Abstract
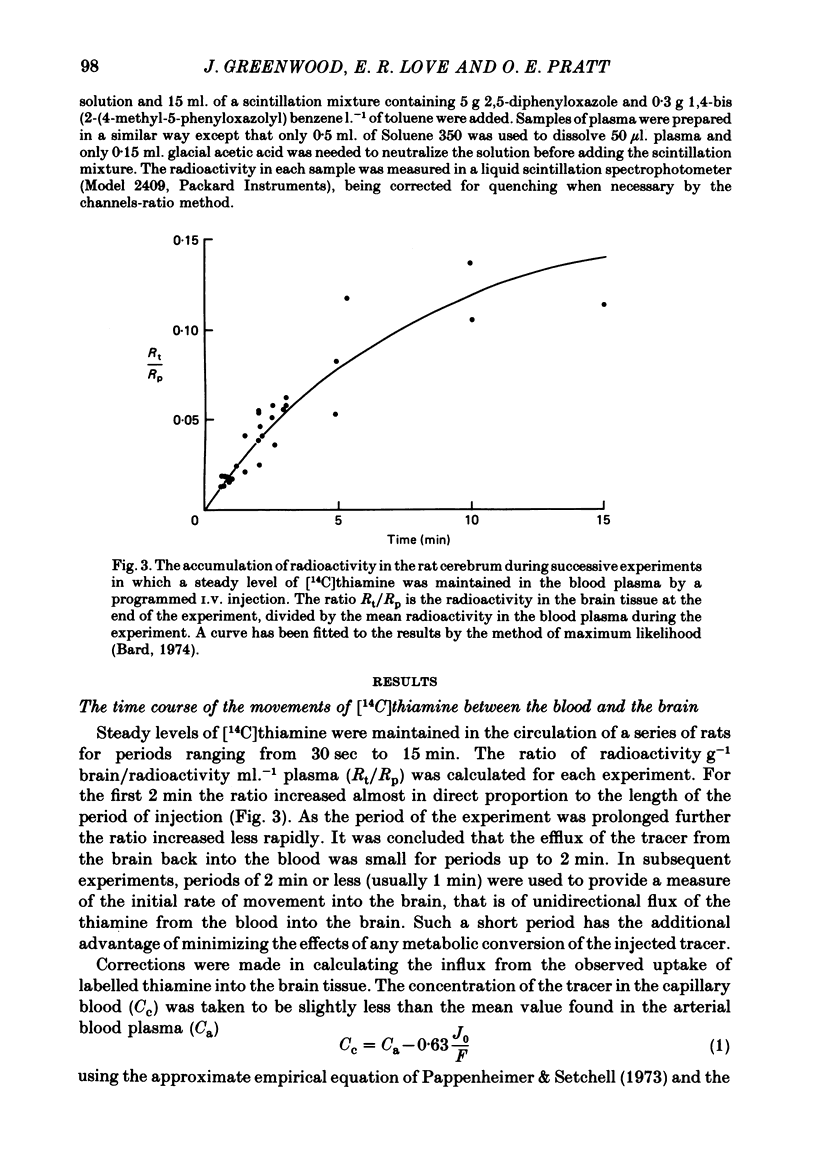
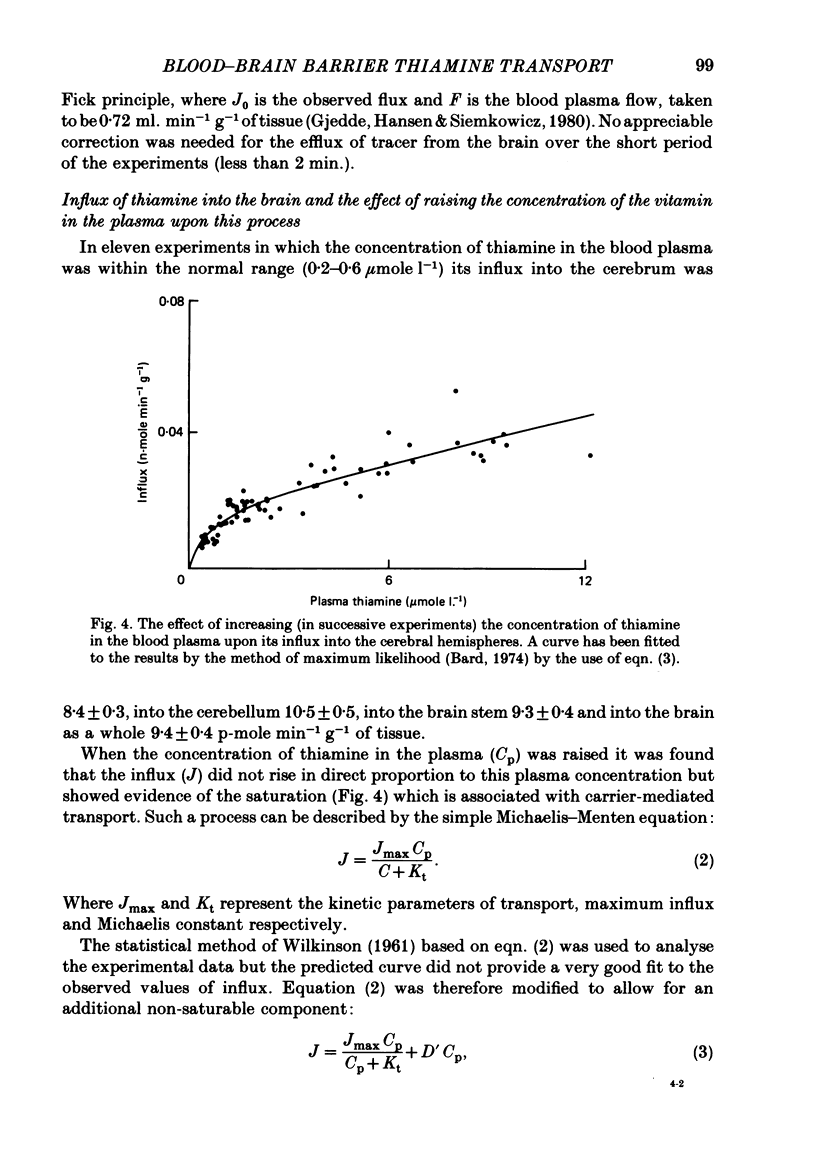
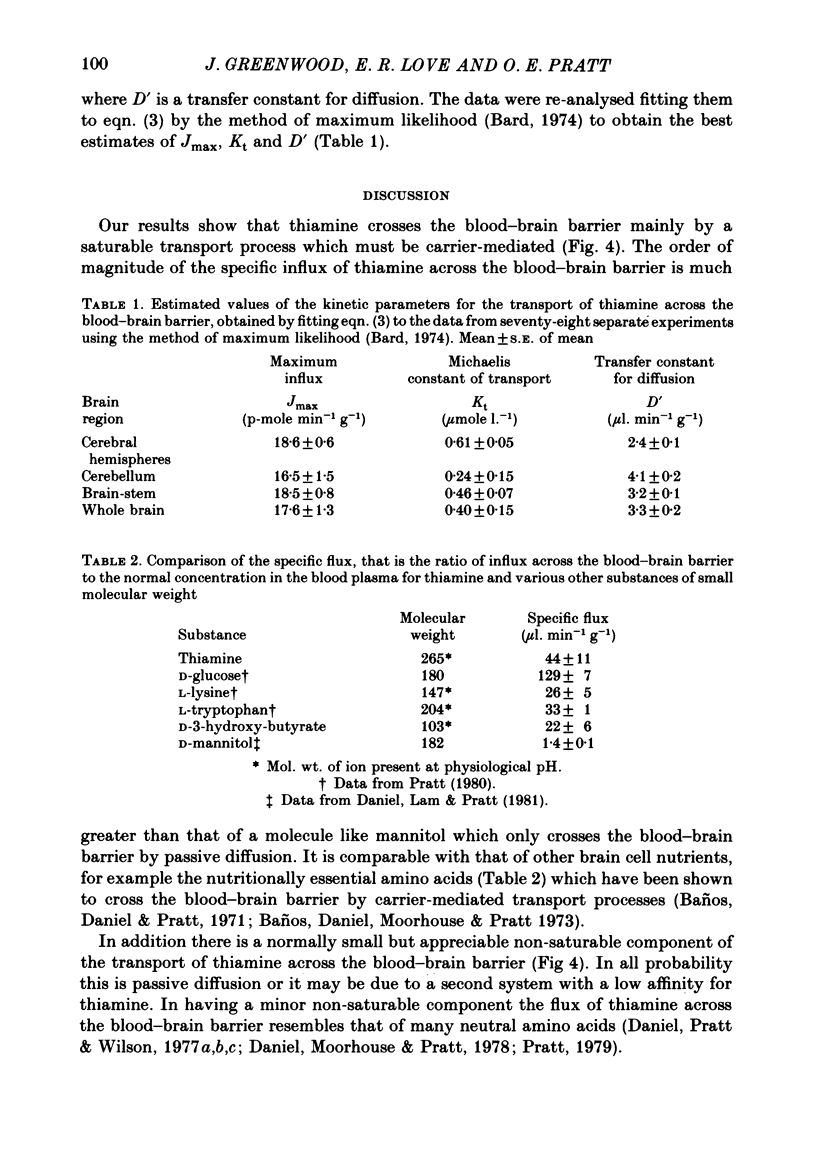
1. By measurement of the rate of disappearance of injected tracer thiamine from the bloodstream, a programme for the continuous injection of thiamine at a variable rate has been devized by which a steady raised level can be achieved rapidly and maintained in the circulation. By this means the flux of radioactive thiamine across the blood-brain barrier has been measured. 2. In separate experiments progressively higher levels of thiamine were maintained in the bloodstream. Evidence was obtained that the transport of thiamine across the blood-brain barrier is a carrier-mediated process which can be saturated by raised levels of thiamine. 3. The saturation of the transport process was incomplete: kinetic analysis showed that there was a non-saturable component of the transport which was probably due to passive diffusion. 4. The contribution of the non-saturable component was normally small and is probably insufficient to meet the needs of the brain for the vitamin unless the concentration of the vitamin in the blood is raised considerably above normal. 5. This two-component transport process had substantially similar kinetic parameters in different regions of the brain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baños G., Daniel P. M., Moorhouse S. R., Pratt O. E. The influx of amino acids into the brain of the rat in vivo: the essential compared with some non-essential amino acids. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):59–70. doi: 10.1098/rspb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Baños G., Daniel P. M., Pratt O. E. Inhibition of entry of L-arginine into the brain of the rat, in vivo, by L-lysine or L-ornithine. J Physiol. 1971;214 (Suppl):24P–25P. [PubMed] [Google Scholar]
- Christensen H. N. Metabolite transport at cell membranes. Adv Exp Med Biol. 1976;69:3–12. doi: 10.1007/978-1-4684-3264-0_1. [DOI] [PubMed] [Google Scholar]
- DREYFUS P. M. The quantitative histochemical distribution of thiamine in deficient rat brain. J Neurochem. 1961 Nov;8:139–145. doi: 10.1111/j.1471-4159.1961.tb13535.x. [DOI] [PubMed] [Google Scholar]
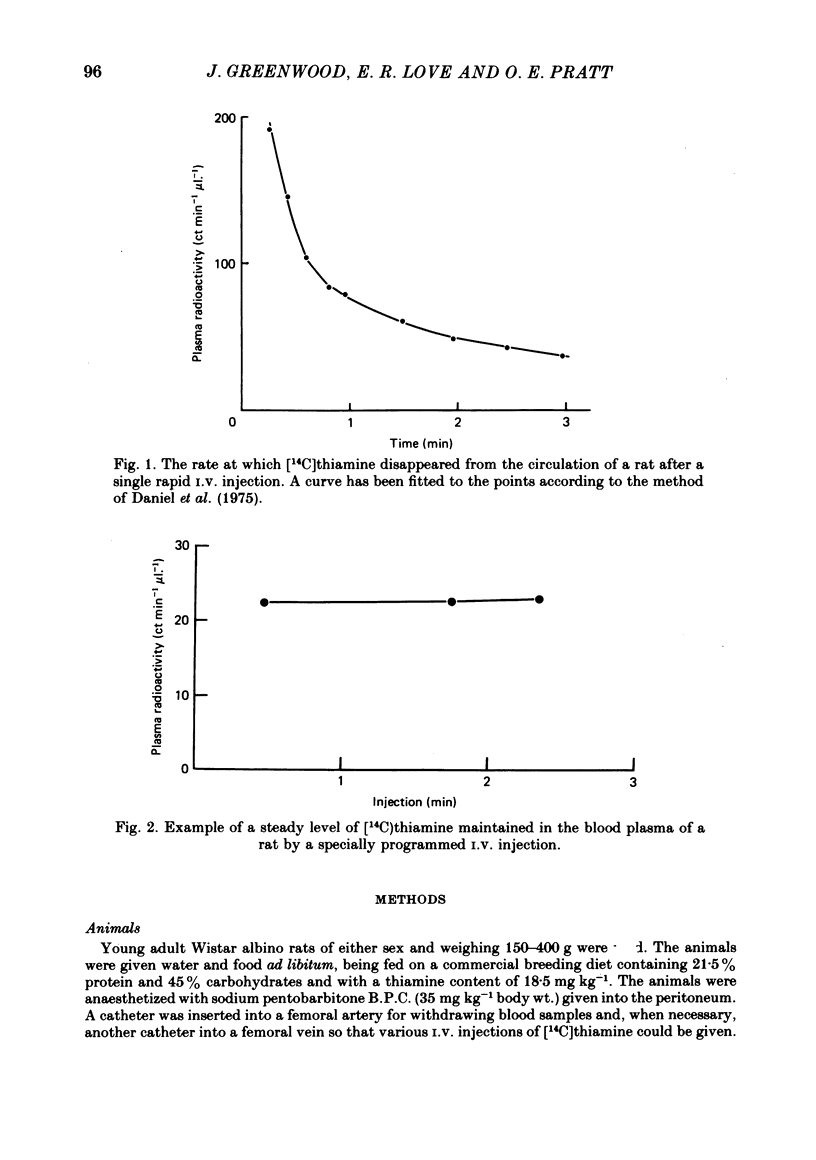
- Daniel P. M., Donaldson J., Pratt O. E. A method for injecting substances into the circulation to reach rapidly and to maintain a steady level. With examples of its application in the study of carbohydrate and amino acid metabolism. Med Biol Eng. 1975 Mar;13(2):214–227. doi: 10.1007/BF02477731. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Lam D. K., Pratt O. E. Changes in the effectiveness of the blood-brain and blood-spinal cord barriers in experimental allergic encephalomyelitis. J Neurol Sci. 1981 Nov-Dec;52(2-3):211–219. doi: 10.1016/0022-510x(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Moorhouse S. R., Pratt O. E., Wilson P. A method for rapidly washing the blood out of an organ or tissue of the anaesthetized living animal. J Physiol. 1974 Mar;237(2):11P–12P. [PubMed] [Google Scholar]
- Daniel P. M., Pratt O. E., Wilson P. A. The exclusion of L-isoleucine or of L-leucine from the brain of the rat, caused by raised levels of L-valine in the circulation, and the manner in which this exclusion can be partially overcome. J Neurol Sci. 1977 Apr;31(3):421–431. doi: 10.1016/0022-510x(77)90219-2. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Pratt O. E., Wilson P. A. The influx of isoleucine into the cerebral hemispheres and cerebellum: carrier-mediated transport and diffusion. Q J Exp Physiol Cogn Med Sci. 1977 Apr;62(2):163–173. doi: 10.1113/expphysiol.1977.sp002386. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Pratt O. E., Wilson P. A. The transport of L-leucine into the brain of the rat in vivo: saturable and non-saturable components of influx. Proc R Soc Lond B Biol Sci. 1977 Mar 18;196(1124):333–346. doi: 10.1098/rspb.1977.0044. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Hansen A. J., Siemkowicz E. Rapid simultaneous determination of regional blood flow and blood-brain glucose transfer in brain of rat. Acta Physiol Scand. 1980 Apr;108(4):321–330. doi: 10.1111/j.1748-1716.1980.tb06540.x. [DOI] [PubMed] [Google Scholar]
- Itokawa Y., Schulz R. A., Cooper J. R. Thiamine in nerve membranes. Biochim Biophys Acta. 1972 Apr 14;266(1):293–299. doi: 10.1016/0005-2736(72)90144-7. [DOI] [PubMed] [Google Scholar]
- LEIGH D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry. 1951 Aug;14(3):216–221. doi: 10.1136/jnnp.14.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D., Faulkner W. R., Price J. W., Smeby R. R. Intermittent cerebellar ataxia associated with hyperpyruvic acidemia, hyperalaninemia, and hyperalaninuria. Pediatrics. 1969 Jun;43(6):1025–1034. [PubMed] [Google Scholar]
- Lumeng L., Edmondson J. W., Schenker S., Li T. K. Transport and metabolism of thiamin in isolated rat hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7265–7268. [PubMed] [Google Scholar]
- McCandless D. W., Schenker S., Cook M. Encephalopathy of thiamine deficieny: studies of intracerebral mechanisms. J Clin Invest. 1968 Oct;47(10):2268–2280. doi: 10.1172/JCI105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock D. S., Gubler C. J. Effects of thiamine deficiency and treatment with the antagonists, oxythiamine and pyrithiamine, on the levels and distribution of thiamine derivatives in rat brain. J Nutr Sci Vitaminol (Tokyo) 1973;19(3):237–249. doi: 10.3177/jnsv.19.237. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Setchell B. P. Cerebral glucose transport and oxygen consumption in sheep and rabbits. J Physiol. 1973 Sep;233(3):529–551. doi: 10.1113/jphysiol.1973.sp010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrini C., Rindi G. An improved method for the electrophoretic separation and fluorometric determination of thiamine and its phosphates in animal tissues. Int J Vitam Nutr Res. 1980;50(1):10–18. [PubMed] [Google Scholar]
- Pratt O. E. An electronically controlled syringe drive for giving an injection at a variable rate according to a preset programme. J Physiol. 1974 Mar;237(2):5P–6P. [PubMed] [Google Scholar]
- Pratt O. E. Kinetics of tryptophan transport across the blood-brain barrier. J Neural Transm Suppl. 1979;(15):29–42. doi: 10.1007/978-3-7091-2243-3_3. [DOI] [PubMed] [Google Scholar]
- Rindi G., De Giuseppe L., Sciorelli G. Thiamine monophosphate, a normal constituent of rat plasma. J Nutr. 1968 Apr;94(4):447–454. doi: 10.1093/jn/94.4.447. [DOI] [PubMed] [Google Scholar]
- Rindi G., Patrini C., Comincioli V., Reggiani C. Thiamine content and turnover rates of some rat nervous regions, using labeled thiamine as a tracer. Brain Res. 1980 Jan 13;181(2):369–380. doi: 10.1016/0006-8993(80)90619-8. [DOI] [PubMed] [Google Scholar]
- Rindi G., Ventura U. Phosphorylation and uphill intestinal transport of thiamine, in vitro. Experientia. 1967 Mar 15;23(3):175–176. doi: 10.1007/BF02136267. [DOI] [PubMed] [Google Scholar]
- Rindi G., Ventura U. Thiamine intestinal transport. Physiol Rev. 1972 Oct;52(4):821–827. doi: 10.1152/physrev.1972.52.4.821. [DOI] [PubMed] [Google Scholar]
- SHARMA S. K., QUASTEL J. H. TRANSPORT AND METABOLISM OF THIAMINE IN RAT BRAIN CORTEX IN VITRO. Biochem J. 1965 Mar;94:790–800. doi: 10.1042/bj0940790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R. Thiamine transport in the central nervous system. Am J Physiol. 1976 Apr;230(4):1101–1107. doi: 10.1152/ajplegacy.1976.230.4.1101. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


