Abstract
1. Mice were hemihysterectomized on day 8 of pregnancy to reduce the number of feto-placental units.
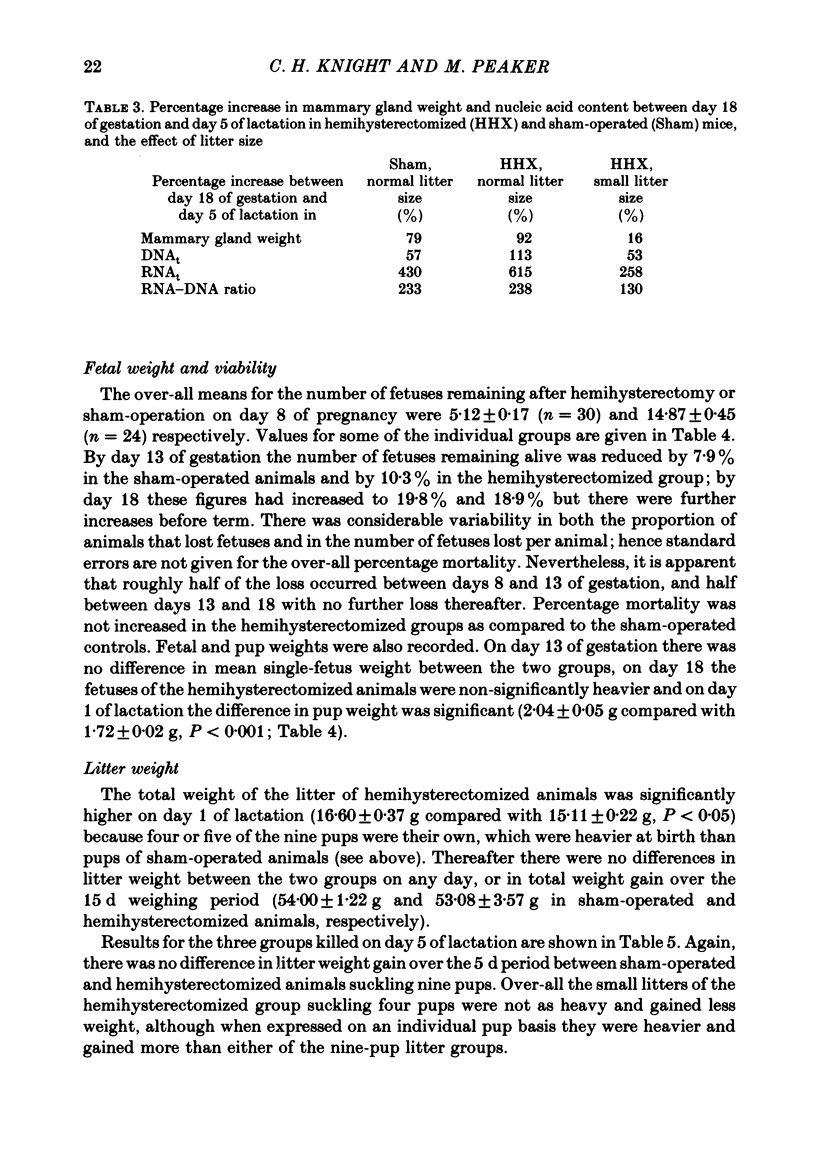
2. Fetal mortality was not affected by hemihysterectomy; mean single-pup weight at birth was increased when compared with sham-operated controls.
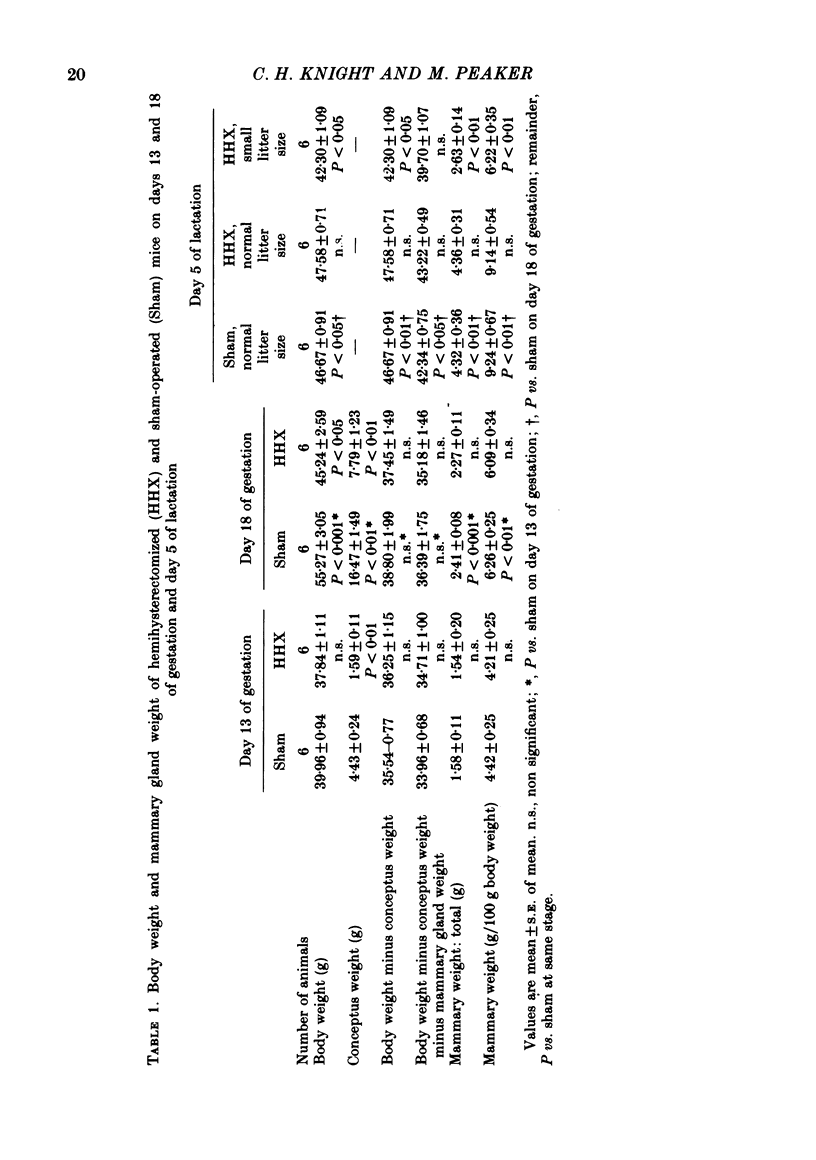
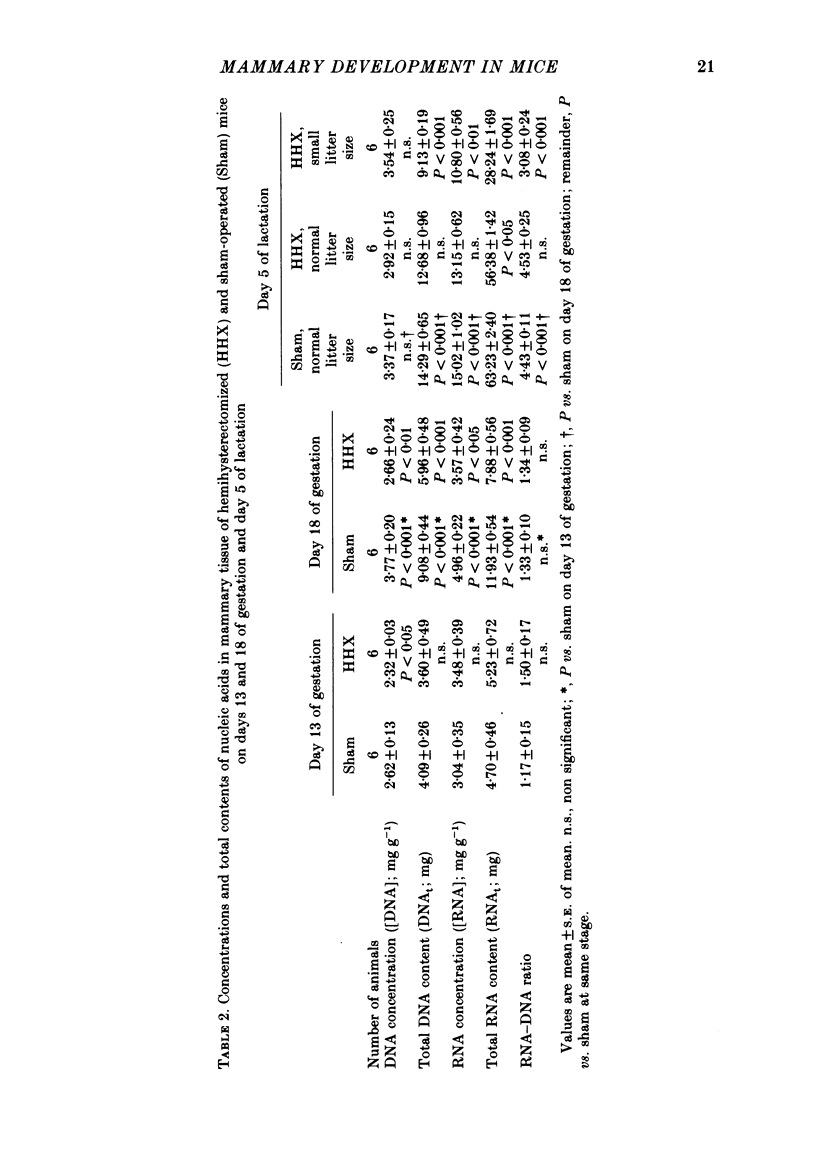
3. Pregnant sham-operated and hemihysterectomized animals were killed on days 13 and 18 of gestation, and their mammary glands were analysed for total DNA (DNAt) and RNA (RNAt). Both were significantly lower in the hemihysterectomized group on day 18, but not on day 13.
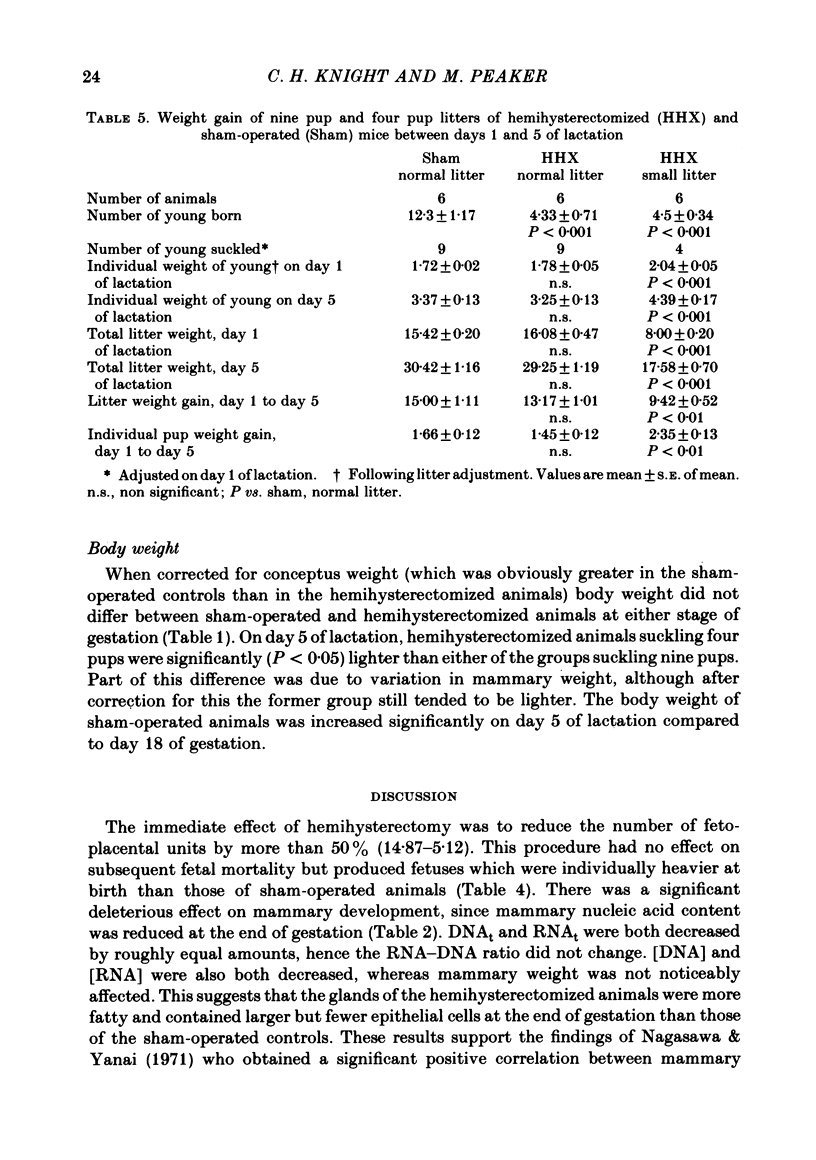
4 Milk yield was assessed, by daily weighing of the litter, in groups of sham-operated and hemihysterectomized lactating animals suckling nine pups each. There was no difference in yield between the two groups.
5. One group of sham-operated mice suckling nine pups, one of hemihysterectomized mice suckling nine pups and one of hemihysterectomized mice suckling four pups were killed on day 5 of lactation for mammary gland analysis. There was no significant difference in mammary weight or DNAt between the sham-operated and hemihysterectomized animals suckling nine pups, although RNAt was still reduced in the latter. Mammary weight, DNAt and RNAt were all significantly lower in the hemihysterectomized group suckling four pups than in either of the other two groups.
6. It is concluded that the less well developed mammary glands of mice which give birth to small litters are capable of compensatory growth during the first few days of lactation if a sufficiently strong suckling stimulus is given.
7. It is suggested that control of mammary development by the fetus during gestation and by the suckling young during early lactation are both mechanisms designed to ensure that milk yield is appropriate to the needs of the young.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. R. Mammary gland growth in the hypophysectomized pregnant rat (38522). Proc Soc Exp Biol Med. 1975 Jan;148(1):283–287. doi: 10.3181/00379727-148-38522. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. R., Mepham T. B., Lock K. J. Relative importance of pre-partum and post-partum factors in the control of milk yield in the guinea-pig. J Dairy Res. 1979 Nov;46(4):613–621. doi: 10.1017/s0022029900020677. [DOI] [PubMed] [Google Scholar]
- Eisen E. J., Nagai J., Bakker H., Hayes J. F. Effect of litter size at birth on lactation in mice. J Anim Sci. 1980 Apr;50(4):680–688. doi: 10.2527/jas1980.504680x. [DOI] [PubMed] [Google Scholar]
- Hayden T. J., Thomas C. R., Forsyth I. A. Effect of number of young born (litter size) on milk yield of goats: role for placental lactogen. J Dairy Sci. 1979 Jan;62(1):53–63. doi: 10.3168/jds.S0022-0302(79)83201-4. [DOI] [PubMed] [Google Scholar]
- Hayden T. J., Thomas C. R., Smith S. V., Forsyth I. A. Placental lactogen in the goat in relation to stage of gestation, number of fetuses, metabolites, progesterone and time of day. J Endocrinol. 1980 Aug;86(2):279–290. doi: 10.1677/joe.0.0860279. [DOI] [PubMed] [Google Scholar]
- Knight C. H., Peaker M. Mammary cell proliferation in mice during pregnancy and lactation in relation to milk yield. Q J Exp Physiol. 1982 Jan;67(1):165–177. doi: 10.1113/expphysiol.1982.sp002610. [DOI] [PubMed] [Google Scholar]
- Markoff E., Talamantes F. Serum placental lactogen in mice in relation to day of gestation and number of conceptuses. Biol Reprod. 1981 May;24(4):846–851. doi: 10.1095/biolreprod24.4.846. [DOI] [PubMed] [Google Scholar]
- Moon R. C. Mammary growth and milk yield as related to litter size. Proc Soc Exp Biol Med. 1969 Apr;130(4):1126–1128. doi: 10.3181/00379727-130-33734. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Yanai R. Quantitative participation of placental mammotropic hormones in mammary development during pregnancy of mice. Endocrinol Jpn. 1971 Dec;18(6):507–510. doi: 10.1507/endocrj1954.18.507. [DOI] [PubMed] [Google Scholar]
- Rattray P. V., Garrett W. N., East N. E., Hinman N. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J Anim Sci. 1974 Mar;38(3):613–626. doi: 10.2527/jas1974.383613x. [DOI] [PubMed] [Google Scholar]
- Roth L. L., Rosenblatt J. S. Self-licking and mammary development during pregnancy in the rat. J Endocrinol. 1968 Nov;42(3):363–378. doi: 10.1677/joe.0.0420363. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Jenkin G., Robinson J. S., Thorburn G. D., Friesen H., Chan J. S. Concentrations of placental lactogen in chronically catheterized ewes and fetuses in late pregnancy. J Endocrinol. 1980 Apr;85(1):27–34. doi: 10.1677/joe.0.0850027. [DOI] [PubMed] [Google Scholar]
- Tucker H. A. Regulation of mammary nucleic acid content by various suckling intensities. Am J Physiol. 1966 Jun;210(6):1209–1214. doi: 10.1152/ajplegacy.1966.210.6.1209. [DOI] [PubMed] [Google Scholar]


