Abstract
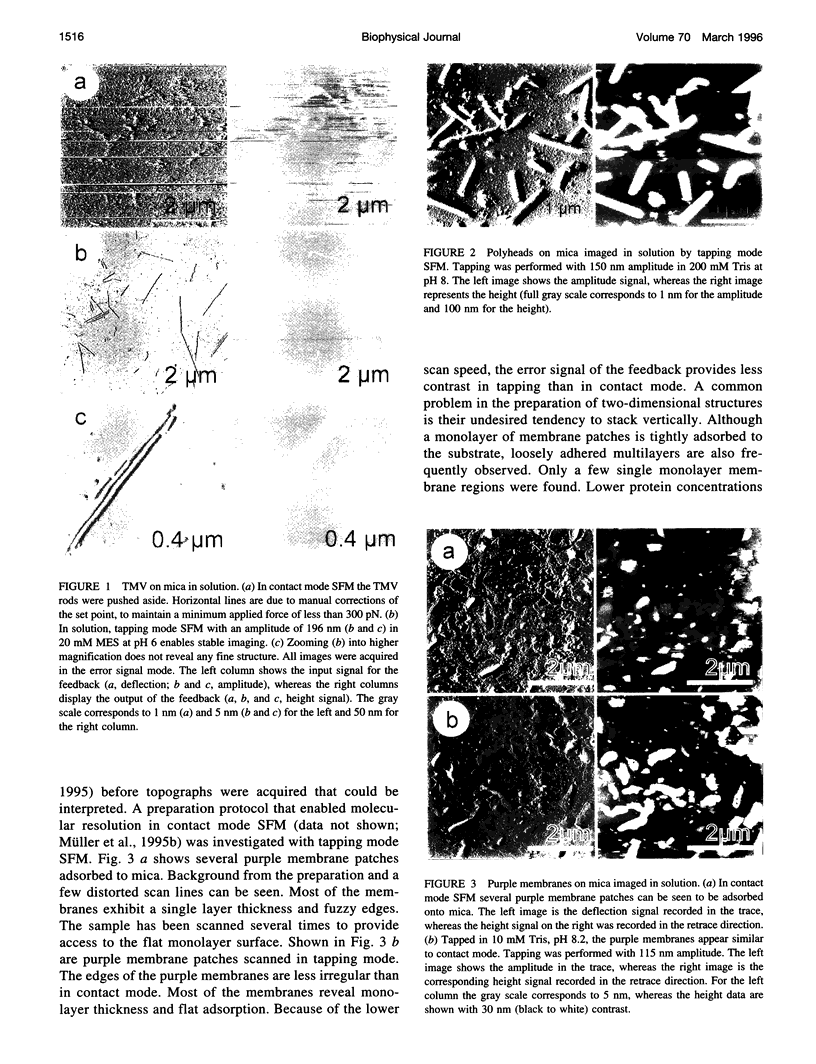
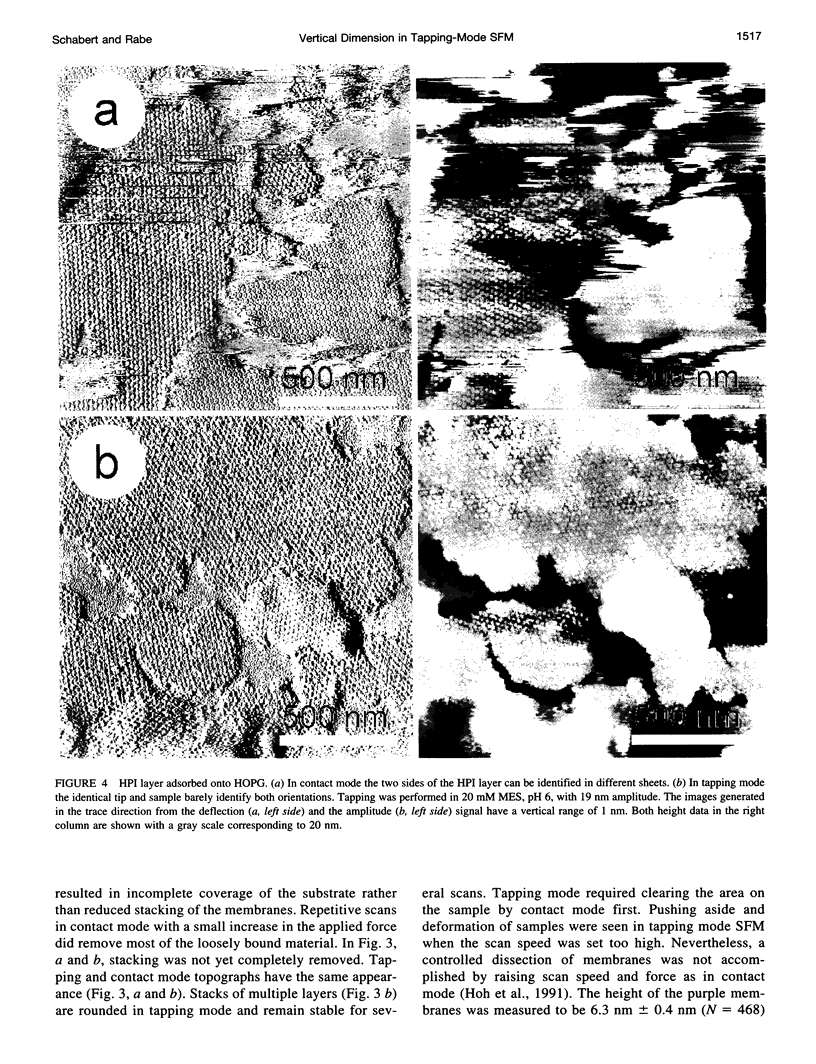
The vertical dimensions of the well-characterized test samples tobacco mosaic virus, T4 bacteriophage polyhead, purple membrane, and hexagonally packed intermediate (HPI) layer were investigated by tapping mode scanning force microscopy (SFM) in solution. Purple membrane and HPI layer were imaged in both contact mode and tapping mode SFM for direct comparison. All vertical dimensions match the known heights. The practical implications of the absence of frictional forces in tapping mode are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister W., Barth M., Hegerl R., Guckenberger R., Hahn M., Saxton W. O. Three-dimensional structure of the regular surface layer (HPI layer) of Deinococcus radiodurans. J Mol Biol. 1986 Jan 20;187(2):241–250. doi: 10.1016/0022-2836(86)90231-7. [DOI] [PubMed] [Google Scholar]
- Bezanilla M., Drake B., Nudler E., Kashlev M., Hansma P. K., Hansma H. G. Motion and enzymatic degradation of DNA in the atomic force microscope. Biophys J. 1994 Dec;67(6):2454–2459. doi: 10.1016/S0006-3495(94)80733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Downing K. H., Hansma P. K. Imaging the membrane protein bacteriorhodopsin with the atomic force microscope. Biophys J. 1990 Dec;58(6):1473–1480. doi: 10.1016/S0006-3495(90)82492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. Biological applications of scanning probe microscopes. Annu Rev Biophys Biophys Chem. 1991;20:79–108. doi: 10.1146/annurev.bb.20.060191.000455. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Hoh J. H. Biomolecular imaging with the atomic force microscope. Annu Rev Biophys Biomol Struct. 1994;23:115–139. doi: 10.1146/annurev.bb.23.060194.000555. [DOI] [PubMed] [Google Scholar]
- Hegner M., Wagner P., Semenza G. Immobilizing DNA on gold via thiol modification for atomic force microscopy imaging in buffer solutions. FEBS Lett. 1993 Dec 28;336(3):452–456. doi: 10.1016/0014-5793(93)80854-n. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Lal R., John S. A., Revel J. P., Arnsdorf M. F. Atomic force microscopy and dissection of gap junctions. Science. 1991 Sep 20;253(5026):1405–1408. doi: 10.1126/science.1910206. [DOI] [PubMed] [Google Scholar]
- Karrasch S., Dolder M., Schabert F., Ramsden J., Engel A. Covalent binding of biological samples to solid supports for scanning probe microscopy in buffer solution. Biophys J. 1993 Dec;65(6):2437–2446. doi: 10.1016/S0006-3495(93)81327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch S., Hegerl R., Hoh J. H., Baumeister W., Engel A. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):836–838. doi: 10.1073/pnas.91.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D. J., Büldt G., Engel A. Force-induced conformational change of bacteriorhodopsin. J Mol Biol. 1995 Jun 2;249(2):239–243. doi: 10.1006/jmbi.1995.0292. [DOI] [PubMed] [Google Scholar]
- Müller D. J., Schabert F. A., Büldt G., Engel A. Imaging purple membranes in aqueous solutions at sub-nanometer resolution by atomic force microscopy. Biophys J. 1995 May;68(5):1681–1686. doi: 10.1016/S0006-3495(95)80345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K., Stubbs G. Structure of tobacco mosaic virus at 3.6 A resolution: implications for assembly. Science. 1986 Mar 21;231(4744):1401–1406. doi: 10.1126/science.3952490. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ohnesorge F., Binnig G. True atomic resolution by atomic force microscopy through repulsive and attractive forces. Science. 1993 Jun 4;260(5113):1451–1456. doi: 10.1126/science.260.5113.1451. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Fritz M., Hansma H. G., Hansma P. K. Direct observation of enzyme activity with the atomic force microscope. Science. 1994 Sep 9;265(5178):1577–1579. doi: 10.1126/science.8079171. [DOI] [PubMed] [Google Scholar]
- Schabert F. A., Engel A. Reproducible acquisition of Escherichia coli porin surface topographs by atomic force microscopy. Biophys J. 1994 Dec;67(6):2394–2403. doi: 10.1016/S0006-3495(94)80726-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabert F. A., Henn C., Engel A. Native Escherichia coli OmpF porin surfaces probed by atomic force microscopy. Science. 1995 Apr 7;268(5207):92–94. doi: 10.1126/science.7701347. [DOI] [PubMed] [Google Scholar]
- Shao Z., Yang J. Progress in high resolution atomic force microscopy in biology. Q Rev Biophys. 1995 May;28(2):195–251. doi: 10.1017/s0033583500003061. [DOI] [PubMed] [Google Scholar]
- Stemmer A., Engel A. Imaging biological macromolecules by STM: quantitative interpretation of topographs. Ultramicroscopy. 1990 Dec;34(3):129–140. doi: 10.1016/0304-3991(90)90067-v. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Klug A. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. I. Three-dimensional image reconstruction. J Mol Biol. 1974 Aug 25;87(4):641–656. doi: 10.1016/0022-2836(74)90075-8. [DOI] [PubMed] [Google Scholar]
- Vesenka J., Manne S., Giberson R., Marsh T., Henderson E. Colloidal gold particles as an incompressible atomic force microscope imaging standard for assessing the compressibility of biomolecules. Biophys J. 1993 Sep;65(3):992–997. doi: 10.1016/S0006-3495(93)81171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H., Hartmann T., Baumeister W., Guckenberger R. Thickness determination of biological samples with a zeta-calibrated scanning tunneling microscope. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9343–9347. doi: 10.1073/pnas.87.23.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegräbe W., Nonnenmacher M., Guckenberger R., Wolter O. Atomic force microscopy of a hydrated bacterial surface protein. J Microsc. 1991 Jul;163(Pt 1):79–84. doi: 10.1111/j.1365-2818.1991.tb03161.x. [DOI] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Tillack T. W., Shao Z. New approach for atomic force microscopy of membrane proteins. The imaging of cholera toxin. J Mol Biol. 1993 Jan 20;229(2):286–290. doi: 10.1006/jmbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- Zenhausern F., Adrian M., Emch R., Taborelli M., Jobin M., Descouts P. Scanning force microscopy and cryo-electron microscopy of tobacco mosaic virus as a test specimen. Ultramicroscopy. 1992 Jul;42-44(Pt B):1168–1172. doi: 10.1016/0304-3991(92)90419-k. [DOI] [PubMed] [Google Scholar]






