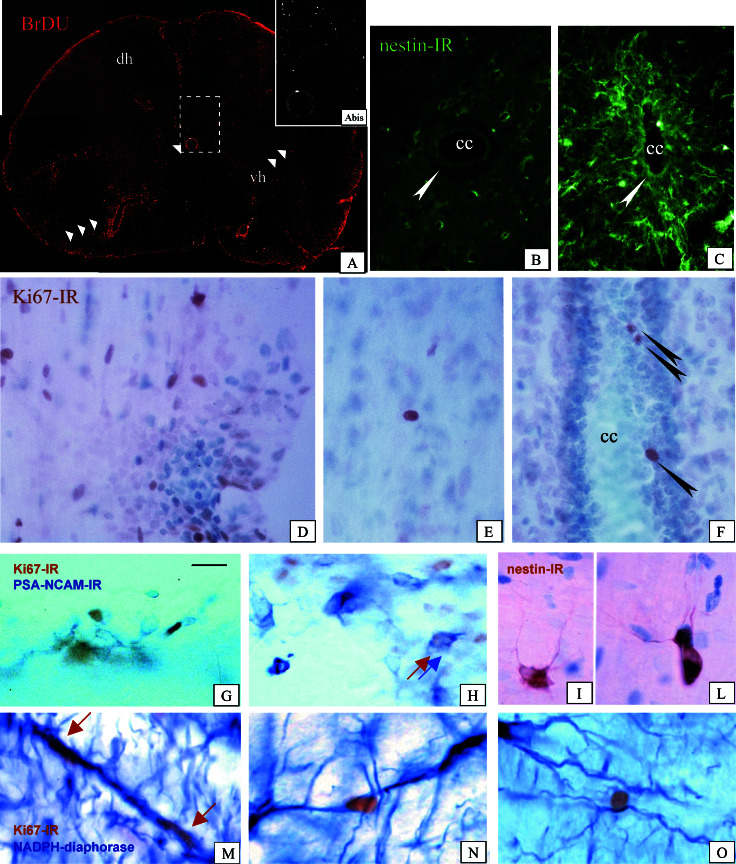
Proliferating nuclei were visualized by using both BrdUrd uptake and an antibody against the cell cycle-associated antigen Ki67. A very high number of dividing cells was found in EAE animals, not only in SVZ, but also in the white and gray matter in brain and spinal cord and between ependymal cells in the central canal (Fig. 1 A and D–F). Ki67- and BrdUrd-positive cells were associated with inflammatory foci (Fig. 1D), but also isolated far from blood vessels and inflammation cellular aggregates (Fig. 1E). Double-labeling experiments revealed that only a few of them expressed OX42 (not shown), a marker for macrophage/microglial lineage, which is strongly expressed in the acute stage of EAE, or glial fibrillary acidic protein positivity, associated with astrocytes. Moreover, cycling cells were also found between ependymal cells in the central canal (Fig. 1 A and F). In the spinal cord, nestin-immunoreactivity was found in the ependymal layer of the central canal (Fig. 1 B and C), in reactive astrocytes, as identified by double-labeling immunostaining for glial fibrillary acidic protein, and in small, finely branched intraparenchymal cells (Fig. 1 I and L). PSA-NCAM-immunore-active cells were also found in the spinal cord. These cells were grouped in small aggregates, and double-labeling experiments using antibody against the proliferation-associated marker Ki67 revealed that they were newly generated cells (Fig. 1 G and H). Ki67-positive nuclei were also associated with NADPH diaphorase-reactive processes tangentially directed between the central canal and the external surface of the spinal cord (Fig. 1 M–O) as well as with nestin-positive elements (not shown).
Figure 1.
(A) Immunocytochemical detection of proliferating, BrdUrd-positive cells in the lumbar tract of the spinal cord of EAE-affected rat. One arrow indicates a positive element between ependymal cells in the central canal; two arrows indicate elements having spread in the white matter; three arrows indicate a dense, perivascular aggregate of positive cells. (Inset) Higher magnification. (B and C) Nestin-immunoreactivity in the areas around the central canal in control (B) and EAE-affected (C) animals. Arrows indicate the ependymal cells. A dramatic increase in immunostaining, also involving ependymal cells, is found in EAE animals. (D–F) Ki67-positive nuclei in EAE, associated with an inflammatory cellular infiltrate (D) isolated in the white matter (E) and between ependymal cells (F) in the central canal of the spinal cord. (G and H) Codistribution of Ki67 nuclear positivity (brown) and PSA-NCAM membrane positivity (blue) in the spinal cord of EAE-affected rats. (I and L) Small, finely branched nestin-immunoreactive cells in the spinal cord of EAE animals. (M–O) Close association between Ki67-positive nuclei and NADPH-reactive process extending between the ependymal layer and the pial surface in the spinal cord of EAE rat. cc, central canal; dh, dorsal horn; vh, ventral horn. (Magnifications: A, ×25; A Inset, ×50; B and C, ×250; D–O, ×400.)
Thyroid Hormone Administration, Cell Proliferation, and Oligodendrocyte Lineage in Spinal Cord and SVZ During EAE.
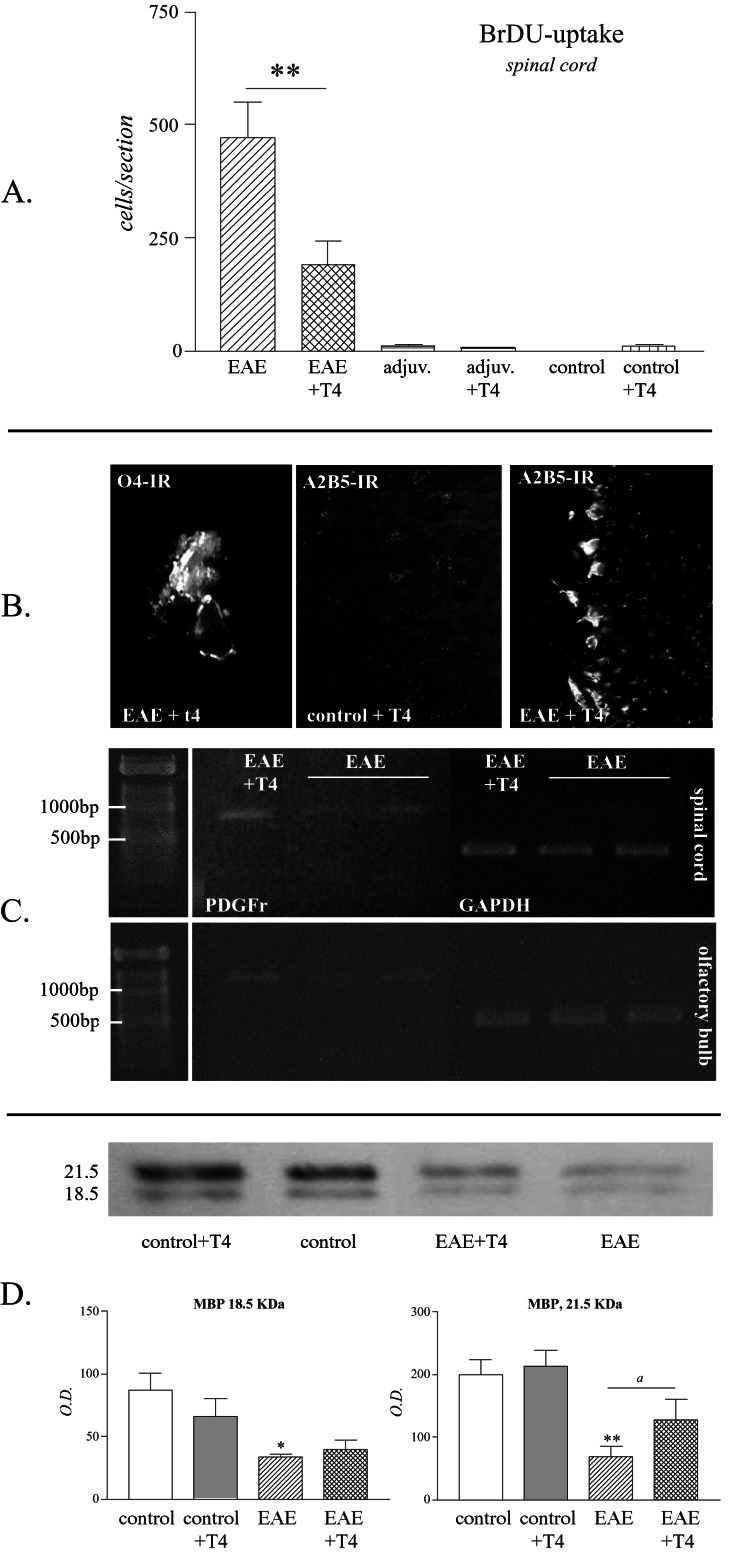
The proliferation rate in lumbar spinal cord was measured by counting the number of BrdUrd-positive cells in five sections in five animals in each experimental group. In EAE animals, up to 500 positive cells/section were counted (Fig. 2A), whereas only single cells were found in control groups. T4 treatment induced a significant decrease in the number of BrdUrd-positive cells in the spinal cord of EAE-affected animals. Moreover, only in EAE T4-treated rats were we able to detect O4-positive cells in the spinal cord; they were small, with 2–3 short branches (Fig. 2B). We also observed a strong up-regulation of A2B5-IR in the olfactory bulb in the same experimental conditions (Fig. 2B). We then investigated PDGFRα expression mRNA, which is slightly but clearly up-regulated in the spinal cord and olfactory bulb of T4-treated EAE animals compared with EAE rats (Fig. 2C). Finally, Western blot analysis of MBP reveals that both 21.5- and 18.5-kDA isoforms recognized by the antibody are severely down-regulated in EAE compared with control animals. T4 treatment, as applied in this experiment, is able to induce a significant increase in MBP in EAE, but not in control animals (Fig. 2D).
Figure 2.
T4 treatment decreases proliferation and favors oligodendroglial lineage and olygodendrocyte maturation in the spinal cord of EAE animals. (A) The histogram reports the count of BrdUrd-positive cells in the lumbar tract of the spinal cord in experimental groups. Data are means ± SE from six animals per group. Statistical analysis: Student's t test, **, P < 0.01. (B) Micrographs illustrate O4-positive cell in the spinal cord of EAE animals treated with T4 and the up-regulation of A2B5 in the same experimental condition in the olfactory bulb. (C) Reverse transcription–PCR analysis of PDGFRα mRNA in EAE and after T4 treatment in the spinal cord and olfactory bulb. (D) Western blot and quantitative analysis of 21.5- and 18.5-kDA MBP proteins. Data are means ± SE from five animals per group. Statistical analysis: Anova and Dunnett test, *, P < 0.05, **, P < 0.01; Student's t test for T4 effect, a, P < 0.05. (Magnifications: 04: ×500; A2B5: ×250.)
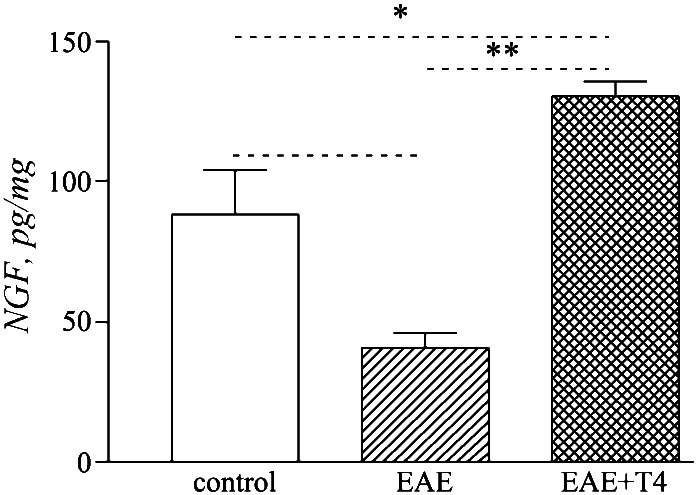
We also measured NGF content in the spinal cord. As already reported (26), NGF content dramatically decreases in the spinal cord during the acute phase of EAE. T4 treatment counteracts any fall in NGF tissue level, increasing NGF levels above that of control animals (Fig. 3). The level of NGF also increases in the control animals, but this difference does not reach statistical significance.
Figure 3.
NGF content in the spinal cord of EAE animals and after T4 treatment. Data are means ± SE from five animals per group. Statistical analysis: Anova and Dunnett test, *, P < 0.05, **, P < 0.01; Student's t test for T4 effect, a, P < 0.05.
To speculate on the possible origin of proliferating elements in the spinal cord during EAE (resident vs. recruited precursors), we also analyzed the SVZ in all experimental groups. Proliferation in the SVZ was measured by Western blotting Ki67 protein. The level of Ki67-immunoreactive protein increased during EAE and T4 treatment significantly reduced expression of this cell cycle-associated protein in EAE-affected rats (Fig. 4A). Nestin expression, which is associated with Ki67-immunoreactive elements in the SVZ as illustrated by the confocal images, increased in EAE animals and was down-regulated by T4 treatment (Fig. 4B). The slight increase observed in control animals treated with T4 is not significant.
Discussion
Thyroid hormone is essential for normal oligodendrocyte maturation and myelination (16, 27). Cellular action of thyroid hormone is mediated to thyroid hormone receptors that are part of the family of nuclear receptors, which are transcription factors capable of repressing or inducing transcription of target genes (28). There are two thyroid hormone receptor genes (α and β) each generating two isoforms (α1, α2, β1, β2) by alternative spicing. Oligodendrocyte precursor cells express α isoforms, whereas expression of β isoforms is confined to differentiated oligodendrocytes (27). Early in development, thyroid hormone functions as an instructive agent, triggering cell cycle exit (17, 18). In postmitotic oligodendrocytes, it increases morphological and functional maturation by stimulating expression of various genes, such as the myelin-oligodendrocyte glycoprotein, MBP, and glutamine synthase (17). Myelination is actually delayed in hypothyroid animals and accelerated in hyperthyroid animals (29, 30).
Thyroid Hormone Administration Decreases Proliferation and Expression of Markers Associated with Nondifferentiated Precursors During EAE.
One significant indication provided by our study is the presence of a very high number of dividing Ki67- and BrdUrd-positive nuclei in EAE animals compared with control animals, which are characterized by nestin and PSA-NCAM positivity. It is known that during the acute phase of multiple sclerosis and EAE (31) proliferating cells also belong to perivascular inflammatory cells and parenchymal glial cells having entered the cell proliferation cycle (32), even if most of them give rise to oligodendroglia (33). In our experiments, very few dividing cells in EAE animals displayed microphage/microglial characteristics as identified by OX42-immunoreactivity, whereas nestin, a marker for neuroectodermal cells (34), was strongly up-regulated in EAE. This finding suggests that there is a recruitment of newly generated precursors in the active phase of EAE. The recruitment of progenitor cells from the SVZ into the areas of demyelination has been demonstrated in chemically induced demyelination (35). These precursors seem to find a migration pathway. Indeed, Ki67-positive nuclei are closely associated with NADPH diaphorase-reactive processes, which possibly correspond to radial astrocytes (36). Processes from these cells span the white matter from the pial surface to the gray-white matter interface and could represent a migratory path, as suggested by Horner et al. (12).
Thyroid hormone reduces proliferation in EAE animals, in both spinal cord and SVZ, and also down-regulates expression for markers of nondifferentiated precursors. This effect, which is obtained by exogenous administration of three boluses of thyroid hormone, seems to mirror thyroid hormone action during development, when it acts by inducing exit from the cell cycle and entry to the differentiation process (17, 18). We observed that three boluses of T4 decreased the expression of nestin, when up-regulated in EAE, and this corresponds to cell differentiation (10, 37). The nestin gene promoter contains putative binding sites for nuclear receptors, which thyroid hormone binds in neural progenitor cells of the embryonic CNS (38). Using different olygodendrocyte lineage cells, it was shown that high levels of nestin protein are expressed in proliferating progenitors, but the protein is down-regulated in differentiated oligodendrocytes (39). Moreover, expression of PSA-NCAM on these precursors seems to coincide with restriction to a glial fate (40). Thus, thyroid hormone administered in vivo in EAE animals decreases proliferation and favors differentiation toward the oligodendroglial lineage. This is found only in EAE and not in control animals. In particular, three T4 administrations induced a slight, but insignificant, increase in control animals, whereas prolonged hyperthyroidism up-regulated nestin expression (15). We already have suggested that in EAE animals the breakdown of the blood–brain barrier exposes precursors to unique environmental conditions, perhaps bringing into the CNS molecules able to alter the balance between quiescent and active progenitors in favor of the active one (14). On the other hand, it has been suggested that inflammation itself promotes survival and migration of transplanted oligodendrocyte progenitors in the adult CNS (41).
Thyroid Hormone Administration Favors in Vivo Oligodendrocyte Lineage and Maturation During EAE.
Generation of oligodendrocyte precursors (31, 42) and the presence of occasional myelin-forming oligodendrocyte in EAE have been described (43). Here we report that thyroid hormone administration significantly improves this process, as indicated by increased expression of markers for different stages of oligodendrocyte differentiation and maturation, i.e., O4, A2B5, and PDGFRα, and this corresponds to in vitro studies (44, 45). Oligodendrocyte progenitors arising postnatally from the SVZ can migrate long distances away from this zone (46), even if the nature of attractive molecules is still unclear. However, the expression of PSA-NCAM seems to allows cells to respond to extracellular cues in appropriate time and space (47). It is also known that the capability to differentiate into myelin-forming oligodendrocytes is intrinsic to the lineage oligodendrocyte progenitors (48) and a myelin-gene induction is observed in vivo by neuronal contact (49).
Moreover, we indicated that a regulation of myelin-forming proteins is possible in EAE through T4 administration. MBP is a target gene for thyroid hormone receptor (50), but our data support the idea that MBP up-regulation is caused by newly formed oligodendrocytes and not only by a direct regulation of MBP gene expression. In fact, this effect is not observed in control animals. Moreover, re-expression of the 21.5-kDa isoform, which appears early in embryogenesis, is associated with myelogenesis (51). On the other hand, magnetic resonance spectroscopy study has shown that thyroxine therapy can reverse abnormal myelination in congenital hypothyroidism (52).
Thyroid Hormone Restores NGF Levels in the Spinal Cord of EAE-Affected Animals.
We previously reported that NGF content in the spinal cord drops in the acute phase of EAE (26). Here we indicate that T4 administration restores NGF to control animal content. It is known that under physiological conditions thyroid hormone regulates endogenous synthesis of NGF (53–55), and a single injection of T4 is able to raise NGF content in the brain (56). NGF administration ameliorates the clinical course of EAE in marmoset (57), and the protection of oligodendrocyte from cell death-inducing agents could be part of this effect. In fact, NGF protects oligodendrocyte from injury induced by tumor necrosis factor (58), a proinflammatory cytokine strongly implicated in the pathogenesis of EAE (59). There is evidence that oligodendrocytes are receptive to the action of NGF, that NGF protects oligodendrocytes from cell death and NGF prevents and/or reduces the neuro pathological events in EAE marmoset brain. Therefore, NGF may be implicated in oligodendrocyte protection. A possible function of the enhanced NGF after T4 administration is the regulation of endogenous brain NGF synthesis, instead of use traumatic exogenous administration.
Conclusions.
Very little is known about spontaneous remyelination and molecules able to affect it. Moreover, we are still far from having demonstrated that thyroid hormone promotes remyelination in demyelinating diseases, but in this study we further suggest that under certain conditions it is possible to influence endogenous precursors by exogenous administration of appropriate signaling molecules.
Acknowledgments
We are grateful to Prof. Rita Levi-Montalcini for her invaluable support, suggestions, and assistance. Technical assistance of Nadia De Sordi and Stefania Pirondi is gratefully acknowledged. This work was supported by Telethon (Grant 1336, to L.C.) and Fondazione Cassa di Risparmio in Bologna, Bologna, Italy (to L.C. and L.A.).
Abbreviations
- CNS
central nervous system
- SVZ
subventricular zone
- EAE
experimental allergic encephalomyelitis
- PSA-NCAM
polysialylated-neural cell adhesion molecule
- PDGF
platelet-derived growth factor
- PDGFRα
PDGF receptor α
- MBP
myelin basic protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NGF
nerve growth factor
Footnotes
To whom reprint requests should be addressed. E-mail: lcalza@vet.unibo.it.
References
- 1.Scolding N J, Zajicek J P, Wood N, Compston D A S. Prog Neurobiol. 1994;43:143–173. doi: 10.1016/0301-0082(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Levine J L, Reynolds R, Fawcett J W. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 3.Perry V H. Brain. 1998;121:2219–2220. doi: 10.1093/brain/121.12.2219. [DOI] [PubMed] [Google Scholar]
- 4.Bruck W, Schmied M, Suchanek G, Bruck Y, Breitschopf H, Poser S, Piddlesden S, Lassmann H. Ann Neurol. 1994;35:65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- 5.Scolding N, Franklin R, Stevens S, Heldin C-H, Compston A, Newcombe J. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- 6.Wolswijk G. Brain. 1998;123:105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Baumann N, Pham-Dinh D. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 10.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 11.Kehl L J, Fairbanks C A, Laughlin T M, Wilcox G L. Science. 1997;276:586–589. doi: 10.1126/science.276.5312.586. [DOI] [PubMed] [Google Scholar]
- 12.Horner P J, Power A E, Kempermann G, Kuhn H G, Palmer T D, Winkler J, Thai L J, Gage F. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson C B, Momma S, Clarke D L, Risling M, Lendahl U, Frisen J. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 14.Calzà L, Giardino L, Pozza M, Bettelli C, Micera A. Proc Natl Acad Sci USA. 1998;95:3209–3214. doi: 10.1073/pnas.95.6.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardino L, Bettelli C, Calzà L. Neurosci Lett. 2000;295:17–20. doi: 10.1016/s0304-3940(00)01580-9. [DOI] [PubMed] [Google Scholar]
- 16.Rogister B, Ben-Hur T, Dobois-Dalcq M. Mol Cell Neurosci. 1999;14:287–300. doi: 10.1006/mcne.1999.0790. [DOI] [PubMed] [Google Scholar]
- 17.Baas D, Bourbeau D, Sarlieve L L, Ittel M E, Dussault J H, Puymirat J. Glia. 1997;19:324–332. doi: 10.1002/(sici)1098-1136(199704)19:4<324::aid-glia5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Durand B, Raff M. BioEssays. 2000;22:64–71. doi: 10.1002/(SICI)1521-1878(200001)22:1<64::AID-BIES11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 20.Asakura K, Miller D J, Pease L R, Rodriguez M. J Neurosci. 1998;18:7700–7708. doi: 10.1523/JNEUROSCI.18-19-07700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K H, Bowen Pope D F, Rood R R. Mol Cell Biol. 1990;10:2237–2246. doi: 10.1128/mcb.10.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tso J Y, Sun X-H-, Kao T-H, Reece K S, Wu R. Nucleic Acid Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weskamp G, Otten U. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- 24.Bracci-Laudiero L, Aloe L, Levi-Montalcini R, Buttinelli C, Schilter D, Gillessen S, Otten U. Neurosci Lett. 1992;147:9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- 25.Tirassa P, Manni L, Stenfors C, Lundeberg T, Aloe L. J Biotechnol. 2000;84:259–272. doi: 10.1016/s0168-1656(00)00370-9. [DOI] [PubMed] [Google Scholar]
- 26.Calzà L, Giardino L, Pozza M, Micera A, Aloe L. Proc Natl Acad Sci USA. 1997;94:3368–3373. doi: 10.1073/pnas.94.7.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Pena A. J Neurobiol. 1999;40:497–512. doi: 10.1002/(sici)1097-4695(19990915)40:4<497::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Lazar M A. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 29.Dussault J H, Ruel J. Annu Rev Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Pena A, Ibarrola N, Iniguez M A, Munoz A, Bemal J. J Clin Invest. 1993;91:812–818. doi: 10.1172/JCI116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds R, Cenci di Bello I, Dawson M, Levine J. Prog Brain Res. 2001;132:165–174. doi: 10.1016/s0079-6123(01)32073-3. [DOI] [PubMed] [Google Scholar]
- 32.Dowling P, Husar W, Menonna J, Donnenfeld H, Cook S, Sidhu M. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- 33.Solanky M, Maede Y, Ming X, Husar W, Li W, Cook S, Dowling P. J Neurosci Res. 2001;65:308–317. doi: 10.1002/jnr.1155. [DOI] [PubMed] [Google Scholar]
- 34.Almazan G, Vela M, Molina-Holgado E, Guaza C. Microsc Res Tech. 2001;52:753–765. doi: 10.1002/jemt.1060. [DOI] [PubMed] [Google Scholar]
- 35.Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Baron-Van Evercooren A. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 36.Liuzzi F J, Miller R H. Brain Res. 1987;403:385–388. doi: 10.1016/0006-8993(87)90081-3. [DOI] [PubMed] [Google Scholar]
- 37.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson A, Reynolds B. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lezoualch F, Seugnet I, Monnier A L, Ghysdael J, Behr J P, Demeneix B A. J Biol Chem. 1995;270:12100–12108. doi: 10.1074/jbc.270.20.12100. [DOI] [PubMed] [Google Scholar]
- 39.Gallo V, Armstrong R C. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. J Neurosci. 1998;18:5777–5788. doi: 10.1523/JNEUROSCI.18-15-05777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tourbah A, Linninton C, Bachelin C, Avellana-Adalid V, Wekerle H, Baron-Van Evercooren A. J Neurosci Res. 1997;50:853–861. doi: 10.1002/(SICI)1097-4547(19971201)50:5<853::AID-JNR21>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Di Bello I C, Dawson M R, Levine J M, Reynolds R. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen K B, Pender M P. Acta Neuropathol. 1999;98:39–47. doi: 10.1007/s004010051049. [DOI] [PubMed] [Google Scholar]
- 44.Thompson C C, Potter G B. Cereb Cortex. 2000;10:939–945. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- 45.Murray K, Dubois-Dalcq M. J Neurosci Res. 1997;50:146–156. doi: 10.1002/(SICI)1097-4547(19971015)50:2<146::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds R, Wilkin G P. Development (Cambridge, UK) 1988;102:409–425. doi: 10.1242/dev.102.2.409. [DOI] [PubMed] [Google Scholar]
- 47.Rutishauser U, Landmesser L. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- 48.Temple S, Raff M. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 49.Kidd G J, Hauer P E, Trapp B D. J Neurosci Res. 1990;26:409–418. doi: 10.1002/jnr.490260403. [DOI] [PubMed] [Google Scholar]
- 50.Farsetti A, Mitsubashi T, Desvergne B, Robbins J, Nikodem V M. J Biol Chem. 1991;266:23226–23232. [PubMed] [Google Scholar]
- 51.Capello E, Voskuhl R R, McFarland H F, Raine C S. Ann Neurol. 1997;41:797–805. doi: 10.1002/ana.410410616. [DOI] [PubMed] [Google Scholar]
- 52.Jagannathan N R, Tandon N, Raghunathan P, Kochupillai N. Brain Res Dev Brain Res. 1998;109:179–186. doi: 10.1016/s0165-3806(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 53.Calzà L, Giardino L, Ceccatelli S, Hokfelt T. Eur J Neurosci. 1996;8:1873–1881. doi: 10.1111/j.1460-9568.1996.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 54.Calzà L, Giardino L, Aloe L. Exp Neurol. 1997;143:196–206. doi: 10.1006/exnr.1996.6361. [DOI] [PubMed] [Google Scholar]
- 55.Calzà L, Giardino L, Aloe L. Mol Brain Res. 1997;51:60–68. doi: 10.1016/s0169-328x(97)00213-1. [DOI] [PubMed] [Google Scholar]
- 56.Walker P, Weichsel M E, Jr, Fisher D A, Guo S M, Fisher D A. Science. 1979;204:427–429. doi: 10.1126/science.441732. [DOI] [PubMed] [Google Scholar]
- 57.Villoslada P, Hauser S L, Bartke I, Unger J, Heald N, Rosenberg D, Cheung S W, Mobley W C, Fisher S, Genain C P. J Exp Med. 2000;191:1799–1806. doi: 10.1084/jem.191.10.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takano R, Hisahara S, Namikawa K, Kiyama H, Okano H, Miura M. J Biol Chem. 2000;275:16360–16365. doi: 10.1074/jbc.M910419199. [DOI] [PubMed] [Google Scholar]
- 59.Hohlfeld R. Mult Scler. 1996;1:376–378. doi: 10.1177/135245859600100619. [DOI] [PubMed] [Google Scholar]