Abstract
The regulation of neurotrophin (NT) secretion is critical for many aspects of NT-mediated neuronal plasticity. Neurons release NTs by activity-regulated secretion pathways, initiated either by neurotransmitters and/or by existing NTs by a positive-feedback mechanism. This process depends on calcium release from intracellular stores. Little is known, however, about potential pathways that down-regulate NT secretion. Here we demonstrate that nitric oxide (NO) induces a rapid down-regulation of brain-derived neurotrophic factor (BDNF) secretion in cultured hippocampal neurons. Similar effects occur by activating a downstream target of intracellular NO, the soluble guanylyl cyclase, or by increasing the levels of its product, cGMP. Furthermore, down-regulation of BDNF secretion is mediated by cGMP-activated protein kinase G, which prevents calcium release from inositol 1,4,5-trisphosphate-sensitive stores. Our data indicate that the NO/cGMP/protein kinase G pathway represents a signaling mechanism by which neurons can rapidly down-regulate BDNF secretion and suggest that, in hippocampal neurons, NT secretion is finely tuned by both stimulatory and inhibitory signals.
Neurotrophins (NTs), such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-4/5 (NT-4/5), and neurotrophin-3 (NT-3), regulate neuronal survival and differentiation during embryonic development (1, 2). In addition to their trophic role, NTs are thought to participate in certain brain functions such as modulation of synaptic transmission and memory formation (3–6). NTs have been shown to modulate synaptic transmission across a broad temporal spectrum ranging from short-term modulation, which occurs in the order of seconds to minutes (7–17), to a prolonged effect that persists for many hours, such as the long-term potentiation (LTP) (18–23) or long-term depression (24–27) response. In fact, NTs are required for the maintenance of LTP in hippocampal slices, because inhibition of BDNF signaling by using receptor bodies applied early after LTP induction restored potentiated synaptic transmission to baseline levels (22). In addition, pretreatment of hippocampal neuron slices with anti-NT receptor antiserum prevented the late phase of the LTP (22). It has been suggested that BDNF concentrations in CA3/CA1 hippocampal slices must reach a critical threshold level to initiate and maintain the LTP response (18). This phenomenon has been demonstrated in heterozygous BDNF-defective mice (18, 20) that, having impaired endogenous NT production, require either the exogenous administration (20) or local re-expression (19) of BDNF to initiate the LTP response. These observations emphasize the important role played by NTs in modulating synaptic activity and the need to understand better the mechanisms that regulate NT secretion.
Recent studies have investigated how neuronal activity can modulate NT secretion. NGF and BDNF secretion is induced in hippocampal slices and cultured hippocampal neurons in response to excitatory neurotransmitters such as glutamate (28–31) or acetylcholine (29), and secretion of NTs is sustained by a positive-feedback mechanism (30, 32). Recent studies also have demonstrated that electrical activity alone can mediate BDNF secretion in primary sensory neurons (33), which is consistent with studies in which increased intracellular cAMP levels (34) or potassium-mediated depolarization (28, 31, 35), applied to mimic neuronal activity, mediated NT secretion in both neuronal and nonneuronal settings.
At the molecular level, the secretion of NTs has been initiated by the activation of selected neurotransmitter (29, 36) and NT receptors (30, 32, 36). Downstream events mediated by a defined intracellular signaling pathway lead to NT secretion that depends on calcium mobilization from intracellular stores (28–31) by way of the activation of a specific phospholipase C (36). The intracellular localization of NTs also correlates with their potential to undergo regulated release (37–41), which ultimately requires the docking of vesicles at the plasma membrane by way of the cooperation of the N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor proteins, soluble NSF attachment protein-25 and syntaxin (42).
Despite the large body of evidence that describes positive regulatory mechanisms, nothing is known about the negative modulation of NT secretion. We demonstrate here that nitric oxide (NO) induces a rapid negative regulation of BDNF secretion in hippocampal neurons, by way of the activation of a cGMP-dependent signal transduction pathway.
Materials and Methods
Primary Culture of Hippocampal Neurons.
Hippocampal neurons were prepared from embryonic day 17 rat embryos (43). Neurons (200,000) were plated on 13-mm glass coverslips precoated with poly-dl-ornithine (0.5 mg/ml). Neurons were used for experimental purposes after 5 days of in vitro development. The purity of the culture was determined by immunocytochemistry by using neuronal and astroglial-specific markers. Cells were used for experimental purposes when more than 90% of the cells expressed the neuronal marker microtubule-associated protein-2 and less than 5% expressed the glial fibrillary acidic protein specific for astroglial cells (data not shown).
BDNF Release Experiments.
Because cultured hippocampal neurons do not express sufficient BDNF for a reliable quantification, we proceeded to overexpress BDNF by using an adenoviral gene-transfer system (30). Before the experiments, neurons were infected for 36 h with 1010 to 1011 plaque-forming units/ml of the AdCMV-BDNF viral vector containing the cDNA sequence encoding mouse prepro-BDNF (30–31, 36). BDNF release experiments were performed as reported (31). In brief, the infected neurons were equilibrated for 60 min in Hanks' buffer; 500 μl of the same buffer was then added to the cultures, incubated at 37°C or 15°C, and subjected to BDNF quantification. The time course of BDNF secretion was performed by replacing and collecting the buffer every 10 min. Three fractions were collected, and the average value was taken as the “control” level. BDNF content, expressed as a percent of the control, was evaluated in the absence (basal) or presence of 10 μM sodium nitroprusside (SNP)/300 μM (±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide (NOR3)/100 μM 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC1) /1 mM 8-bromo-cGMP (8Br-cGMP)/40 μM KT5823/1 mM L-arginine-methyl ester (L-NAME), or a mixture of glutamate receptor antagonists 100 μM 6-cyano-7-nitroquinoxaline-2,3-dione/50 μM D(−)-2-amino-5-phosphonopentanoic acid/1 mM 1-aminoindan-1,5-dicarboxyl acid added to the incubation buffer. Three additional fractions were collected after the stimulation was removed.
BDNF Detection.
BDNF detection was performed by ELISA. Two mAbs derived from immunized mice were used (30). Samples were loaded onto the plates, previously coated with the primary antibody, together with the horseradish peroxidase-conjugated secondary antibody, and incubated overnight at 4°C. After washing, bound secondary antibody was detected by 3,3′,5,5′-tetramethylbenzidine peroxidase for 30 min. A standard curve was generated by using recombinant BDNF, and the ELISA reached a sensitivity of 0.5 pg/ml.
NO Release from NO Donors.
Kinetics of NO release from NO donors was evaluated in vitro in Hanks' buffer at 37°C and 15°C. The signal developed from the interaction between the NO donors, SNP and NOR3, and the specific fluorescent (excitation 495; emission 515) NO probe, diaminofluorescein, was acquired in a multilabel, temperature-controlled plate reader.
Western Blot.
Proteins were extracted from hippocampal neurons with lysis buffer containing 20 mM Tris⋅HCl (pH 8), 100 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1 mM benzamidine, 1% Nonidet P-40, 1 mM PMSF, 10 mM p-nitrophenylphosphate, 1 mM DTT, 10 mM glycerophosphate, and 1 g/ml aprotinin, leupeptin, and pepstatin. Aliquots containing 80 μg of protein were analyzed by SDS/PAGE (7.5% acrylamide in the resolving gel). Resolved proteins were then electrophoretically transferred onto a nitrocellulose membrane for 1 h. The membrane was saturated with 5% low-fat dry milk, 0.05% Tween 20 in 10 mM Tris⋅HCl, pH 8/150 mM NaCl for 1 h, and then incubated with either anti-endothelial NO synthase (eNOS), anti-neuronal (nNOS), or anti-inducible NOS (iNOS) antibody (1:1,000; Transduction Laboratories, Lexington, KY) for 1 h. After being washed three times, membranes were incubated for 30 min in peroxidase-conjugated secondary anti-mouse IgG (1:2,500; Transduction Laboratories) and the specific NOS signal detected by enhanced chemiluminescence.
Immunohistochemistry and Confocal Microscopy.
The cellular distribution of nNOS was analyzed in cultured hippocampal neurons fixed for 20 min with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. The cells were incubated overnight at 4°C with an anti-nNOS antibody (1:250) and subsequently treated with Cy3-conjugated secondary anti-mouse IgG (1:250). After extensive washing, coverslips were sealed in mounting medium and the fluorescent signal was analyzed by confocal microscopy (Bio-Rad 1024).
NOS Assay.
NO synthesis was evaluated by monitoring L-[3H]citrulline formation from L-[2,3-3H]arginine. Cells were equilibrated for 30 min at 37°C in 20 mM Hepes buffer, pH 7.4, and then incubated for 30 min at 37°C in 1 ml of 20 mM Hepes buffer containing 10 μM L-arginine and 1 μCi per plate L-[2,3-3H]arginine (NEN, 40.5 Ci/mmol specific activity). L-NAME (1 mM) was added to selected plates. The reaction was stopped by washing the cells with ice-cold Hepes buffer containing 5 mM L-arginine and 4 mM EDTA (stop buffer). After the supernatant was discarded, 100 μl of ethanol was added to the cells and allowed to evaporate before the addition of 2 ml of 20 mM Hepes, pH 5.5. After 5 min, 1 ml of this supernatant was mixed with 0.4 ml of Dowex slurry AG50W-X8 (Na+ form) equilibrated in stop buffer and vortexed for 30 min. Samples (0.5 ml) were collected from the supernatant, mixed with 2 ml of UltimaGold scintillation mixture, and counted in a liquid scintillation spectrometer (Canberra Packard MINAXI Tri-Carb 4,000 series). Data were expressed as picomoles of NO per sample per 30 min.
Results
Pattern of BDNF Secretion in Cultured Hippocampal Neurons.
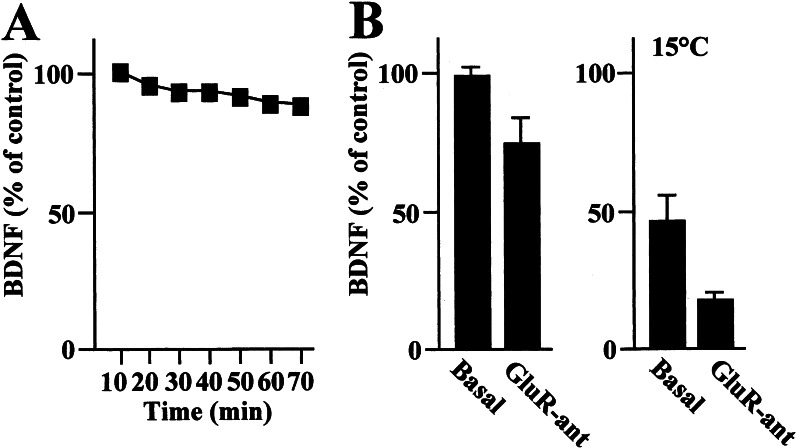
The secretion of BDNF into the medium of cultured rat hippocampal neurons infected with a viral vector containing the mouse prepro-BDNF coding sequence was stable during the experimental time course shown in Fig. 1A. Spontaneous NT secretion from hippocampal neurons has been described as resulting from the combination of a “constitutive” and a “regulated” pathway (29). The constitutive component of this pathway is a temperature-dependent process that is prevented by lowering the temperature from 37°C to 15°C, whereas regulated secretion is largely mediated by glutamate receptor stimulation at both temperatures. To test the extent to which each pathway was active in our system, a mixture of ionotropic and metabotropic glutamate receptor antagonists [GluR-ant; comprising 6-cyano-7-nitroquinoxaline-2,3-dione (100 μM) selective for the glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, D(−)-2-amino-5-phosphonopentanoic acid (50 μM) selective for the glutamate N-methyl-D-aspartate receptor, and 1-aminoindan-1,5-dicarboxyl acid (1 mM) selective for the glutamate metabotropic type I receptor] was added to the incubation. This treatment caused a 26 ± 7% reduction in BDNF secretion from basal levels at 37°C (Fig. 1B) that is likely to reflect the regulated component of NT secretion. Indeed, GluR-ant induced a similar down-regulation of BDNF secretion at the nonpermissive temperature (15°C) for the constitutive secretion (Fig. 1B). In our system, the constitutive component of BDNF secretion represents 54 ± 7% of the total basal output (Fig. 1B).
Figure 1.
BDNF secretion from primary hippocampal neurons. (A) Representative time course of BDNF spontaneously secreted from primary hippocampal neurons. BDNF levels were evaluated during a 70-min period in 10-min fractions collected from neurons cultured at 37°C as described in Materials and Methods. (B) BDNF secretion was evaluated in the absence (Basal) or presence of a mixture of GluR-ant comprising 100 μM 6-cyano-7-nitroquinoxaline-2,3-dione, 50 μM d(−)-2-amino-5-phosphonopentanoic acid, 1 mM 1-aminoindan-1,5-dicarboxyl acid added to the incubation buffer for 20 min at 37°C or 15°C. GluR-ant administration induces a reduction of BDNF levels at both temperatures. Values are given as mean ± SE (n = 12).
Endogenous Production of NO Modulates BDNF Secretion in Cultured Hippocampal Neurons.
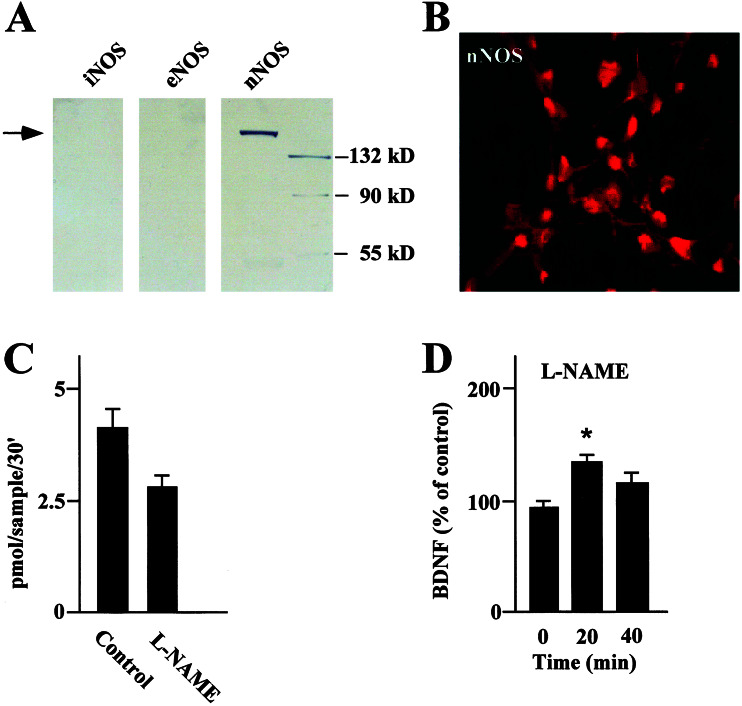
To understand whether endogenous NO could regulate BDNF secretion we used a pharmacological inhibition of NOSs. NO is formed by two constitutively expressed NOSs (endothelial NOS and nNOS) and a third inducible NOS isoform (46). nNOS was the only isoform detected in our hippocampal neurons by Western blot analysis (Fig. 2A). nNOS was expressed in most of the neurons, being distributed throughout the cell body and dendritic processes as demonstrated by immunocytochemistry (Fig. 2B). Finally, nNOS has been shown to mediate NO production by monitoring L-[3H]citrulline formation from L-[2,3-3H]arginine in cultured hippocampal neurons. As expected, pretreatment of cultured hippocampal neurons with 1 mM N-nitro-L-NAME, a specific inhibitor of NOSs (47), decreased the levels of detectable L-[3H]citrulline (Fig. 2C). The possibility of a causal relationship between selective reduction in NO and BDNF secretion was investigated by treating hippocampal neurons with the same concentration of L-NAME and measuring changes in BDNF levels. This protocol increased the levels of BDNF secretion to above baseline levels after 20 min (Fig. 2D), although the effect was transient and underwent desensitization.
Figure 2.
(A) Western blots of NOS isoforms (neuronal NOS, endothelial NOS, and inducible NOS) in extracts of hippocampal neurons performed by using selective antibodies. Only nNOS was significantly expressed at the predicted molecular weight (arrow). (B) Expression of nNOS in hippocampal primary neurons evaluated by immunocytochemistry. Shown is a representative image from a confocal scan along the x-y plane. nNOS displays a uniform distribution in the cell body and in the dendritic processes of most of the neurons. (C) NO synthesis evaluated by monitoring L-[3H]citrulline formation from L-[2,3-3H]arginine. The level of NO production was evaluated in the absence (Control) or presence of 1 mM L-NAME, a NOS inhibitor. Data were expressed as picomoles of NO per sample per 30 min. (D) BDNF secretion evaluated in the absence (0 min) or presence (10 and 20 min) of L-NAME. Values are given as mean ± SE (n = 10). * indicates statistical significance of the difference from the control at P < 0.05.
Exogenous Administration of NO Mediates Down-Regulation of BDNF Secretion.
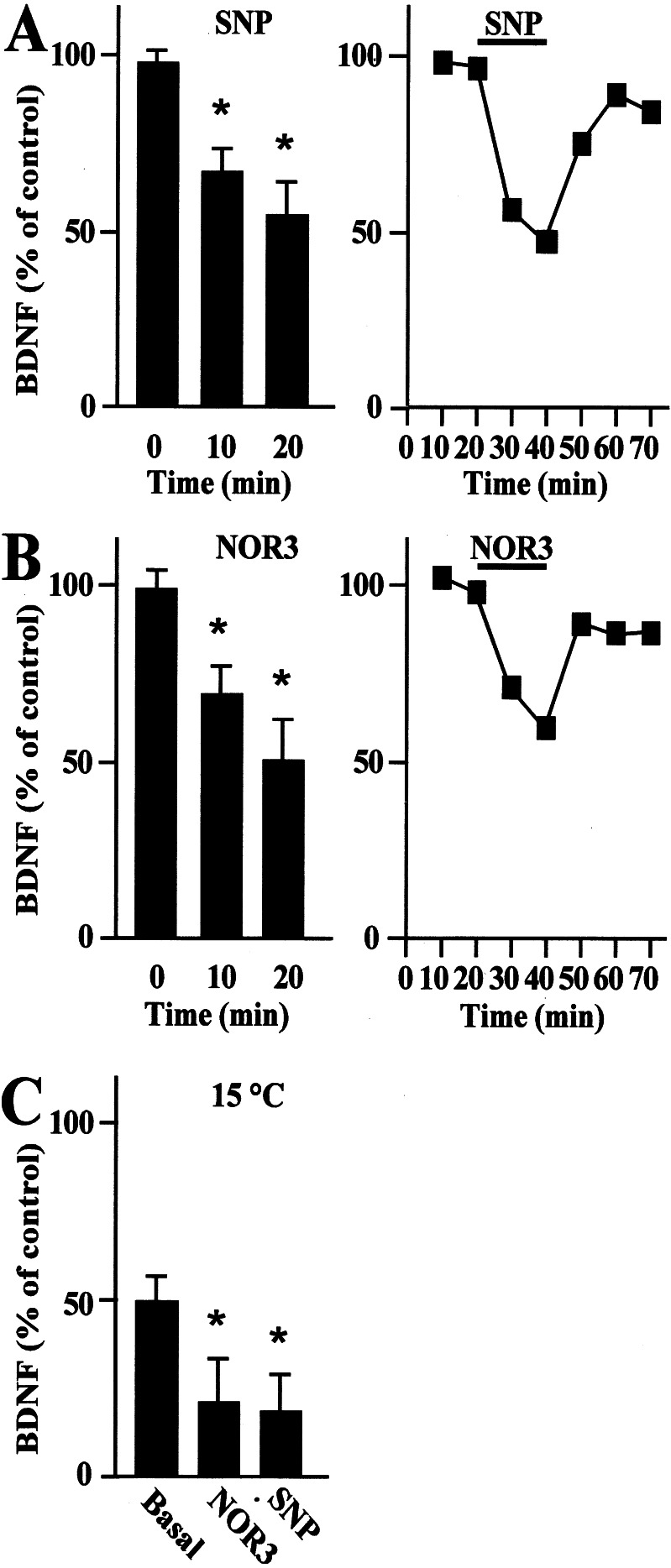
Studies were extended to investigate whether exogenous NO administration to hippocampal neurons regulates BDNF secretion. Cultured hippocampal neurons treated with 10 μM NO-donor SNP showed a 46 ± 6% decrease from basal levels of secreted BDNF after 20 min (Fig. 3A). A similar reduction in BDNF secretion was achieved by using an alternative NO donor, 300 μM NOR3 (Fig. 3B). BDNF secretion returned slowly to baseline levels on donor removal (Fig. 3 A and B). The inhibition of BDNF secretion in response to NO occurred also at 15°C (Fig. 3C), suggesting that only the regulated secretory pathway was affected by this treatment.
Figure 3.
Time course of BDNF secretion from primary hippocampal neurons treated with the NO donors SNP or NOR3. BDNF secretion was evaluated after the administration of 10 μM SNP (A) and 300 μM NOR3 (B) to cultured neurons. BDNF secretion was detected in the absence (0 min) or presence (10 and 20 min) of the indicated donor (Left). Values are given as mean ± SE (n = 10). * indicates statistical significance of the difference from the control at P < 0.02. In a representative experiment (Right), the respective NO donor administrated for 20 min caused a rapid down-regulation of BDNF secretion that partially recovered to baseline on withdrawal of the donor. (C) BDNF secretion evaluated after the administration of 10 μM SNP and 300 μM NOR3 at the reduced temperature of 15°C. Lowering the temperature from 37°C to 15°C caused a reduction of BDNF from basal levels that was further inhibited upon administration of SNP or NOR3 for 20 min. Values are given as mean ± SE (n = 6). * indicates statistical significance of the difference from the basal at P < 0.05.
SNP and NOR3 were shown to generate NO after linear kinetics under the given experimental conditions as was determined by using the specific fluorescent NO probe diaminofluorescein (data not shown).
Increased Levels of cGMP Lead to Down-Regulation of BDNF Secretion.
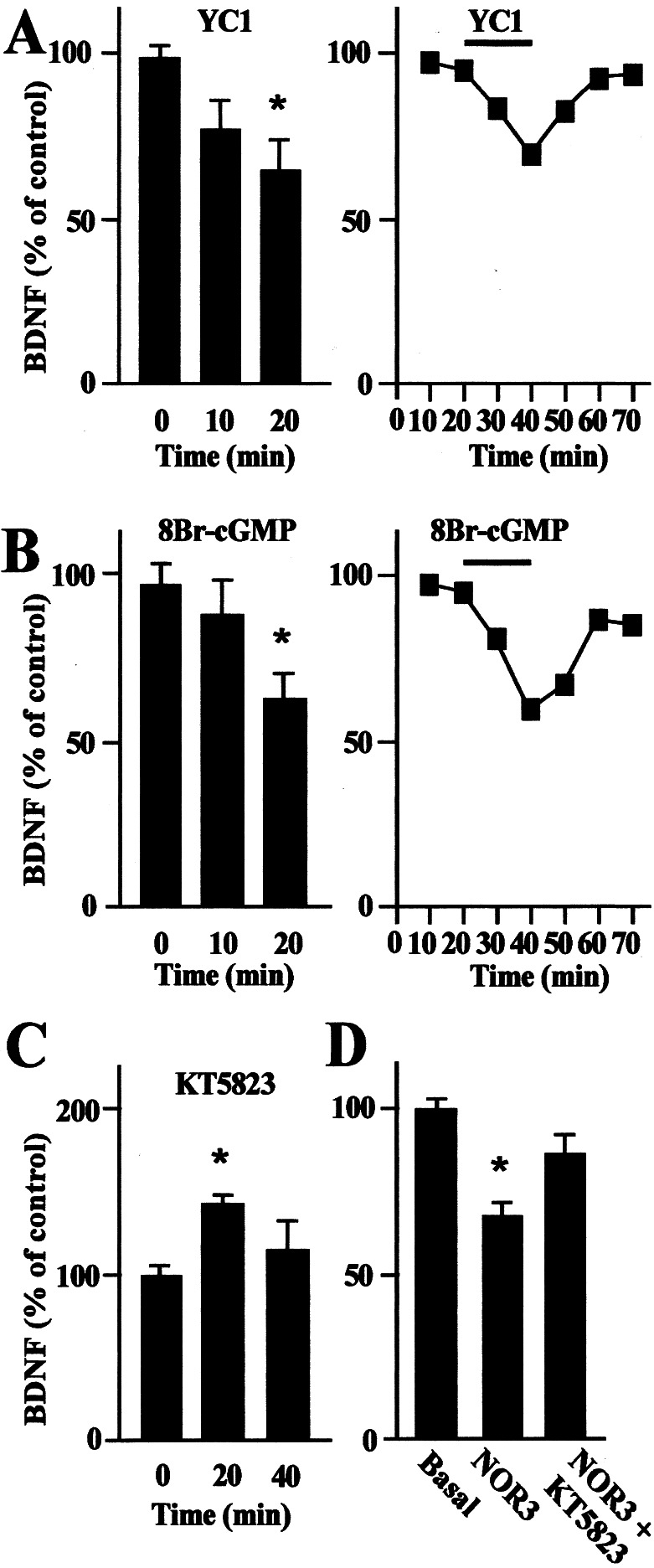
We initially characterized the NO-mediated signal transduction mechanisms that lead to down-regulation of BDNF secretion by activating soluble guanylyl cyclase (sGC). Cultured hippocampal neurons were treated with 100 μM YC1, an agonist of sGC (44). YC1 caused a reduction in basal BDNF secretion of 31 ± 5% after 20 min (Fig. 4A). It was not possible to prevent this effect by pretreatment with the sGC antagonist 1H-[1,2,4]oxadiazole [4,3-a]quinoxalin-1-one (45), because this molecule impaired BDNF quantification by interfering with the detection procedure (data not shown). In addition, we have investigated events downstream of the sGC-mediated signaling pathways. It is known that sGC mediates cGMP production, which prompted an investigation into whether increased levels of cGMP mediate down-regulation of BDNF secretion. Indeed, basal BDNF secretion was reduced by 42 ± 5% by treating cultured neurons directly with 1 mM 8Br-cGMP, a highly membrane-permeable form of cGMP (48) (Fig. 4B). Down-regulation of BDNF secretion was not observed by increasing the intracellular levels of cAMP (data not shown).
Figure 4.
Time course of BDNF secretion mediated by the administration of YC1, 8Br-cGMP, and KT5823. (A) BDNF secretion evaluated in the absence (0 min) or presence (10 and 20 min) of 100 μM YC1, an agonist of sGC (Left). Values are given as mean ± SE (n = 8). * indicates statistical significance of the difference from the control at P < 0.02. In a representative experiment (Right), the administration of YC1 induced a rapid down-regulation of BDNF secretion that slowly recovered to basal level after YC1 removal. (B) BDNF secretion was evaluated in the absence (0 min) or presence (10 min and 20 min) of 1 mM 8Br-cGMP, a permeable form of cGMP (Left). The values given are the mean ± SE (n = 12). * indicates statistical significance of the difference from the control at P < 0.02. In a representative experiment (Right), the administration of 8Br-cGMP decreased the BDNF secretion following the kinetics described in A. (C) BDNF secretion evaluated in the absence (0 min) or presence (10 and 20 min) of 40 μM KT5823, a PKG inhibitor. Values are given as mean ± SE (n = 10). * indicates statistical significance of the difference from the control at P < 0.05. (D) BDNF secretion evaluated in the presence of 300 μM NOR3 and 40 μM KT5823. NOR3 was administered to hippocampal neurons either alone (10 min) or in combination with KT5823 (20 min). NOR3 induced a down-regulation of BDNF secretion, which partially recovered to basal levels after KT5823 administration. Values are given as mean ± SE (n = 6). * indicates statistical significance of the difference from the control at P < 0.02.
PKG-Mediated Down-Regulation of BDNF Secretion.
An established target of cGMP is the family of protein kinases 1α, 1β, and type II (PKG). The NO/cGMP/PKG signaling pathway negatively regulates calcium release from IP3-sensitive stores (49). Because it has been demonstrated that the release of calcium from intracellular stores is the key regulatory mechanism of NT secretion (28–31, 36), we hypothesized that the activation of PKG may represent an important signaling step responsible for the down-regulation of BDNF secretion. To explore this possibility we initially treated hippocampal neurons with 40 μM KT5823, a selective inhibitor of PKG (50). Under this condition, we observed that KT5823 itself induces an up-regulation of BDNF secretion (Fig. 4C), whereas, in experiments conducted in the continuous presence of NOR3, PKG blockade partially prevents the NO-induced down-regulation of BDNF secretion (Fig. 4D).
Discussion
Neuronal activity is thought to promote the delivery of NTs by way of the activation of neurotransmitter (28–29, 31, 36) and NT receptors (30, 32, 36). The mechanism by which NTs reach threshold levels at synaptic sites is thought to be critical for many aspects of neuronal plasticity (7–27). Although a large body of evidence describes mechanisms by which NT secretion is up-regulated (28–37, 41, 42), nothing is known about the existence of a potential negative means of modulating NT secretion. Herein, we demonstrate that the inhibition of NOS activity by L-NAME-reduced de novo synthesis of NO (Fig. 2C) that correlates with the increase from basal levels of BDNF secreted in cultured hippocampal neurons (Fig. 2D). Such NO-dependent up-regulation of BDNF secretion has been demonstrated at both transcriptional and translational levels, where the pharmacological inhibition of NOS activity enhanced BDNF mRNA synthesis and protein expression (51). Similarly, NGF mRNA expression was up-regulated in cultured glial cells and in cortical slices after in vivo inhibition of NOS (52), which is also consistent with the down-regulation of NT expression reported in mixed glial cell preparations in which the release of NGF was reduced from basal levels 24 h after NO administration (52). Such down-regulation of NGF secretion is most likely caused by an inhibition of constitutive release driven by the decrease of intracellular NGF (52).
A different mechanism emerges from our experiments in which the exogenous administration of NO with pharmacological donors down-regulated BDNF secretion within 10–20 min of incubation (Fig. 3 A and B). Furthermore, under conditions that are nonpermissive for constitutive NT secretion (29), such as lowering the temperature from 37°C to 15°C, NO was still able to down-regulate BDNF secretion (Fig. 3C). Although we cannot exclude the possibility that NO may also exert an effect on constitutive secretion, the rapidity with which BDNF secretion was down-regulated at the given temperature suggests that NO most likely acts on a regulated secretory pathway.
At the molecular level, down-regulation of BDNF secretion was mediated by the activation of sGC, an intracellular target of NO, which increases the levels of cGMP (Fig. 4 A and B), and by the downstream activation of cGMP-dependent PKG (Fig. 4 C and D). Activation of PKG raises the possibility that the molecular mechanisms involved in the down-regulation of NT secretion depend on the regulation of intracellular calcium levels. The NO/cGMP/PKG signaling pathway down-regulates the release of calcium from IP3-sensitive stores (53) by forming a complex interaction with the IP3 receptor (49). Calcium release from intracellular stores, by a phospholipase C/IP3-dependent signaling pathway, is the key event for the stimulation of NT secretion by glutamate (29, 36). Hence, two distinct and opposing intracellular signaling mechanisms, namely a glutamate-mediated activation pathway and a NO-mediated inhibition pathway, may converge to regulate calcium mobilization from intracellular stores and BDNF secretion in hippocampal neurons.
Because both endogenous NO and glutamate are released by hippocampal neurons under basal conditions, the spontaneously occurring NT release would correspond to the balance between the transduction mechanisms activated by these two signals. To support this hypothesis, we have demonstrated here that the inhibition of endogenous NO generated by NOS activity mediates an increase above the spontaneously occurring levels of BDNF secretion. Conversely, the inhibition of glutamate neurotransmission in response to treatment of hippocampal cultures with the mixture of GluR-ant results in a reduced BDNF secretion.
In summary, the regulatory system described represents a sensitive mechanism for the rapid up- or down-regulation of NT secretion in response to extracellular messengers.
Acknowledgments
We thank the Interdepartmental Center of Biotechnology for access to the confocal microscope facility and Dr. Natalia Calonghi for her kind assistance. We are grateful for the careful critical review of the manuscript by Dr. Catherine Coleman. BDNF protein, anti-BDNF antibodies, and the AdCMV-BDNF were kindly provided by Hans Thoenen (Max Planck Institute, Munich). This research was partially sponsored by the Italian Association for Cancer Research and Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- LTP
long-term potentiation
- NT
neurotrophin
- IP3
inositol 1,4,5-trisphosphate
- PKG
protein kinase G
- NGF
nerve growth factor
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- NOR3
(±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide
- YC1
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole
- 8Br-cGMP
8-bromo-cGMP
- l-NAME
l-arginine-methyl ester
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- GluR-ant
glutamate receptor antagonists
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 2.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 5.McAllister A K, Katz L C, Lo D C. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 6.Poo M-M. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 7.Lohof A M, Ip N Y, Poo M-M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 8.Knipper M, Da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 9.Knipper M, Leung L S, Zhao D, Rylett R J. NeuroReport. 1994;5:2433–2436. doi: 10.1097/00001756-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lessmann V, Gottmann K, Heumann R. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk W, Pozzo-Miller L D, Figurov A, Lu B. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y X, Zhang Y O, Lester H A, Schuman E M, Davidson N. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine E S, Crozier R A, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suen P C, Wu K, Levine E S, Mount H T, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafitz K W, Rose C R, Thoenen H, Konnerth A. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 18.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 21.Figurov A, Pozzo M L, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 22.Kang H, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 23.Akaneya Y, Tsumoto T, Hatanaka H. J Neurophysiol. 1996;76:4198–4201. doi: 10.1152/jn.1996.76.6.4198. [DOI] [PubMed] [Google Scholar]
- 24.Bear M F, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 25.Linden D J, Connor J A. Annu Rev Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- 26.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber K M, Sawtell N B, Bear M F. Neuropharmacology. 1998;37:571–579. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 28.Blochl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 29.Blochl A, Thoenen H. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 30.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griesbeck O, Canossa M, Campana G, Gartner A, Hoener M C, Nawa H, Kolbeck R, Thoenen H. Microsc Res Tech. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Kruttgen A, Moller J C, Heymach J V, Shooter E M. Proc Natl Acad Sci USA. 1998;95:15867–15872. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkowiec A, Katz D M. J Neurosci. 2000;12:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymach J V, Kruttgen A, Suter U, Shooter E M. J Biol Chem. 1996;271:25430–25437. doi: 10.1074/jbc.271.41.25430. [DOI] [PubMed] [Google Scholar]
- 35.Goodman L J, Valverde J, Lim F, Geschwind M D, Federoff H J, Geller A I, Hefti F. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 36.Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H. EMBO J. 2001;20:1640–1650. doi: 10.1093/emboj/20.7.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gartner A, Shostak Y, Hackel N, Ethell I M, Thoenen H. Mol Cell Neurosci. 2000;15:215–234. doi: 10.1006/mcne.1999.0825. [DOI] [PubMed] [Google Scholar]
- 38.Fawcett J P, Aloyz R, McLean J H, Pareek S, Miller F D. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- 39.Smith M A, Zhang L X, Lyons W E. NeuroReport. 1997;8:1829–1834. doi: 10.1097/00001756-199705260-00008. [DOI] [PubMed] [Google Scholar]
- 40.Moller J C, Kruttgen A, Heymach J V, Ghori N, Shooter E M. J Neurosci Res. 1998;51:463–472. doi: 10.1002/(SICI)1097-4547(19980215)51:4<463::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Mowla S J, Pareek S, Farhadi H F, Petrecca K, Fawcett J P, Seidah N G, Morris S J, Sossin W S, Murphy R A. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blochl A. NeuroReport. 1998;9:1701–1705. doi: 10.1097/00001756-199806010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ignarro L J. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 45.Moore P K, al-Swayeh O A, Chong N W, Evans R A, Gibson A. Br J Pharmacol. 1990;99:408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulsch A, Bauersachs J, Schafer A, Stasch J P, Kastm R, Busse R. Br J Pharmacol. 1997;120:681–689. doi: 10.1038/sj.bjp.0700982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garthwaite J, Southam E, Boulton C L, Nielsen E B, Schmidt K, Mayer B. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 48.Rapoport R M, Draznin M B, Murad F. Proc Natl Acad Sci USA. 1982;79:6470–6474. doi: 10.1073/pnas.79.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang G X, Allescher H D, Korth M, Wilm M, et al. Nature (London) 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 50.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 51.Xiong H, Yamada K, Han K, Nabeshima T, Enikopolov G, Carnahan J, Nawa H. Eur J Neurosci. 1999;11:1567–1576. doi: 10.1046/j.1460-9568.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- 52.Xiong H, Yamada K, Jourdi H, Kawamura M, Takei N, Han D, Nabeshima T, Nawa H. Mol Pharmacol. 1999;56:339–347. doi: 10.1124/mol.56.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruth P, Wang G X, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Proc Natl Acad Sci USA. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]