Abstract
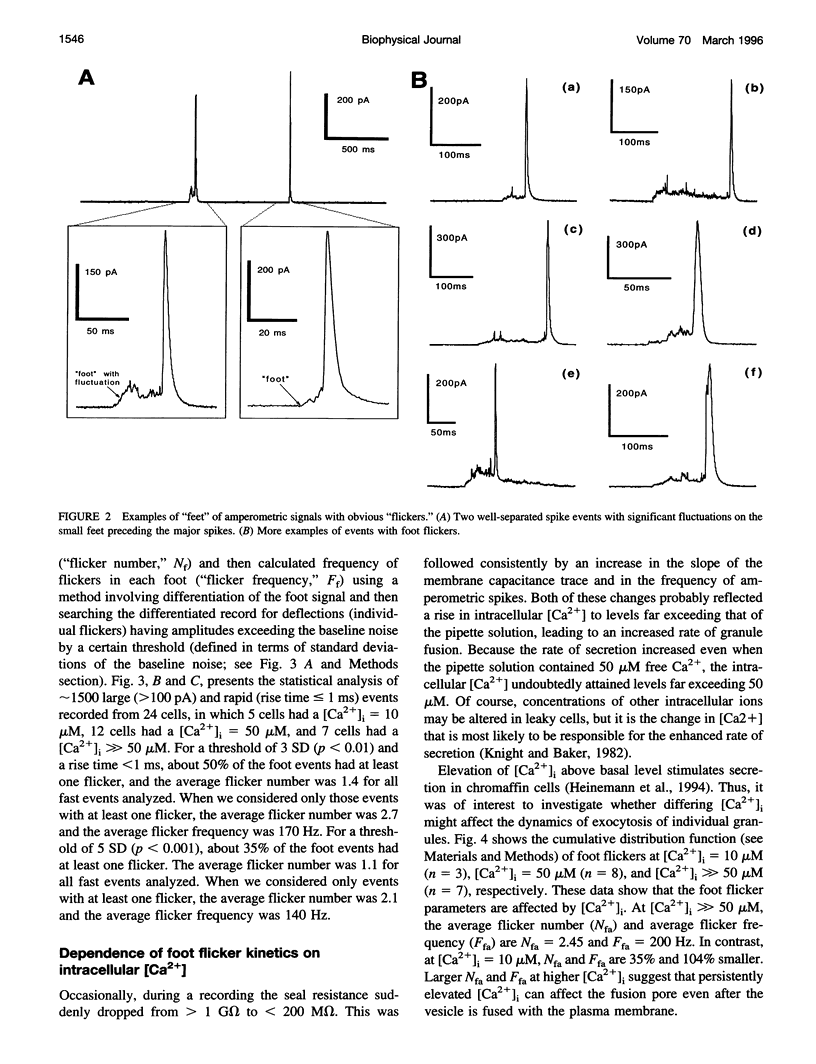
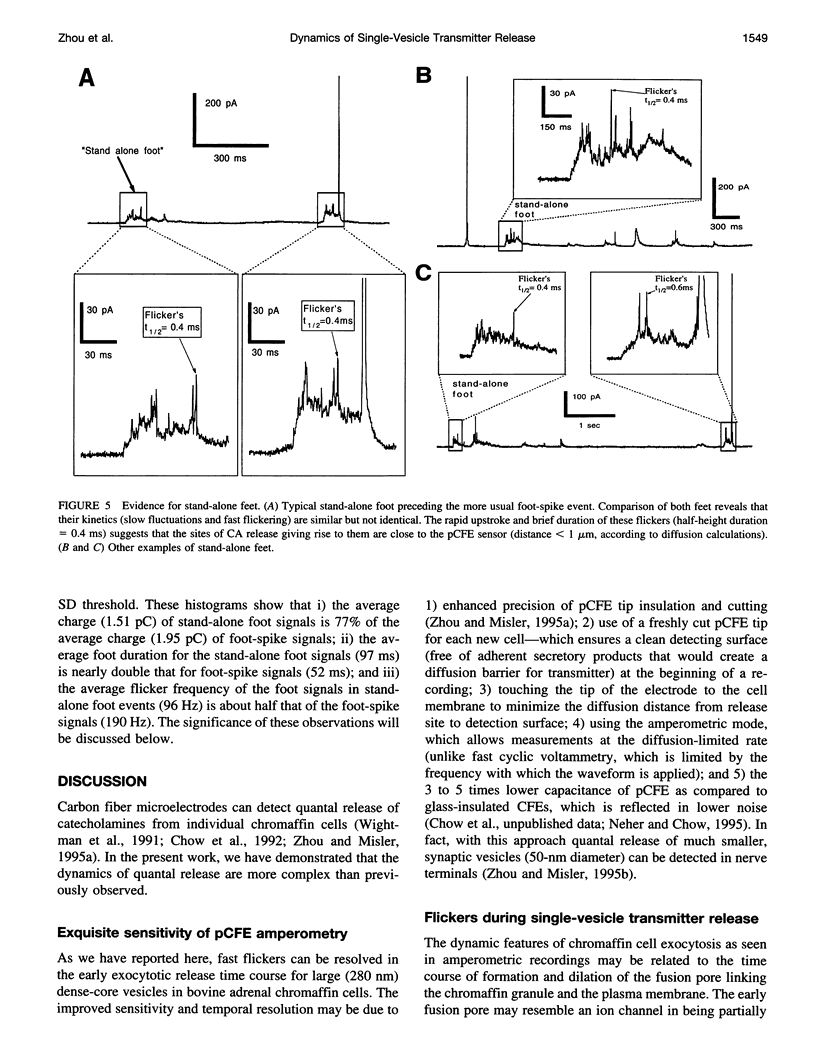
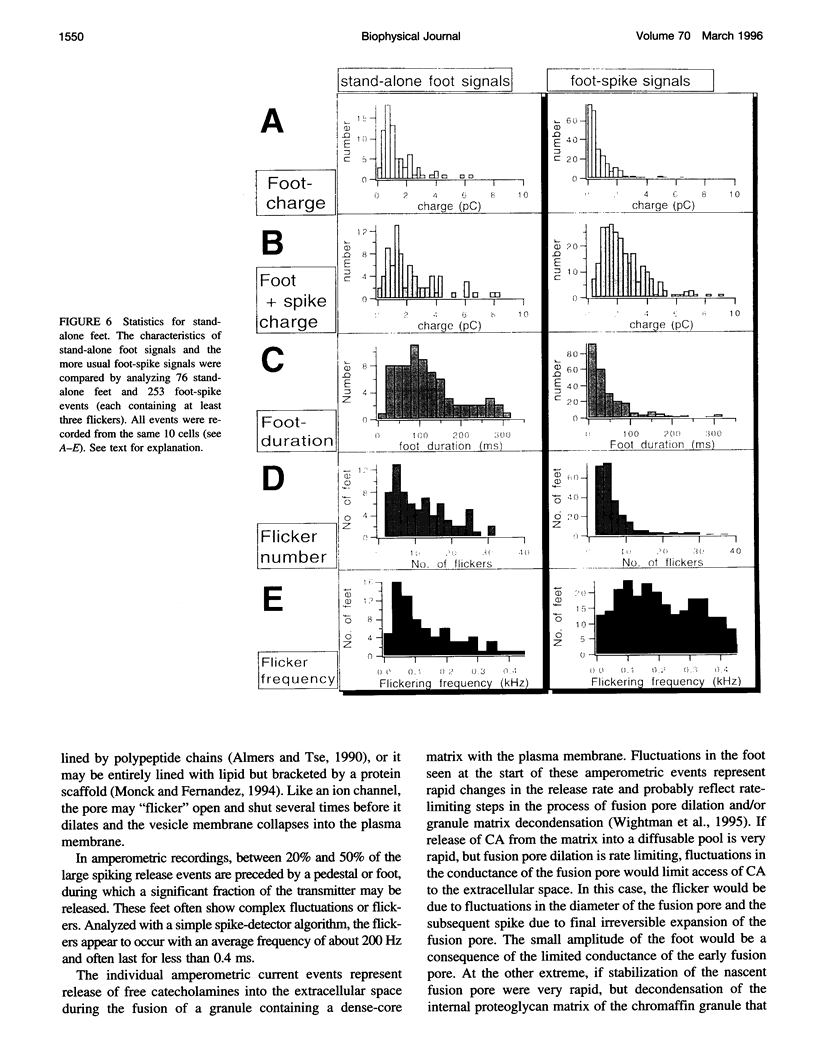
Single-vesicle release of catecholamines from chromaffin cells can be detected in real time as current spikes by the electrochemical method of amperometry. About 70% of spikes are preceded by a small "foot," the trickle of transmitter out of the early fusion pore. In addition, 20-50% of foot signals exhibit rapid fluctuations that we interpret as flickering of the fusion pore. There are also "stand-alone" foot signals, which may reflect transient fusions, in which the vesicles do not collapse completely into the plasma membrane. The number and frequency of the foot flickering are affected by intracellular Ca2+ concentration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Almers W., Tse F. W. Transmitter release from synapses: does a preassembled fusion pore initiate exocytosis? Neuron. 1990 Jun;4(6):813–818. doi: 10.1016/0896-6273(90)90134-2. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernández-Chacón R., Fernández J. M. Release of secretory products during transient vesicle fusion. Nature. 1993 Jun 10;363(6429):554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fesce R., Grohovaz F., Valtorta F., Meldolesi J. Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol. 1994 Jan;4(1):1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore and neurotransmitter release. Neuron. 1994 Apr;12(4):707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Pusch M., Neher E. Washout phenomena in dialyzed mast cells allow discrimination of different steps in stimulus-secretion coupling. Biosci Rep. 1987 Apr;7(4):313–321. doi: 10.1007/BF01121453. [DOI] [PubMed] [Google Scholar]
- Scheller R. H. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995 May;14(5):893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Schroeder T. J., Jankowski J. A., Kawagoe K. T., Wightman R. M., Lefrou C., Amatore C. Analysis of diffusional broadening of vesicular packets of catecholamines released from biological cells during exocytosis. Anal Chem. 1992 Dec 15;64(24):3077–3083. doi: 10.1021/ac00048a003. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995 Jun 22;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Wightman R. M., Jankowski J. A., Kennedy R. T., Kawagoe K. T., Schroeder T. J., Leszczyszyn D. J., Near J. A., Diliberto E. J., Jr, Viveros O. H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R. M., Schroeder T. J., Finnegan J. M., Ciolkowski E. L., Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995 Jan;68(1):383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995 Feb 24;270(8):3498–3505. [PubMed] [Google Scholar]
- Zhou Z., Misler S. Amperometric detection of stimulus-induced quantal release of catecholamines from cultured superior cervical ganglion neurons. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6938–6942. doi: 10.1073/pnas.92.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S. A lipid/protein complex hypothesis for exocytotic fusion pore formation. Ann N Y Acad Sci. 1991;635:307–317. doi: 10.1111/j.1749-6632.1991.tb36501.x. [DOI] [PubMed] [Google Scholar]



