Abstract
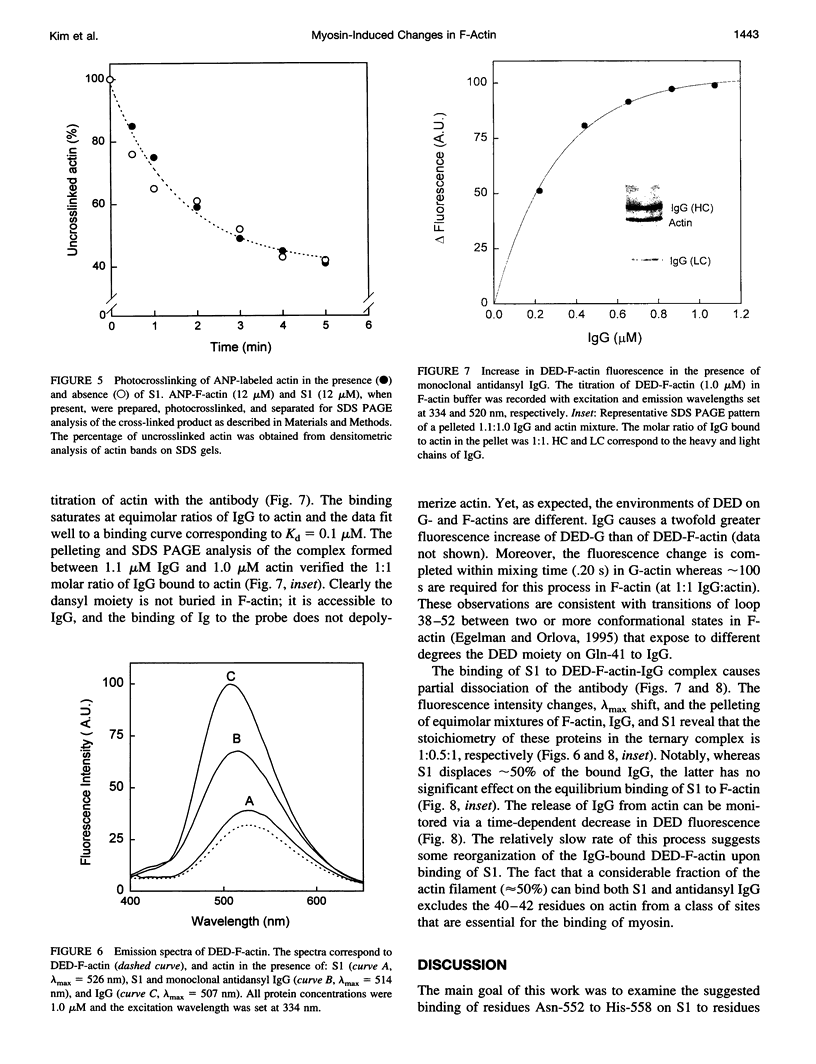
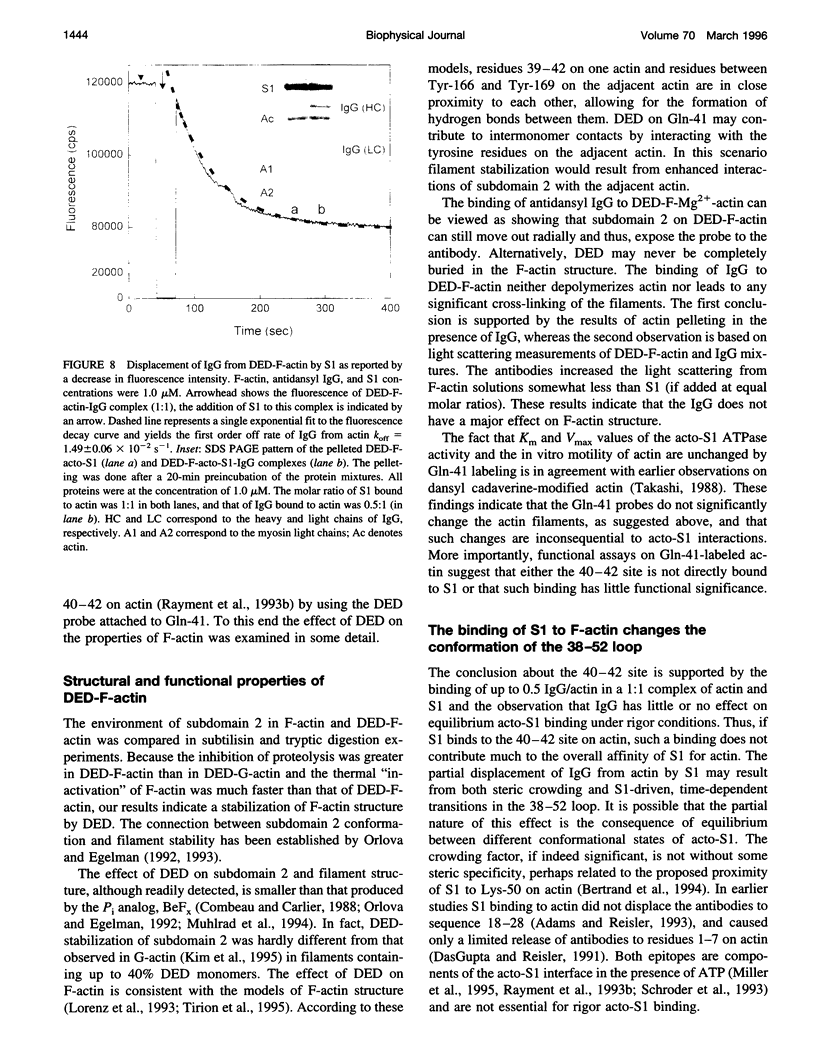
Actin labeled at Gln-41 with dansyl ethylenediamine (DED) via transglutaminase reaction was used for monitoring the interaction of myosin subfragment 1 (S1) with the His-40-Gly-42 site in the 38-52 loop on F-actin. Proteolytic digestions of F-actin with subtilisin and trypsin, and acto-S1 ATPase measurements on heat-treated F-actin revealed that the labeling of Gln-41 had a stabilizing effect on subdomain 2 and the actin filaments. DED on Gln-41 had no effect on the values of K(m) and Vmax of the acto-S1 ATPase and the sliding velocities of actin filaments in the in vitro motility assays. This suggests either that S1 does not bind to the 40-42 site on actin or that such binding is not functionally important. The binding of monoclonal antidansyl IgG to DED-F-actin did not affect acto-S1 binding in the absence of nucleotides, indicating that the 40-42 site does not contribute much to rigor acto-S1 binding. Myosin-induced changes in subdomain 2 on actin were manifested through an increase in the fluorescence of DED-F-actin, a decrease in the accessibility of the probe to collisional quenchers, and a partial displacement of antidansyl IgG from actin by S1. It is proposed that these changes in the 38-52 loop on actin originate from S1 binding to other myosin recognition sites on actin.
Full text
PDF

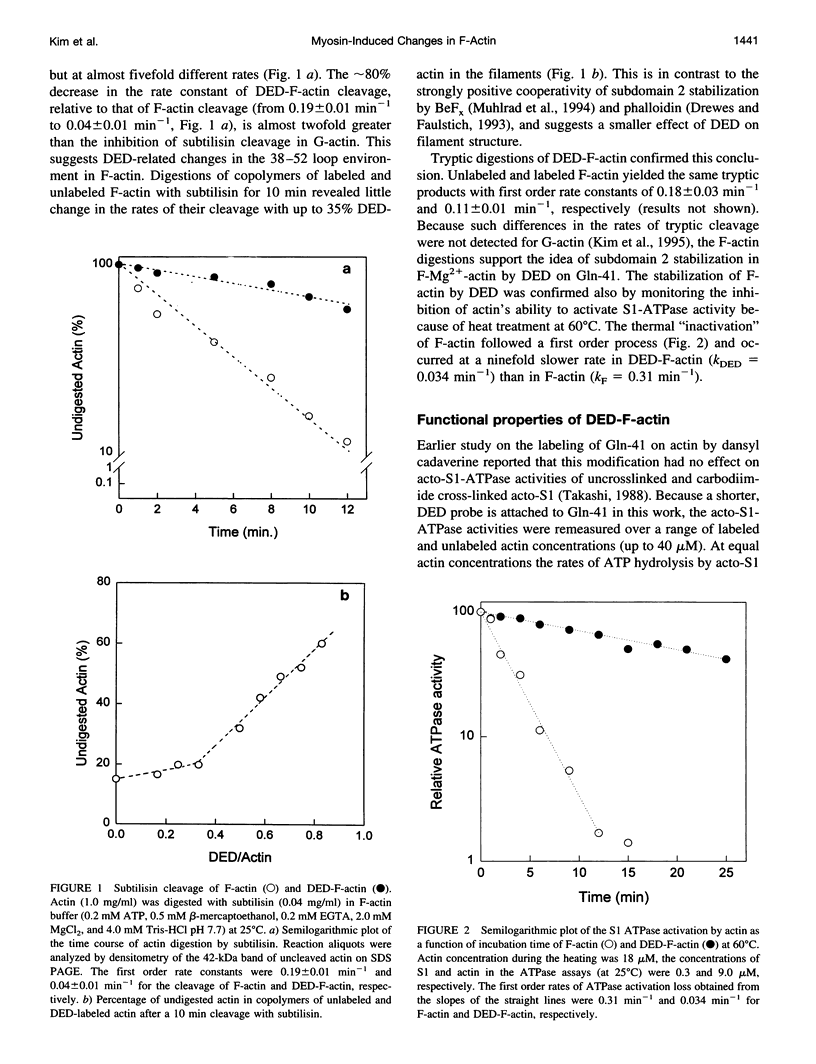
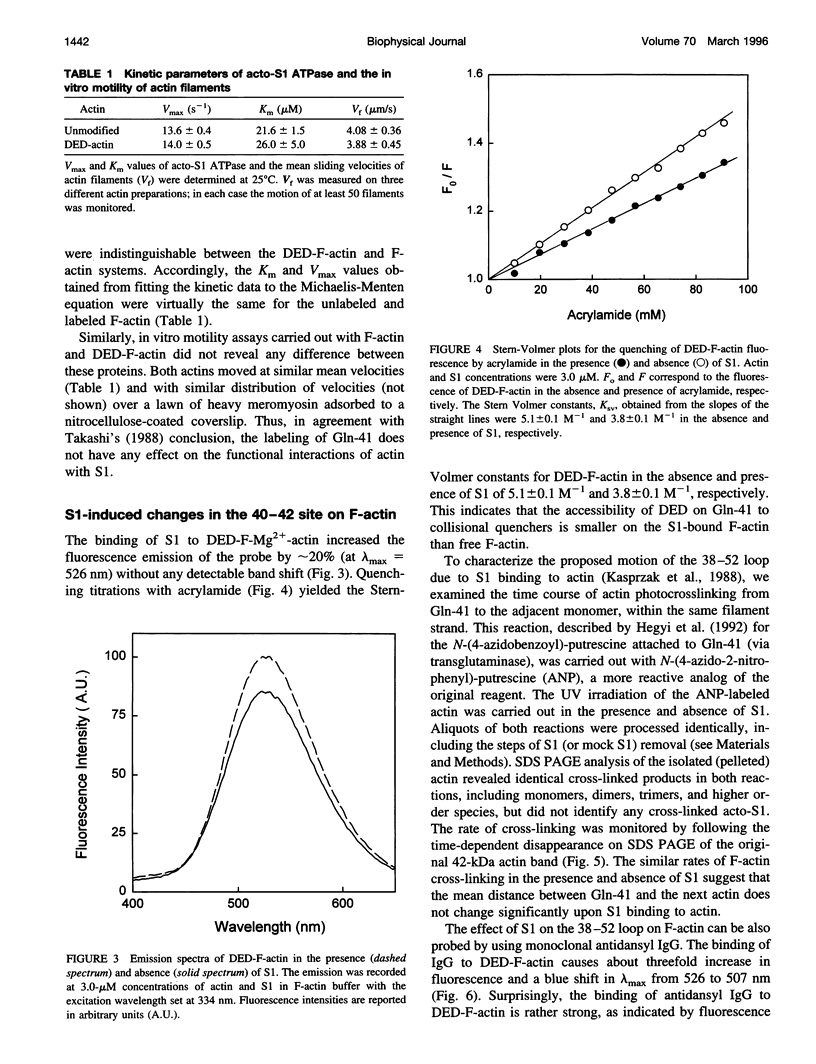


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. B., Reisler E. Sequence 18-29 on actin: antibody and spectroscopic probing of conformational changes. Biochemistry. 1994 Dec 6;33(48):14426–14433. doi: 10.1021/bi00252a008. [DOI] [PubMed] [Google Scholar]
- Adams S., Reisler E. Role of sequence 18-29 on actin in actomyosin interactions. Biochemistry. 1993 May 18;32(19):5051–5056. doi: 10.1021/bi00070a012. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Derancourt J., Kassab R. The covalent maleimidobenzoyl-actin-myosin head complex. Cross-linking of the 50 kDa heavy chain region to actin subdomain-2. FEBS Lett. 1994 May 30;345(2-3):113–119. doi: 10.1016/0014-5793(94)00398-x. [DOI] [PubMed] [Google Scholar]
- Butters C. A., Willadsen K. A., Tobacman L. S. Cooperative interactions between adjacent troponin-tropomyosin complexes may be transmitted through the actin filament. J Biol Chem. 1993 Jul 25;268(21):15565–15570. [PubMed] [Google Scholar]
- Combeau C., Carlier M. F. Probing the mechanism of ATP hydrolysis on F-actin using vanadate and the structural analogs of phosphate BeF-3 and A1F-4. J Biol Chem. 1988 Nov 25;263(33):17429–17436. [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Nucleotide-induced changes in the interaction of myosin subfragment 1 with actin: detection by antibodies against the N-terminal segment of actin. Biochemistry. 1991 Oct 15;30(41):9961–9966. doi: 10.1021/bi00105a021. [DOI] [PubMed] [Google Scholar]
- Drewes G., Faulstich H. Cooperative effects on filament stability in actin modified at the C-terminus by substitution or truncation. Eur J Biochem. 1993 Feb 15;212(1):247–253. doi: 10.1111/j.1432-1033.1993.tb17656.x. [DOI] [PubMed] [Google Scholar]
- Egelman E. H., Orlova A. New insights into actin filament dynamics. Curr Opin Struct Biol. 1995 Apr;5(2):172–180. doi: 10.1016/0959-440x(95)80072-7. [DOI] [PubMed] [Google Scholar]
- Fievez S., Carlier M. F. Conformational changes in subdomain-2 of G-actin upon polymerization into F-actin and upon binding myosin subfragment-1. FEBS Lett. 1993 Jan 25;316(2):186–190. doi: 10.1016/0014-5793(93)81212-i. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- Hegyi G., Michel H., Shabanowitz J., Hunt D. F., Chatterjie N., Healy-Louie G., Elzinga M. Gln-41 is intermolecularly cross-linked to Lys-113 in F-actin by N-(4-azidobenzoyl)-putrescine. Protein Sci. 1992 Jan;1(1):132–144. doi: 10.1002/pro.5560010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huang Y. P., Seguro K., Motoki M., Tawada K. Cross-linking of contractile proteins from skeletal muscle by treatment with microbial transglutaminase. J Biochem. 1992 Aug;112(2):229–234. doi: 10.1093/oxfordjournals.jbchem.a123882. [DOI] [PubMed] [Google Scholar]
- Kasprzak A. A., Takashi R., Morales M. F. Orientation of actin monomer in the F-actin filament: radial coordinate of glutamine-41 and effect of myosin subfragment 1 binding on the monomer orientation. Biochemistry. 1988 Jun 14;27(12):4512–4522. doi: 10.1021/bi00412a044. [DOI] [PubMed] [Google Scholar]
- Kim E., Motoki M., Seguro K., Muhlrad A., Reisler E. Conformational changes in subdomain 2 of G-actin: fluorescence probing by dansyl ethylenediamine attached to Gln-41. Biophys J. 1995 Nov;69(5):2024–2032. doi: 10.1016/S0006-3495(95)80072-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M., Popp D., Holmes K. C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993 Dec 5;234(3):826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- Miller C. J., Reisler E. Role of charged amino acid pairs in subdomain-1 of actin in interactions with myosin. Biochemistry. 1995 Feb 28;34(8):2694–2700. doi: 10.1021/bi00008a037. [DOI] [PubMed] [Google Scholar]
- Muhlrad A., Cheung P., Phan B. C., Miller C., Reisler E. Dynamic properties of actin. Structural changes induced by beryllium fluoride. J Biol Chem. 1994 Apr 22;269(16):11852–11858. [PubMed] [Google Scholar]
- Orlova A., Egelman E. H. A conformational change in the actin subunit can change the flexibility of the actin filament. J Mol Biol. 1993 Jul 20;232(2):334–341. doi: 10.1006/jmbi.1993.1393. [DOI] [PubMed] [Google Scholar]
- Orlova A., Egelman E. H. Structural basis for the destabilization of F-actin by phosphate release following ATP hydrolysis. J Mol Biol. 1992 Oct 20;227(4):1043–1053. doi: 10.1016/0022-2836(92)90520-t. [DOI] [PubMed] [Google Scholar]
- Orlova A., Prochniewicz E., Egelman E. H. Structural dynamics of F-actin: II. Cooperativity in structural transitions. J Mol Biol. 1995 Feb 3;245(5):598–607. doi: 10.1006/jmbi.1994.0049. [DOI] [PubMed] [Google Scholar]
- Owen C., DeRosier D. A 13-A map of the actin-scruin filament from the limulus acrosomal process. J Cell Biol. 1993 Oct;123(2):337–344. doi: 10.1083/jcb.123.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Schutt C. E., Myslik J. C., Rozycki M. D., Goonesekere N. C., Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993 Oct 28;365(6449):810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- Schwyter D. H., Kron S. J., Toyoshima Y. Y., Spudich J. A., Reisler E. Subtilisin cleavage of actin inhibits in vitro sliding movement of actin filaments over myosin. J Cell Biol. 1990 Aug;111(2):465–470. doi: 10.1083/jcb.111.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Takashi R. A novel actin label: a fluorescent probe at glutamine-41 and its consequences. Biochemistry. 1988 Feb 9;27(3):938–943. doi: 10.1021/bi00403a015. [DOI] [PubMed] [Google Scholar]
- Tirion M. M., ben-Avraham D., Lorenz M., Holmes K. C. Normal modes as refinement parameters for the F-actin model. Biophys J. 1995 Jan;68(1):5–12. doi: 10.1016/S0006-3495(95)80156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]