Abstract
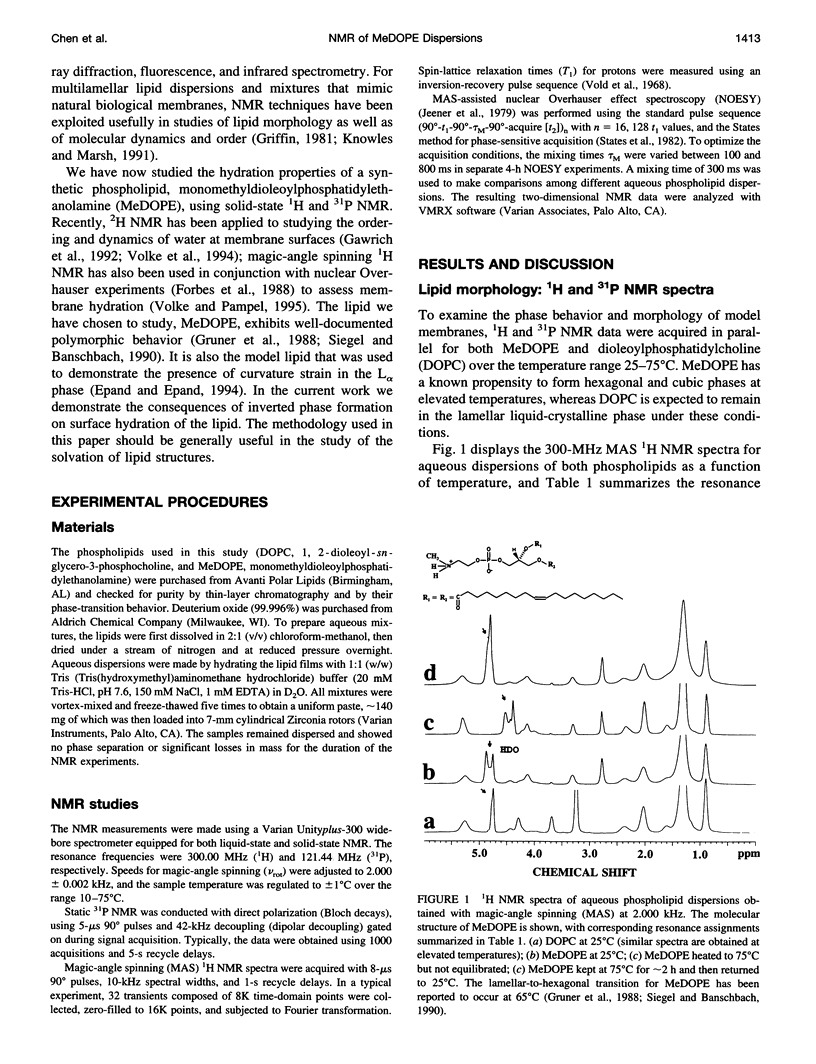
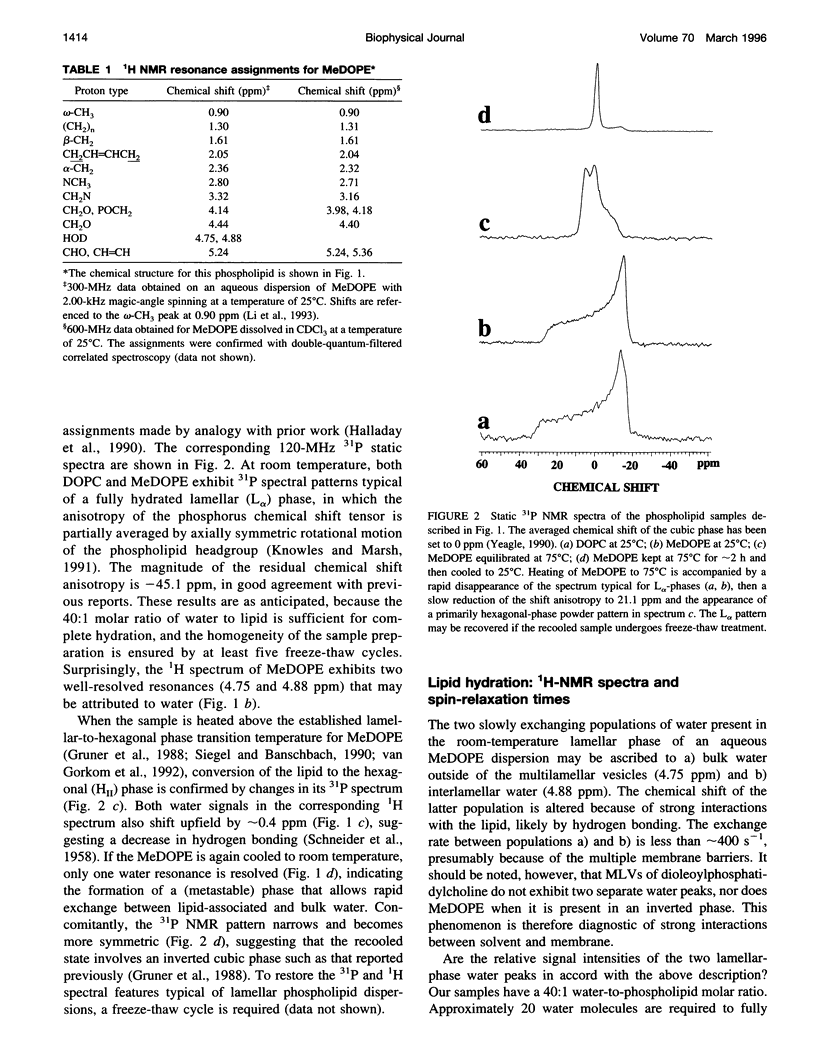
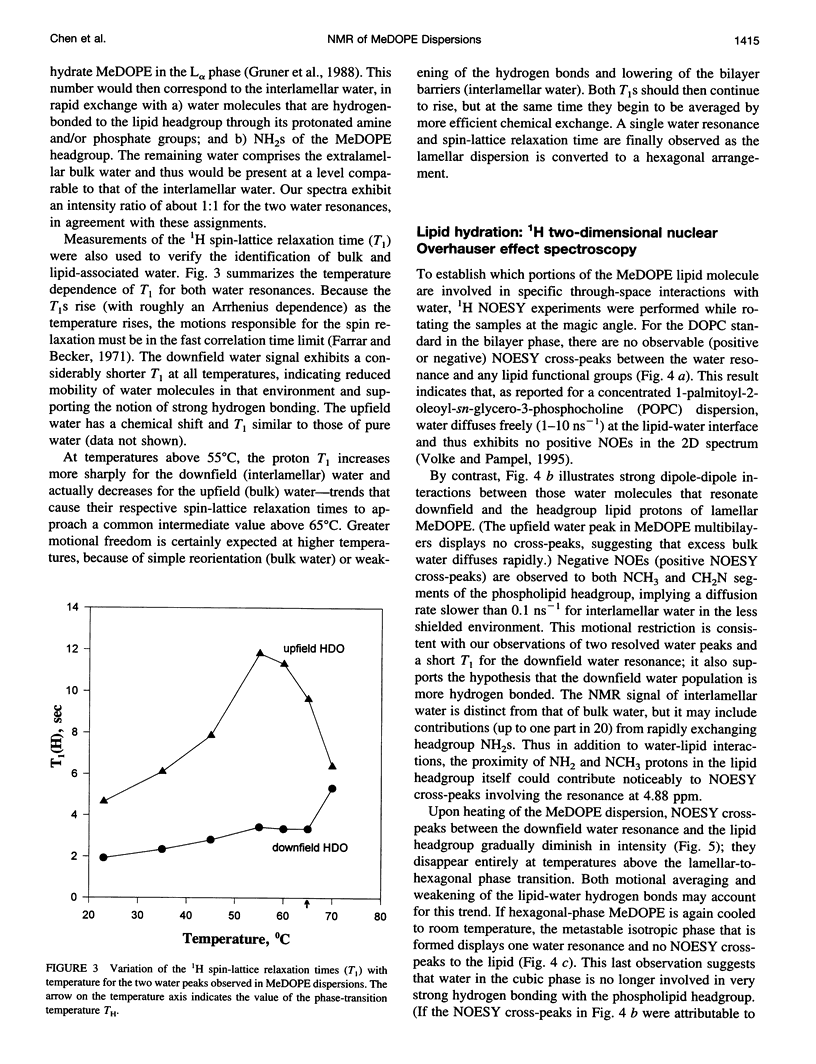
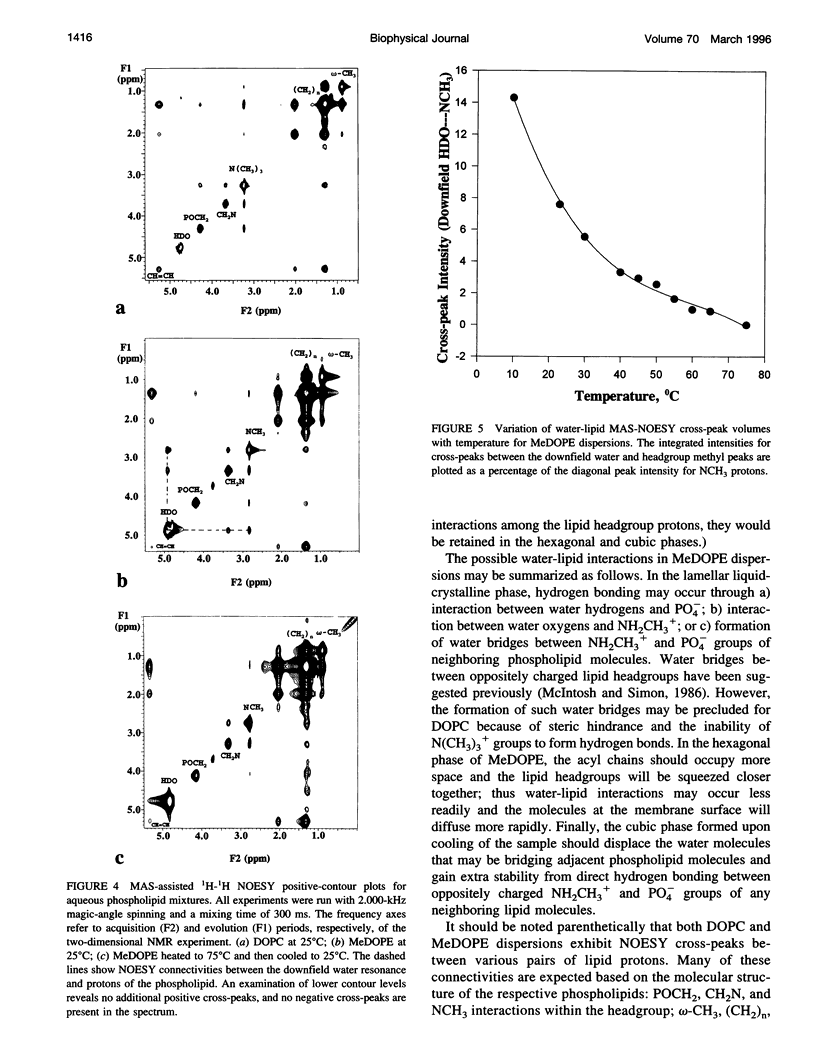
Solid-state proton nuclear magnetic resonance has been used to examine surface hydration in suspensions of monomethyldioleoylphosphatidylethanolamine (MeDOPE). The magic-angle spinning (MAS) 1H spectra for aqueous suspensions of MeDOPE in the L alpha phase exhibited two resonances of roughly equal intensity that could be ascribed to water protons, but both their spin-lattice relaxation times and chemical shifts converged upon conversion to the hexagonal phase. Only a single water peak was observed for analogous samples of dioleoylphosphatidylcholine (DOPC). MAS-assisted two-dimensional nuclear Overhauser effect spectroscopy (NOESY) was conducted for multibilayers of both MeDOPE and DOPC. Through-space interactions were identified between pairs of lipid protons, as expected from their chemical structure. For lamellar suspensions of MeDOPE, positive NOESY cross-peaks were observed between the downfield-shifted water resonance (only) and both CH2N and NH2CH3+ protons of the lipid headgroup. These cross-peaks were not observed in the NOESY spectra of MeDOPE in its hexagonal or cubic phases or for lamellar DOPC reference samples. Taken together, the observation of two water peaks, spin-lattice relaxation behavior, and NOESY connectivities in MeDOPE suspensions support the interpretation that the low-field water peak corresponds to hydrogen-bonded interlamellar water interacting strongly with the lipid. Such a population of water molecules exists in association with MeDOPE in the lamellar phase but not for its inverted phases or for lamellar dispersions of DOPC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornell R. B. Regulation of CTP:phosphocholine cytidylyltransferase by lipids. 2. Surface curvature, acyl chain length, and lipid-phase dependence for activation. Biochemistry. 1991 Jun 18;30(24):5881–5888. doi: 10.1021/bi00238a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 1985 Dec 3;24(25):7092–7095. doi: 10.1021/bi00346a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F. Calorimetric detection of curvature strain in phospholipid bilayers. Biophys J. 1994 May;66(5):1450–1456. doi: 10.1016/S0006-3495(94)80935-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Leon B. T. Hexagonal phase forming propensity detected in phospholipid bilayers with fluorescent probes. Biochemistry. 1992 Feb 11;31(5):1550–1554. doi: 10.1021/bi00120a036. [DOI] [PubMed] [Google Scholar]
- Gabriel N. E., Roberts M. F. Short-chain lecithin/long-chain phospholipid unilamellar vesicles: asymmetry, dynamics, and enzymatic hydrolysis of the short-chain component. Biochemistry. 1987 May 5;26(9):2432–2440. doi: 10.1021/bi00383a006. [DOI] [PubMed] [Google Scholar]
- Gawrisch K., Ruston D., Zimmerberg J., Parsegian V. A., Rand R. P., Fuller N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J. 1992 May;61(5):1213–1223. doi: 10.1016/S0006-3495(92)81931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. G. Solid state nuclear magnetic resonance of lipid bilayers. Methods Enzymol. 1981;72:108–174. doi: 10.1016/s0076-6879(81)72010-x. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Tate M. W., Kirk G. L., So P. T., Turner D. C., Keane D. T., Tilcock C. P., Cullis P. R. X-ray diffraction study of the polymorphic behavior of N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1988 Apr 19;27(8):2853–2866. doi: 10.1021/bi00408a029. [DOI] [PubMed] [Google Scholar]
- Halladay H. N., Stark R. E., Ali S., Bittman R. Magic-angle spinning NMR studies of molecular organization in multibilayers formed by 1-octadecanoyl-2-decanoyl-sn-glycero-3-phosphocholine. Biophys J. 1990 Dec;58(6):1449–1461. doi: 10.1016/S0006-3495(90)82490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. L., Bezrukov S. M., Gruner S. M., Tate M. W., Vodyanoy I., Parsegian V. A. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993 Jul;65(1):23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles P. F., Marsh D. Magnetic resonance of membranes. Biochem J. 1991 Mar 15;274(Pt 3):625–641. doi: 10.1042/bj2740625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom G., Hauksson J. B., Rilfors L., Bergenståhl B., Wieslander A., Eriksson P. O. Membrane lipid regulation in Acholeplasma laidlawii grown with saturated fatty acids. Biosynthesis of a triacylglucolipid forming reversed micelles. J Biol Chem. 1993 Aug 5;268(22):16198–16207. [PubMed] [Google Scholar]
- McCallum C. D., Epand R. M. Insulin receptor autophosphorylation and signaling is altered by modulation of membrane physical properties. Biochemistry. 1995 Feb 14;34(6):1815–1824. doi: 10.1021/bi00006a001. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 1986 Aug 26;25(17):4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration and steric pressures between phospholipid bilayers. Annu Rev Biophys Biomol Struct. 1994;23:27–51. doi: 10.1146/annurev.bb.23.060194.000331. [DOI] [PubMed] [Google Scholar]
- Osterberg F., Rilfors L., Wieslander A., Lindblom G., Gruner S. M. Lipid extracts from membranes of Acholeplasma laidlawii A grown with different fatty acids have a nearly constant spontaneous curvature. Biochim Biophys Acta. 1995 Jun 27;1257(1):18–24. doi: 10.1016/0005-2760(95)00042-b. [DOI] [PubMed] [Google Scholar]
- Rietveld A. G., Chupin V. V., Koorengevel M. C., Wienk H. L., Dowhan W., de Kruijff B. Regulation of lipid polymorphism is essential for the viability of phosphatidylethanolamine-deficient Escherichia coli cells. J Biol Chem. 1994 Nov 18;269(46):28670–28675. [PubMed] [Google Scholar]
- Rilfors L., Hauksson J. B., Lindblom G. Regulation and phase equilibria of membrane lipids from Bacillus megaterium and Acholeplasma laidlawii strain A containing methyl-branched acyl chains. Biochemistry. 1994 May 24;33(20):6110–6120. doi: 10.1021/bi00186a010. [DOI] [PubMed] [Google Scholar]
- Senisterra G., Epand R. M. Role of membrane defects in the regulation of the activity of protein kinase C. Arch Biochem Biophys. 1993 Jan;300(1):378–383. doi: 10.1006/abbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J. L. Lamellar/inverted cubic (L alpha/QII) phase transition in N-methylated dioleoylphosphatidylethanolamine. Biochemistry. 1990 Jun 26;29(25):5975–5981. doi: 10.1021/bi00477a014. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993 Nov;65(5):2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volke F., Eisenblätter S., Klose G. Hydration force parameters of phosphatidylcholine lipid bilayers as determined from 2H-NMR studies of deuterated water. Biophys J. 1994 Nov;67(5):1882–1887. doi: 10.1016/S0006-3495(94)80670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volke F., Pampel A. Membrane hydration and structure on a subnanometer scale as seen by high resolution solid state nuclear magnetic resonance: POPC and POPC/C12EO4 model membranes. Biophys J. 1995 May;68(5):1960–1965. doi: 10.1016/S0006-3495(95)80373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. C., Cafiso D. S. Phospholipid packing and conformation in small vesicles revealed by two-dimensional 1H nuclear magnetic resonance cross-relaxation spectroscopy. Biophys J. 1986 Mar;49(3):779–783. doi: 10.1016/S0006-3495(86)83705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom L. C., Nie S. Q., Epand R. M. Hydrophobic lipid additives affect membrane stability and phase behavior of N-monomethyldioleoylphosphatidylethanolamine. Biochemistry. 1992 Jan 28;31(3):671–677. doi: 10.1021/bi00118a006. [DOI] [PubMed] [Google Scholar]


