Abstract
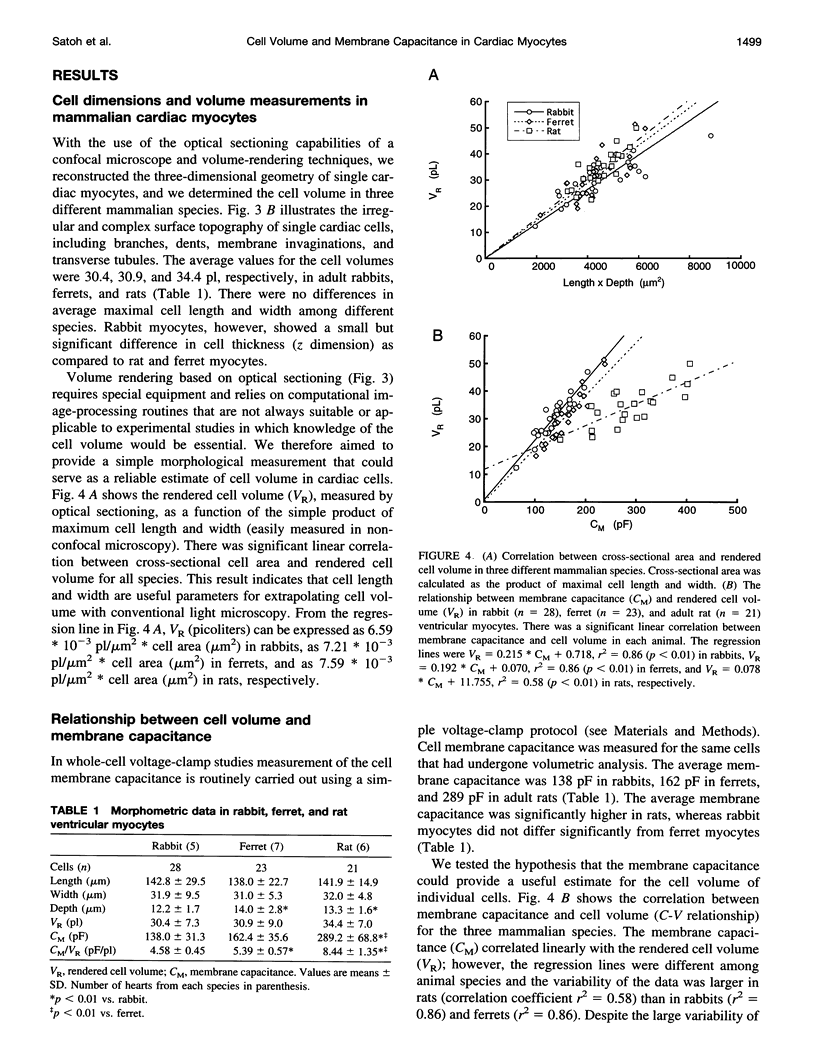
The quantitative analysis of the contribution of ion fluxes through membrane channels to changes of intracellular ion concentrations would benefit from the exact knowledge of the cell volume. It would allow direct correlation of ionic current measurements with simultaneous measurements of ion concentrations in individual cells. Because of various limitations of conventional light microscopy a simple method for accurate cell volume determination is lacking. We have combined the optical sectioning capabilities of fluorescence laser scanning confocal microscopy and the whole-cell patch-clamp technique to study the correlation between cell volume and membrane capacitance. Single cardiac myocytes loaded with the fluorescent dye calcein were optically sectioned to produce a series of confocal images. The volume of cardiac myocytes of three different mammalian species was determined by three-dimensional volume rendering of the confocal images. The calculated cell volumes were 30.4 +/- 7.3 pl (mean +/- SD) in rabbits (n = 28), 30.9 +/- 9.0 pl in ferrets (n = 23), and 34.4 +/- 7.0 pl in rats (n = 21), respectively. There was a positive linear correlation between membrane capacitance and cell volume in each animal species. The capacitance-volume ratios were significantly different among species (4.58 +/- 0.45 pF/pl in rabbit, 5.39 +/- 0.57 pF/pl in ferret, and 8.44 +/- 1.35 pF/pl in rat). Furthermore, the capacitance-volume ratio was dependent on the developmental stage (8.88 +/- 1.14 pF/pl in 6-month-old rats versus 6.76 +/- 0.62 pF/pl in 3-month-old rats). The data suggest that the ratio of surface area:volume of cardiac myocytes undergoes significant developmental changes and differs among mammalian species. We further established that the easily measurable parameters of cell membrane capacitance or the product of cell length and width provide reliable but species-dependent estimates for the volume of individual cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth E., Stämmler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992 Jul;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- Berlin J. R., Bassani J. W., Bers D. M. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994 Oct;67(4):1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–H381. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Agonist-induced [Ca2+]i waves and Ca(2+)-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol. 1992 Aug;263(2 Pt 2):H576–H586. doi: 10.1152/ajpheart.1992.263.2.H576. [DOI] [PubMed] [Google Scholar]
- Bénitah J. P., Gomez A. M., Bailly P., Da Ponte J. P., Berson G., Delgado C., Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J Physiol. 1993 Sep;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. E., Gerdes A. M., Smith T. D. Comparison of regional differences in cardiac myocyte dimensions in rats, hamsters, and guinea pigs. Anat Rec. 1987 Sep;219(1):53–59. doi: 10.1002/ar.1092190110. [DOI] [PubMed] [Google Scholar]
- Chacon E., Reece J. M., Nieminen A. L., Zahrebelski G., Herman B., Lemasters J. J. Distribution of electrical potential, pH, free Ca2+, and volume inside cultured adult rabbit cardiac myocytes during chemical hypoxia: a multiparameter digitized confocal microscopic study. Biophys J. 1994 Apr;66(4):942–952. doi: 10.1016/S0006-3495(94)80904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. R., Roysam B., Turner J. N. Automated tracing and volume measurements of neurons from 3-D confocal fluorescence microscopy data. J Microsc. 1994 Feb;173(Pt 2):103–114. doi: 10.1111/j.1365-2818.1994.tb03433.x. [DOI] [PubMed] [Google Scholar]
- Delcarpio J. B., Claycomb W. C., Moses R. L. Ultrastructural morphometric analysis of cultured neonatal and adult rat ventricular cardiac muscle cells. Am J Anat. 1989 Dec;186(4):335–345. doi: 10.1002/aja.1001860403. [DOI] [PubMed] [Google Scholar]
- Dodge D. E., Plopper C. G., Rucker R. B. Regulation of Clara cell 10 kD protein secretion by pilocarpine: quantitative comparison of nonciliated cells in rat bronchi and bronchioles based on laser scanning confocal microscopy. Am J Respir Cell Mol Biol. 1994 Mar;10(3):259–270. doi: 10.1165/ajrcmb.10.3.8117444. [DOI] [PubMed] [Google Scholar]
- Drewnowska K., Baumgarten C. M. Regulation of cellular volume in rabbit ventricular myocytes: bumetanide, chlorothiazide, and ouabain. Am J Physiol. 1991 Jan;260(1 Pt 1):C122–C131. doi: 10.1152/ajpcell.1991.260.1.C122. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Mottino G., Chen F., Peri V., Holland P., Tuana B. S. Subcellular distribution of dystrophin in isolated adult and neonatal cardiac myocytes. Am J Physiol. 1994 Dec;267(6 Pt 1):C1707–C1716. doi: 10.1152/ajpcell.1994.267.6.C1707. [DOI] [PubMed] [Google Scholar]
- Fraticelli A., Josephson R., Danziger R., Lakatta E., Spurgeon H. Morphological and contractile characteristics of rat cardiac myocytes from maturation to senescence. Am J Physiol. 1989 Jul;257(1 Pt 2):H259–H265. doi: 10.1152/ajpheart.1989.257.1.H259. [DOI] [PubMed] [Google Scholar]
- Gerdes A. M., Moore J. A., Hines J. M., Kirkland P. A., Bishop S. P. Regional differences in myocyte size in normal rat heart. Anat Rec. 1986 Aug;215(4):420–426. doi: 10.1002/ar.1092150414. [DOI] [PubMed] [Google Scholar]
- Guilak F. Volume and surface area measurement of viable chondrocytes in situ using geometric modelling of serial confocal sections. J Microsc. 1994 Mar;173(Pt 3):245–256. doi: 10.1111/j.1365-2818.1994.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Hryshko L. V., Stiffel V., Bers D. M. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Huynh T. V., Chen F., Wetzel G. T., Friedman W. F., Klitzner T. S. Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res. 1992 Mar;70(3):508–515. doi: 10.1161/01.res.70.3.508. [DOI] [PubMed] [Google Scholar]
- Keung E. C., Toll L., Ellis M., Jensen R. A. L-type cardiac calcium channels in doxorubicin cardiomyopathy in rats morphological, biochemical, and functional correlations. J Clin Invest. 1991 Jun;87(6):2108–2113. doi: 10.1172/JCI115241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlof L., Forsgren P. O. Confocal microscopy: important considerations for accurate imaging. Methods Cell Biol. 1993;38:79–95. doi: 10.1016/s0091-679x(08)61000-6. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Tatham P. E., Powell T., Twist V. W., Speller R. D., Loverock L. T. Size measurements on isolated rat heart cells using Coulter analysis and light scatter flow cytometry. Biochim Biophys Acta. 1979 Sep 20;587(1):99–III. doi: 10.1016/0304-4165(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., McCallister L. P. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol. 1973 Feb;31(2):172–181. doi: 10.1016/0002-9149(73)91030-8. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Page E., Surdyk-Droske M. Distribution, surface density, and membrane area of diadic junctional contacts between plasma membrane and terminal cisterns in mammalian ventricle. Circ Res. 1979 Aug;45(2):260–267. doi: 10.1161/01.res.45.2.260. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A. The dimensions of human cardiac myocytes; confusion caused by methodology and pathology. J Mol Cell Cardiol. 1995 Mar;27(3):863–865. doi: 10.1016/0022-2828(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Rich T. L., Langer G. A., Klassen M. G. Two components of coupling calcium in single ventricular cell of rabbits and rats. Am J Physiol. 1988 May;254(5 Pt 2):H937–H946. doi: 10.1152/ajpheart.1988.254.5.H937. [DOI] [PubMed] [Google Scholar]
- Scamps F., Mayoux E., Charlemagne D., Vassort G. Calcium current in single cells isolated from normal and hypertrophied rat heart. Effects of beta-adrenergic stimulation. Circ Res. 1990 Jul;67(1):199–208. doi: 10.1161/01.res.67.1.199. [DOI] [PubMed] [Google Scholar]
- Scheynius A., Dalenbring M., Carlsson K., England R., Lindberg M. Quantitative analysis of Langerhans' cells in epidermis at irritant contact reactions using confocal laser scanning microscopy. Acta Derm Venereol. 1992 Sep;72(5):348–351. [PubMed] [Google Scholar]
- Smith S. H., Bishop S. P. Regional myocyte size in compensated right ventricular hypertrophy in the ferret. J Mol Cell Cardiol. 1985 Oct;17(10):1005–1011. doi: 10.1016/s0022-2828(85)80081-x. [DOI] [PubMed] [Google Scholar]
- Tinel H., Wehner F., Sauer H. Intracellular Ca2+ release and Ca2+ influx during regulatory volume decrease in IMCD cells. Am J Physiol. 1994 Jul;267(1 Pt 2):F130–F138. doi: 10.1152/ajprenal.1994.267.1.F130. [DOI] [PubMed] [Google Scholar]
- Tomita F., Bassett A. L., Myerburg R. J., Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circ Res. 1994 Aug;75(2):296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- Vandewoude M. F., Buyssens N. Effect of ageing and malnutrition on rat myocardium. I. The myocyte. Virchows Arch A Pathol Anat Histopathol. 1992;421(3):179–188. doi: 10.1007/BF01611173. [DOI] [PubMed] [Google Scholar]
- Vliegen H. W., van der Laarse A., Huysman J. A., Wijnvoord E. C., Mentar M., Cornelisse C. J., Eulderink F. Morphometric quantification of myocyte dimensions validated in normal growing rat hearts and applied to hypertrophic human hearts. Cardiovasc Res. 1987 May;21(5):352–357. doi: 10.1093/cvr/21.5.352. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Winka L., Langer G. A. Role of calcium current and sarcoplasmic reticulum calcium release in control of myocardial contraction in rat and rabbit myocytes. J Mol Cell Cardiol. 1993 Nov;25(11):1339–1347. doi: 10.1006/jmcc.1993.1146. [DOI] [PubMed] [Google Scholar]
- Wettwer E., Amos G., Gath J., Zerkowski H. R., Reidemeister J. C., Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovasc Res. 1993 Sep;27(9):1662–1669. doi: 10.1093/cvr/27.9.1662. [DOI] [PubMed] [Google Scholar]
- Wright S. J., Centonze V. E., Stricker S. A., DeVries P. J., Paddock S. W., Schatten G. Introduction to confocal microscopy and three-dimensional reconstruction. Methods Cell Biol. 1993;38:1–45. doi: 10.1016/s0091-679x(08)60998-x. [DOI] [PubMed] [Google Scholar]




