Abstract
1. We studied the effects of a toxic concentration of ouabain on transmembrane electrical activity and on mechanical behaviour of right ventricular papillary muscles from ferrets in a single sucrose-gap using current clamp and voltage clamp.
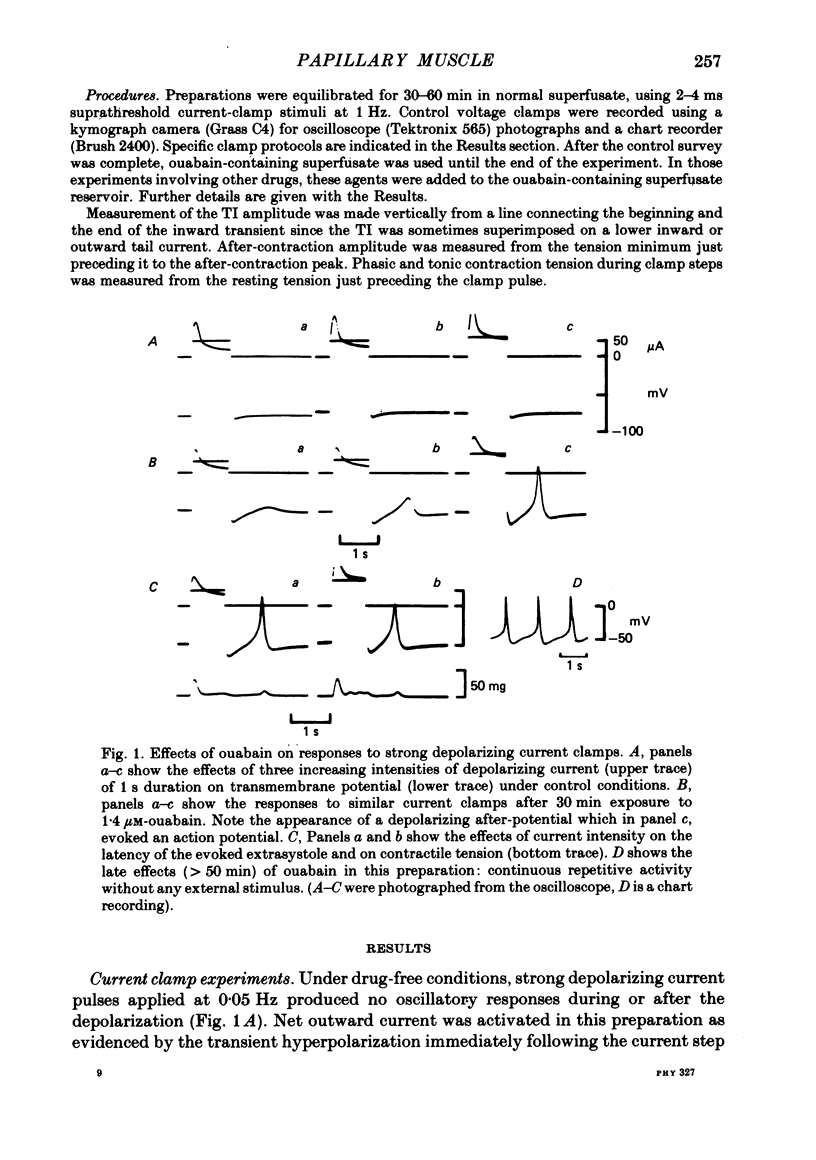
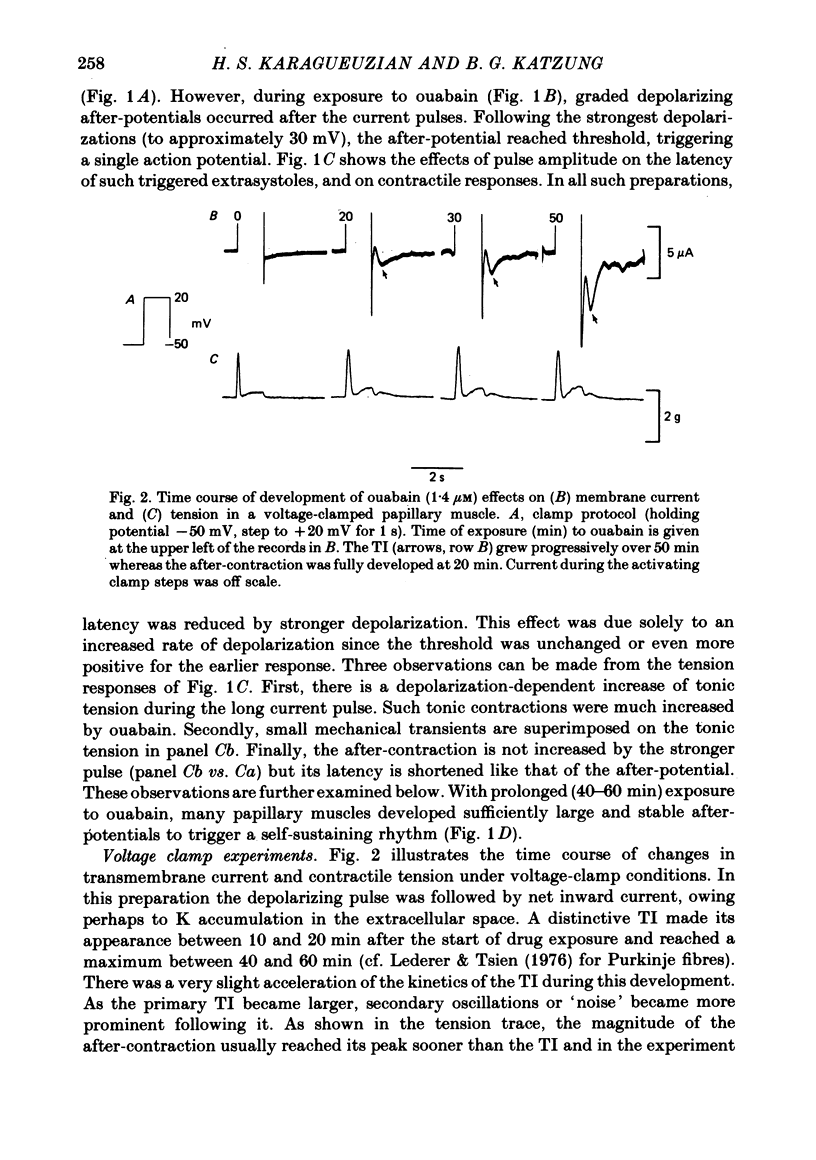
2. Ouabain (1·4-1·8 μM) induced oscillatory after-potentials and after-concentrations in current-clamp experiments. Voltage clamp showed that the oscillatory after-potential was caused by a transient inward current, similar to that in Purkinje fibres.
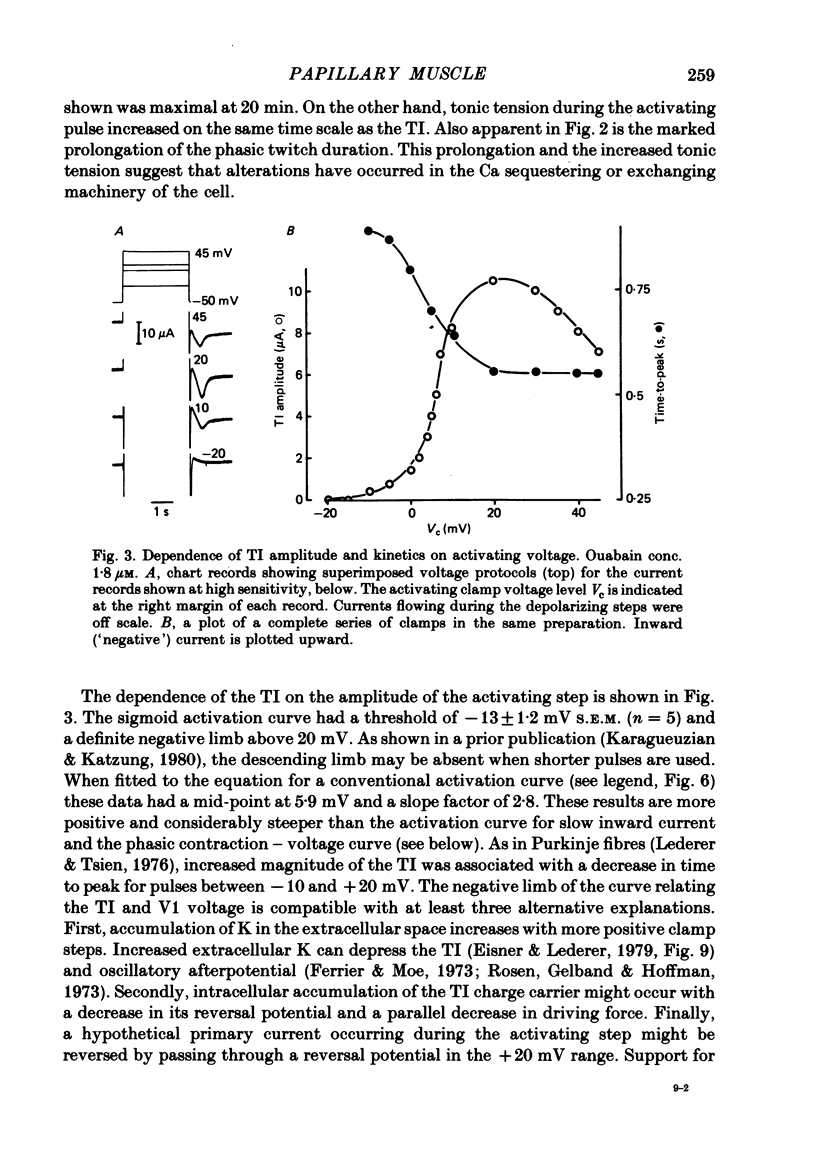
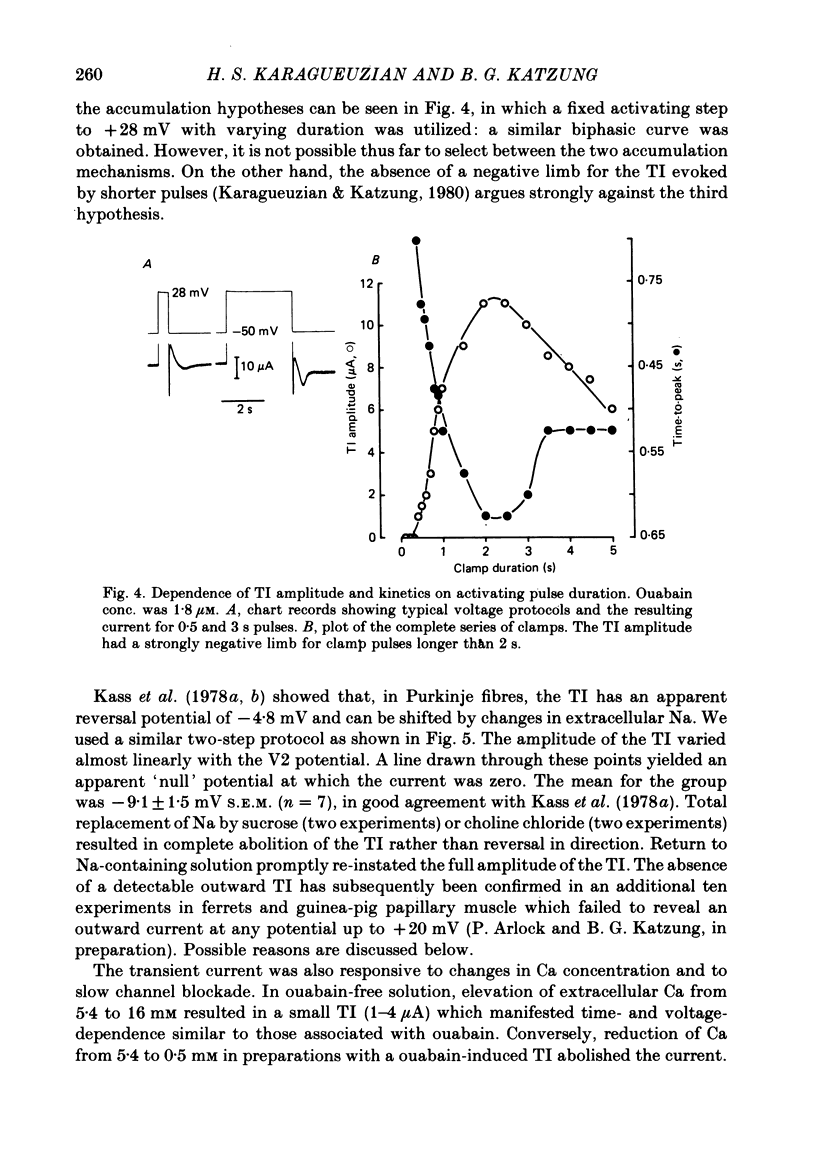
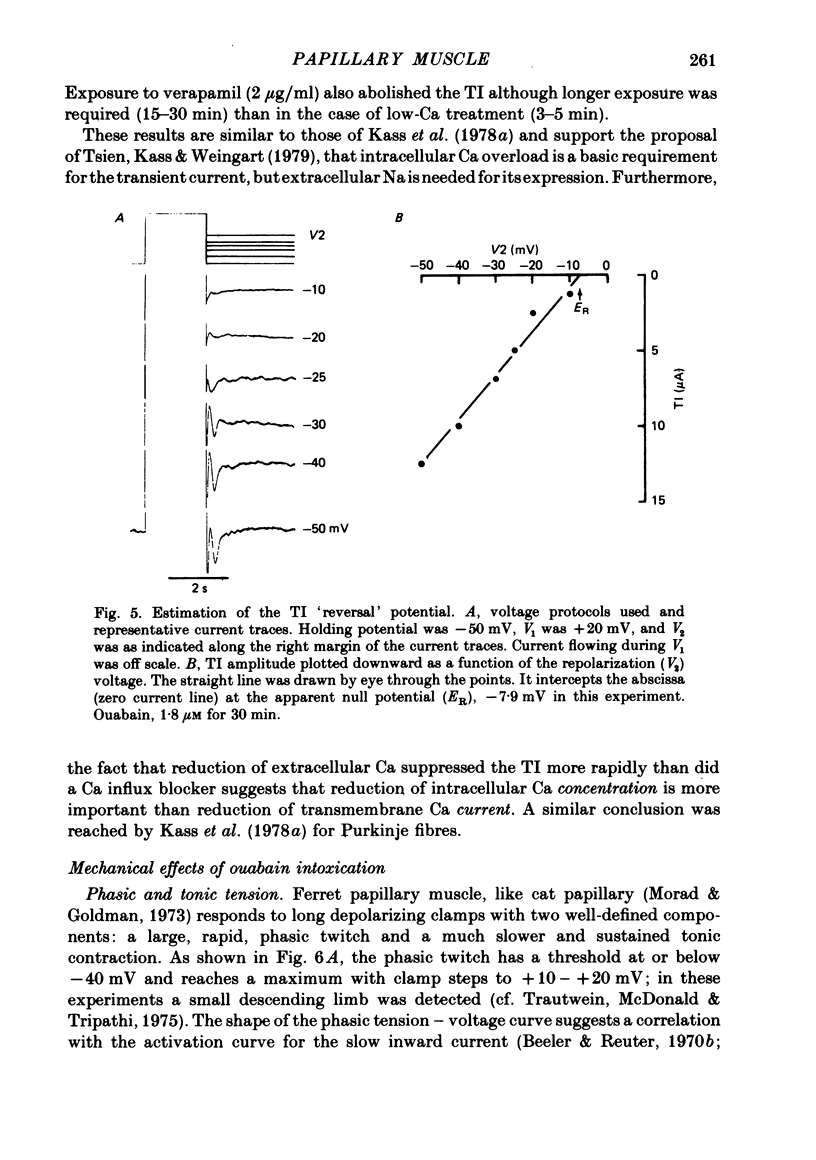
3. The transient current had a sigmoidal dependence on the preceding (activating) voltage step V1, with a treshold around -13 mV and a plateau between +10 and 20 mV. There was a decline in current amplitude for more positive clamps. When activated by a fixed V1 voltage step, and measured at different repolarization levels V2, the transient current manifested an inverse dependence on V2 between -50 and -10 mV. No outward transient current could be detected. Total replacement of Na in the bathing medium by Tris or by sucrose abolished the transient current.
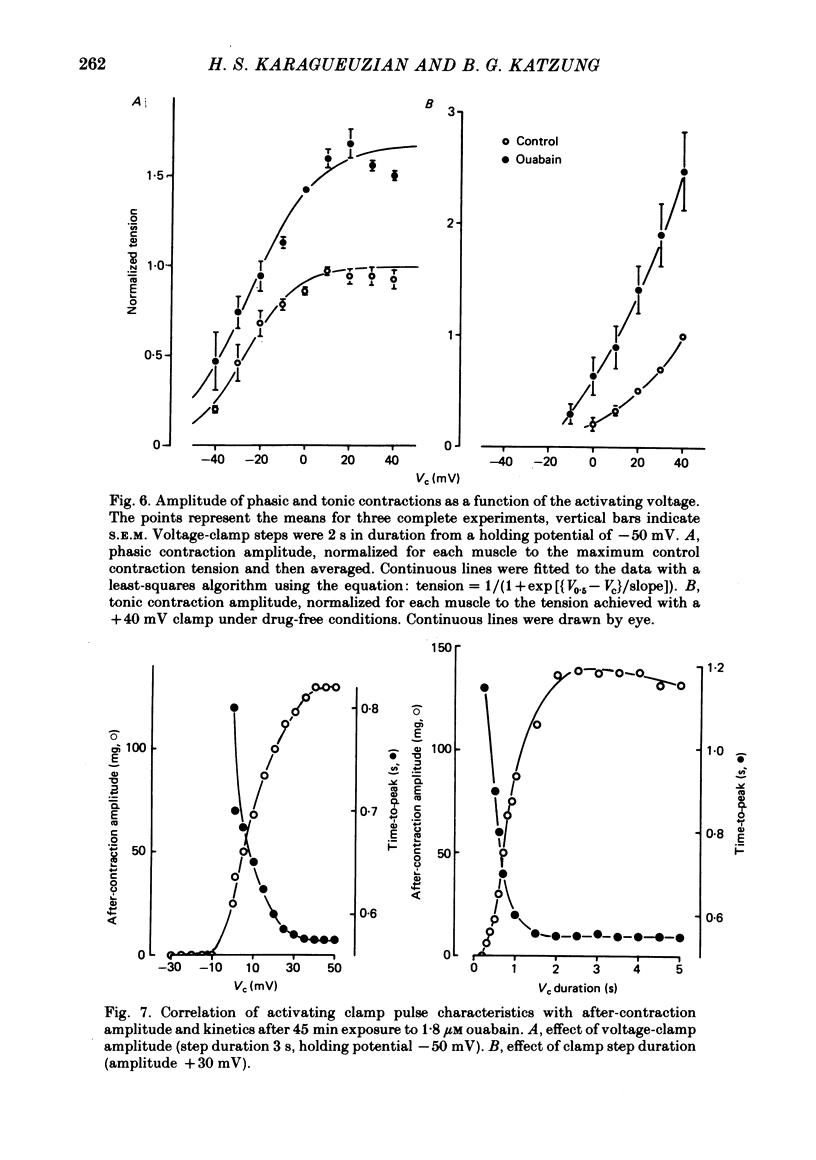
4. Ouabain caused an increase of phasic (twitch) tension responses to voltage steps at all potentials without shifting the curve relating these variables on the voltage axis. The drug evoked an even greater increase in the tonic tension responses.
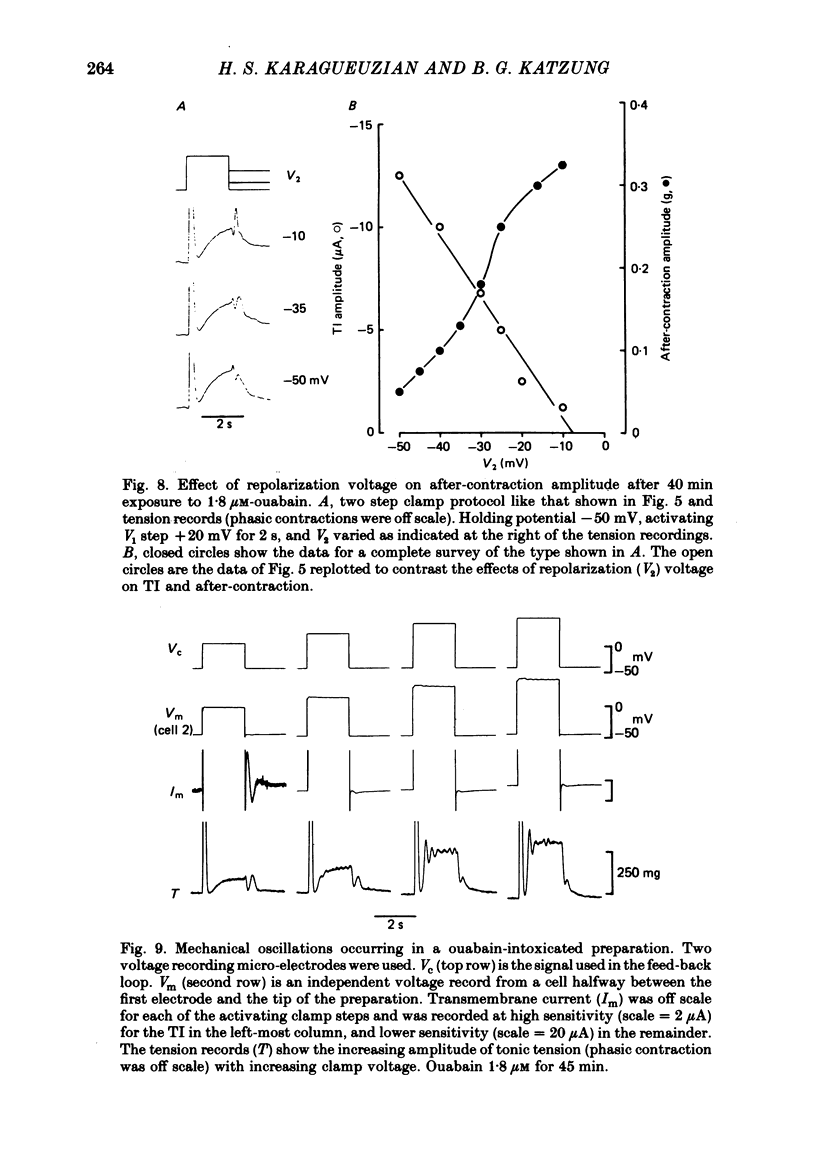
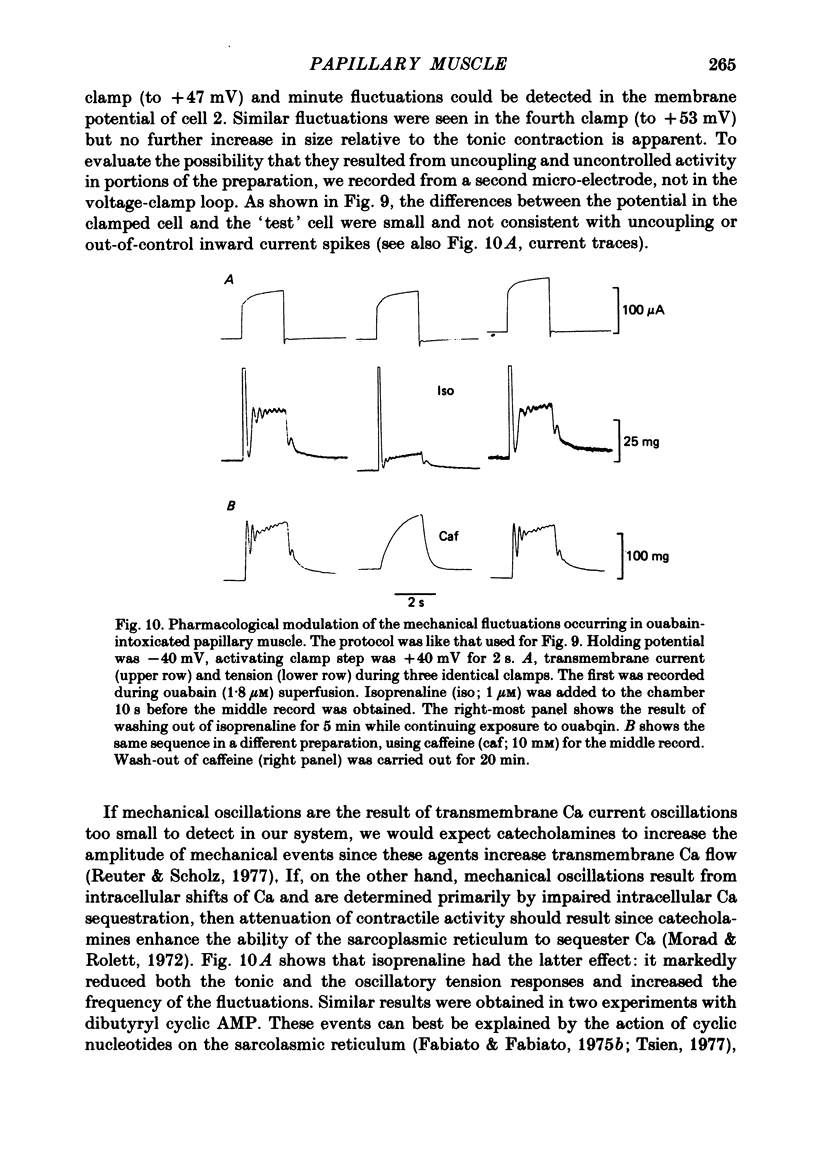
5. After prolonged exposure, oscillatory mechanical responses were frequently recorded during positive voltage steps. Unlike the after-contraction, these mechanical fluctuations were not consistently damped and were not accompanied by detectable synchronous current fluctuations. Catecholamines and dibutyryl cyclic AMP markedly reduced the amplitude of the tonic contraction and the mechanical oscillations but increased their frequency. Caffeine had no effect on the tonic contraction amplitude but abolished the fluctuations.
6. These results support the proposal that Ca is transiently released from the overloaded sarcoplasmic reticulum in ouabain-intoxicated muscle and may evoke oscillatory responses in nearby contractile fibrils. When these transient increases of sarcoplasmic free Ca are large enough, they may induce the transient transmembrane current described above.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akselrod S., Landau E. M., Lass Y. Electromechanical noise in atrial muscle cells of the carp: a possible ionic feed-back mechanism. J Physiol. 1979 May;290(2):387–397. doi: 10.1113/jphysiol.1979.sp012777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. The effect of membrane potential on the calcium transport systems in squid axons [proceedings]. J Physiol. 1976 Sep;260(2):24P–25P. [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Rapp P. E. A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol. 1979 Aug;81:217–279. doi: 10.1242/jeb.81.1.217. [DOI] [PubMed] [Google Scholar]
- Bozler E., Delahayes J. F. Mechanical and electrical oscillations in cardiac muscle of the turtle. J Gen Physiol. 1973 Nov;62(5):523–534. doi: 10.1085/jgp.62.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The interaction of sodium and calcium ions at the cell membrane and the control of contractile strength in frog atrial muscle. J Physiol. 1980 Aug;305:109–123. doi: 10.1113/jphysiol.1980.sp013353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entman M. L., Levey G. S., Epstein S. E. Mechanism of action of epinephrine and glucagon on the canine heart. Evidence for increase in sarcotubular calcium stores mediated by cyclic 3',5'-AMP. Circ Res. 1969 Oct;25(4):429–438. doi: 10.1161/01.res.25.4.429. [DOI] [PubMed] [Google Scholar]
- Ettinger P. O., Calabro J., Regan T. J., Oldewurtel H. A. Origin of acetyl strophanthidin-induced ventricular arrhythmias. J Clin Invest. 1977 Feb;59(2):193–202. doi: 10.1172/JCI108629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Relaxing and inotropic effects of cyclic AMP on skinned cardiac cells. Nature. 1975 Feb 13;253(5492):556–558. doi: 10.1038/253556b0. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 1977 May-Jun;19(6):459–474. doi: 10.1016/0033-0620(77)90010-x. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R. Effects of transmembrane potential on oscillatory afterpotentials induced by acetylstrophanthidin in canine ventricular tissues. J Pharmacol Exp Ther. 1980 Nov;215(2):332–341. [PubMed] [Google Scholar]
- Ferrier G. R., Moe G. K. Effect of calcium on acetylstrophanthidin-induced transient depolarizations in canine Purkinje tissue. Circ Res. 1973 Nov;33(5):508–515. doi: 10.1161/01.res.33.5.508. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Spontaneous tension oscillations in guinea-pig atrial trabeculae. Pflugers Arch. 1975 Jul 9;358(1):11–25. doi: 10.1007/BF00584566. [DOI] [PubMed] [Google Scholar]
- Greenspan A. M., Morad M. Electromechanical studies on the inotropic effects of acetylstrophanthidin in ventricular muscle. J Physiol. 1975 Dec;253(2):357–384. doi: 10.1113/jphysiol.1975.sp011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Sodium-calcium exchange in regulation of cardiac contractility. Evidence for an electrogenic, voltage-dependent mechanism. J Gen Physiol. 1979 Apr;73(4):403–424. doi: 10.1085/jgp.73.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagueuzian H. S., Katzung B. G. Ionic basis of digitalis toxicity in mammalian ventricle: demonstration of transient inward current. Proc West Pharmacol Soc. 1980;23:281–284. [PubMed] [Google Scholar]
- Karagueuzian H. S., Katzung B. G. Relative inotropic and arrhythmogenic effects of five cardiac steroids in ventricular myocardium: oscillatory afterpotentials and the role of endogenous catecholamines. J Pharmacol Exp Ther. 1981 Aug;218(2):348–356. [PubMed] [Google Scholar]
- Karaguezian H. S., Fenoglio J. J., Jr, Weiss M. B., Wit A. L. Coronary occlusion and reperfusion: effects on subendocardial cardiac fibers. Am J Physiol. 1980 Apr;238(4):H581–H593. doi: 10.1152/ajpheart.1980.238.4.H581. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Repke D. I., Dunnett J., Hasselbach W. Dependence of calcium permeability of sarcoplasmic reticulum vesicles on external and internal calcium ion concentrations. J Biol Chem. 1977 Mar 25;252(6):1950–1956. [PubMed] [Google Scholar]
- Katzung B. G. Effects of extracellular calcium and sodium on depolarization-induced automaticity in guinea pig papillary muscle. Circ Res. 1975 Jul;37(1):118–127. doi: 10.1161/01.res.37.1.118. [DOI] [PubMed] [Google Scholar]
- Kirchberger M. A., Tada M., Katz A. M. Adenosine 3':5'-monophosphate-dependent protein kinase-catalyzed phosphorylation reaction and its relationship to calcium transport in cardiac sarcoplasmic reticulum. J Biol Chem. 1974 Oct 10;249(19):6166–6173. [PubMed] [Google Scholar]
- Lakatta E. G., Lappé D. L. Diastolic scattered light fluctuation, resting force and twitch force in mammalian cardiac muscle. J Physiol. 1981 Jun;315:369–394. doi: 10.1113/jphysiol.1981.sp013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C., Katzung B. G. Mechanism of arrhythmias caused by ouabain in depolarized ventricular myocardium. Proc West Pharmacol Soc. 1977;20:263–268. [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976 Dec;263(2):73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Nawrath H., Trautwein W. Membrane currents and tension in cat ventricular muscle treated with cardiac glycosides. Circ Res. 1975 Nov;37(5):674–682. doi: 10.1161/01.res.37.5.674. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba M. Effects of caffeine on tension development in dog papillary muscle under voltage clamp. Jpn J Physiol. 1973 Feb;23(1):47–58. [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Hoffman B. F. Correlation between effects of ouabain on the canine electrocardiogram and transmembrane potentials of isolated Purkinje fibers. Circulation. 1973 Jan;47(1):65–72. doi: 10.1161/01.cir.47.1.65. [DOI] [PubMed] [Google Scholar]
- Somberg J. C., Barry W. H., Smith T. W. Differing sensitivities of Purkinje fibers and myocardium to inhibition of monovalent cation transport by digitalis. J Clin Invest. 1981 Jan;67(1):116–123. doi: 10.1172/JCI110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Tsien R. W., Kass R. S., Weingart R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979 Aug;81:205–215. doi: 10.1242/jeb.81.1.205. [DOI] [PubMed] [Google Scholar]
- VASSALLE M., KARIS J., HOFFMAN B. F. Toxic effects of ouabain on Purkinje fibers and ventricular muscle fibers. Am J Physiol. 1962 Sep;203:433–439. doi: 10.1152/ajplegacy.1962.203.3.433. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Spontaneous mechanical activity in depolarized frog ventricle. J Gen Physiol. 1976 Aug;68(2):145–157. doi: 10.1085/jgp.68.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]


