Abstract
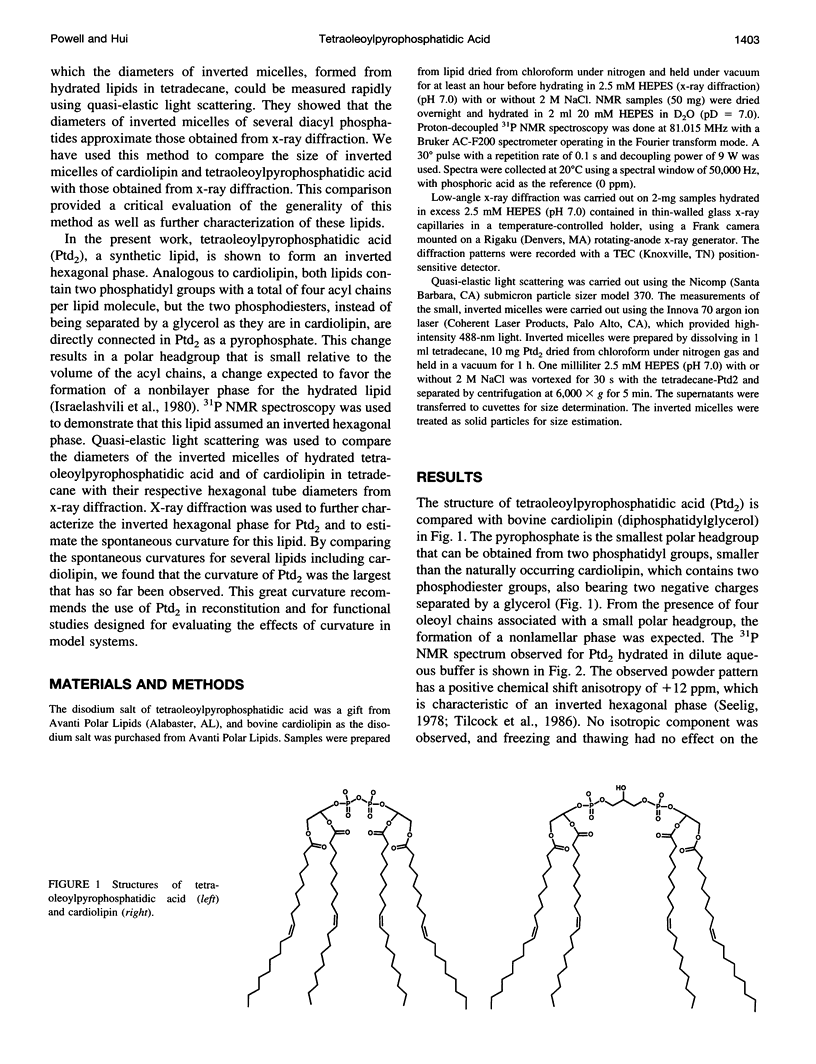
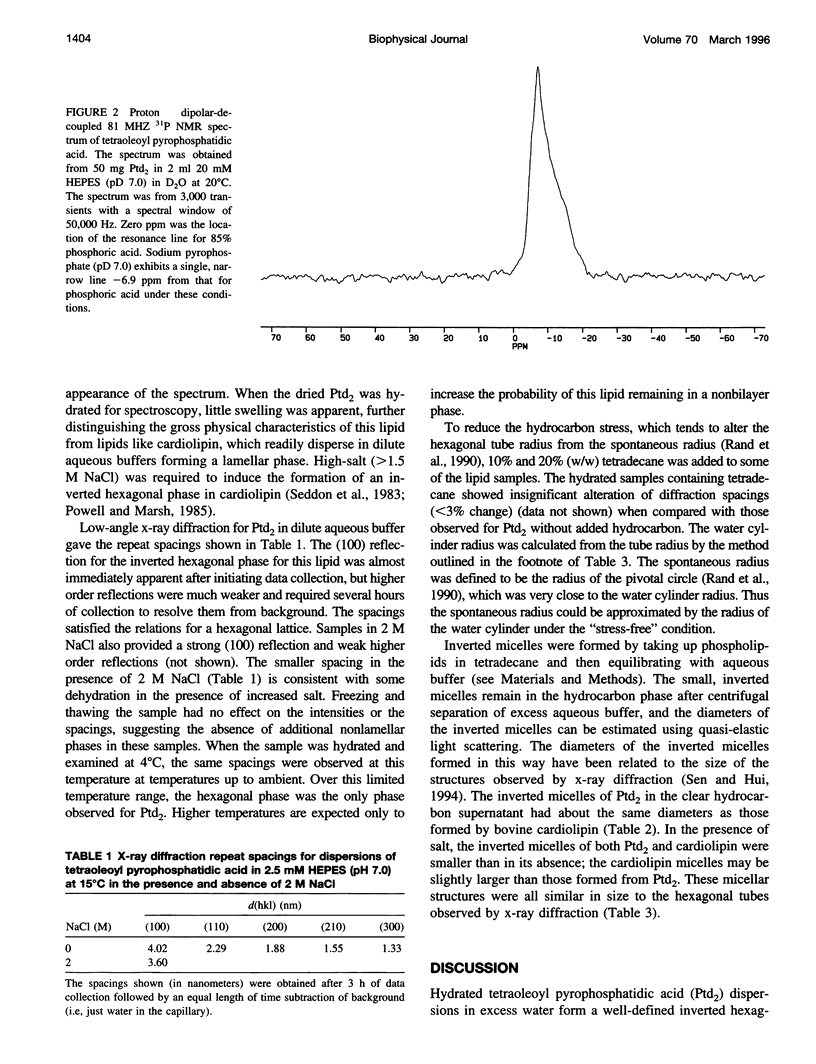
Tetraoleoylpyrophosphatidic acid (bis phosphatidic acid), when hydrated in aqueous buffer, was shown to form an inverted hexagonal phase using 31P NMR. Low-angle x-ray diffraction provided verification of the formation of this phase in dilute aqueous buffer and in 2 M NaCl and permitted comparison of the tube diameter with that of cardiolipin in 2 M NaCl. By using the water cylinder diameters for tetraoleoylpyrophosphatidic acid, bovine cardiolipin, chloroplast monogalactosyl-diglyceride, and dioleoyl phosphatidylethanolamine as a means of estimating the spontaneous curvatures, tetraoleoylpyro-phosphatidic acid was shown to exhibit the greatest curvature of any of the above lipids, equaled only by the calcium salt of cardiolipin. Inverted micelles of hydrated tetraoleoylpyrophosphatidic acid and of cardiolipin in tetradecane were approximately the diameter of the inverted hexagonal tubes. A rationale is given for the differences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitch D. A., Marsh D., Powell G. L. Activation of beef-heart cytochrome c oxidase by cardiolipin and analogues of cardiolipin. Biochim Biophys Acta. 1990 Oct 24;1020(1):34–42. doi: 10.1016/0005-2728(90)90090-q. [DOI] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- De Kruijff B., Nayar R., Cullis P. R. 31P-NMR studies on phospholipid structure in membranes of intact, functionally-active, rat liver mitochondria. Biochim Biophys Acta. 1982 Jan 4;684(1):47–52. doi: 10.1016/0005-2736(82)90047-5. [DOI] [PubMed] [Google Scholar]
- Dorne A. J., Joyard J., Douce R. Do thylakoids really contain phosphatidylcholine? Proc Natl Acad Sci U S A. 1990 Jan;87(1):71–74. doi: 10.1073/pnas.87.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Nir S., Parolin M., Flanagan T. D. The role of the ganglioside GD1a as a receptor for Sendai virus. Biochemistry. 1995 Jan 24;34(3):1084–1089. doi: 10.1021/bi00003a045. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Blocked lipid exchange in bilayers and its possible influence on the shape of vesicles. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):510–515. doi: 10.1515/znc-1974-9-1010. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Taraschi T. F., Janes N. Support for the shape concept of lipid structure based on a headgroup volume approach. Biophys J. 1993 Oct;65(4):1429–1432. doi: 10.1016/S0006-3495(93)81206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum C. D., Epand R. M. Insulin receptor autophosphorylation and signaling is altered by modulation of membrane physical properties. Biochemistry. 1995 Feb 14;34(6):1815–1824. doi: 10.1021/bi00006a001. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Knowles P. F., Marsh D. Spin-label studies on the specificity of interaction of cardiolipin with beef heart cytochrome oxidase. Biochemistry. 1987 Dec 15;26(25):8138–8145. doi: 10.1021/bi00399a018. [DOI] [PubMed] [Google Scholar]
- Powell G. L., Marsh D. Polymorphic phase behavior of cardiolipin derivatives studied by 31P NMR and X-ray diffraction. Biochemistry. 1985 Jun 4;24(12):2902–2908. doi: 10.1021/bi00333a013. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972 Feb 11;255(2):484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Green J. P., Nichols B. W. The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta. 1973 Jul 18;311(4):531–544. doi: 10.1016/0005-2736(73)90128-4. [DOI] [PubMed] [Google Scholar]


