Abstract
1. The purpose of this study was to determine whether the phasic bursting activity, characteristic of certain magnocellular neuropeptidergic neurones in rat hypothalamus, is dependent upon chemical synaptic input.
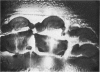
2. Slices of hypothalamus were placed in an in vitro chamber with hippocampal slices. The synaptic response in the CA1 cell layer from Schaffer collateral stimulation was monitored before, during and after synaptic transmission was blocked by superfusion of medium containing high Mg2+ (either 18·7 or 9·3 mM) and low Ca2+ (0·05 mM). This well studied pathway was chosen as an assay of synaptic blockade because hypothalamic circuitry is relatively unknown.
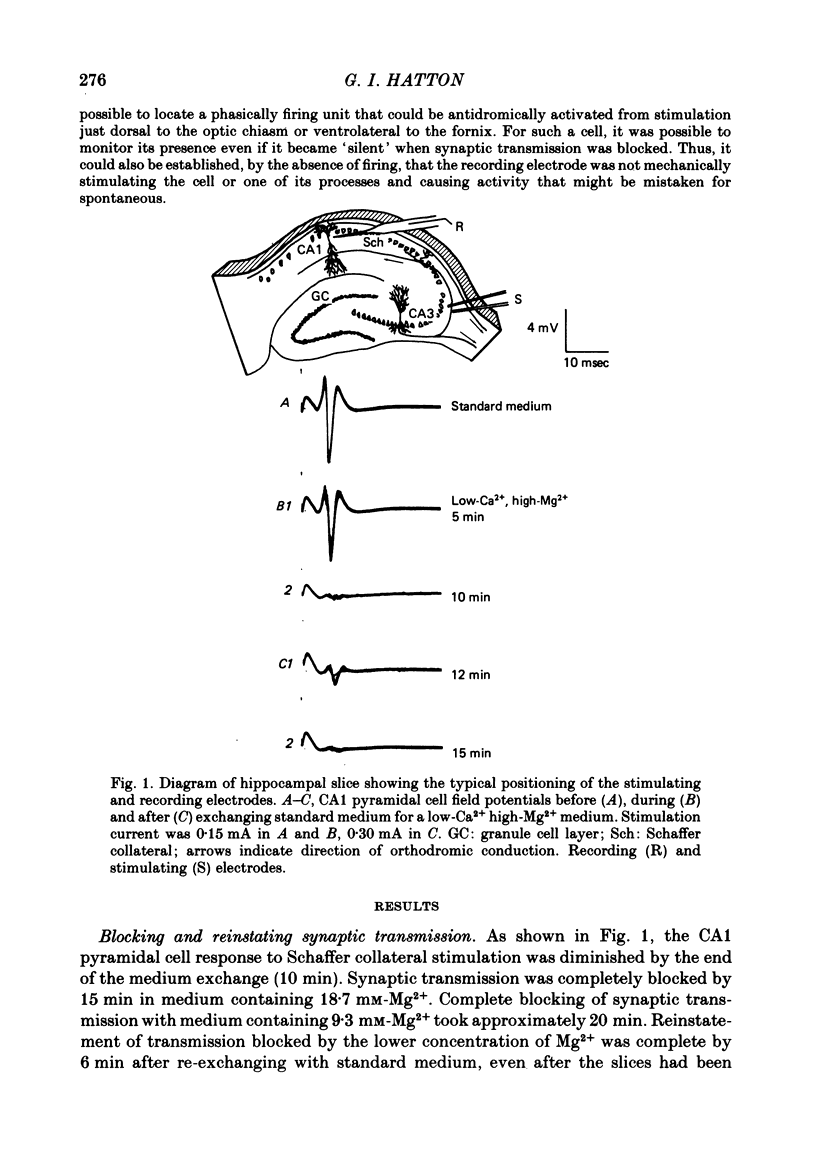
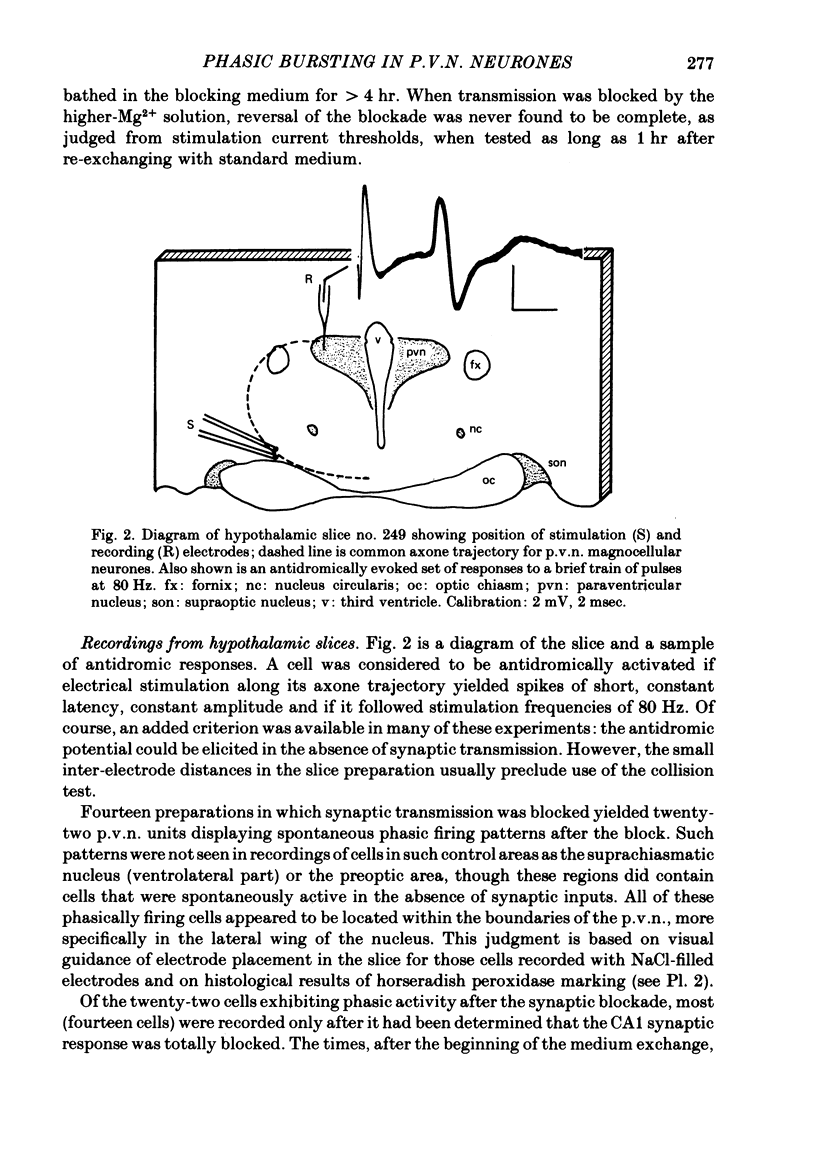
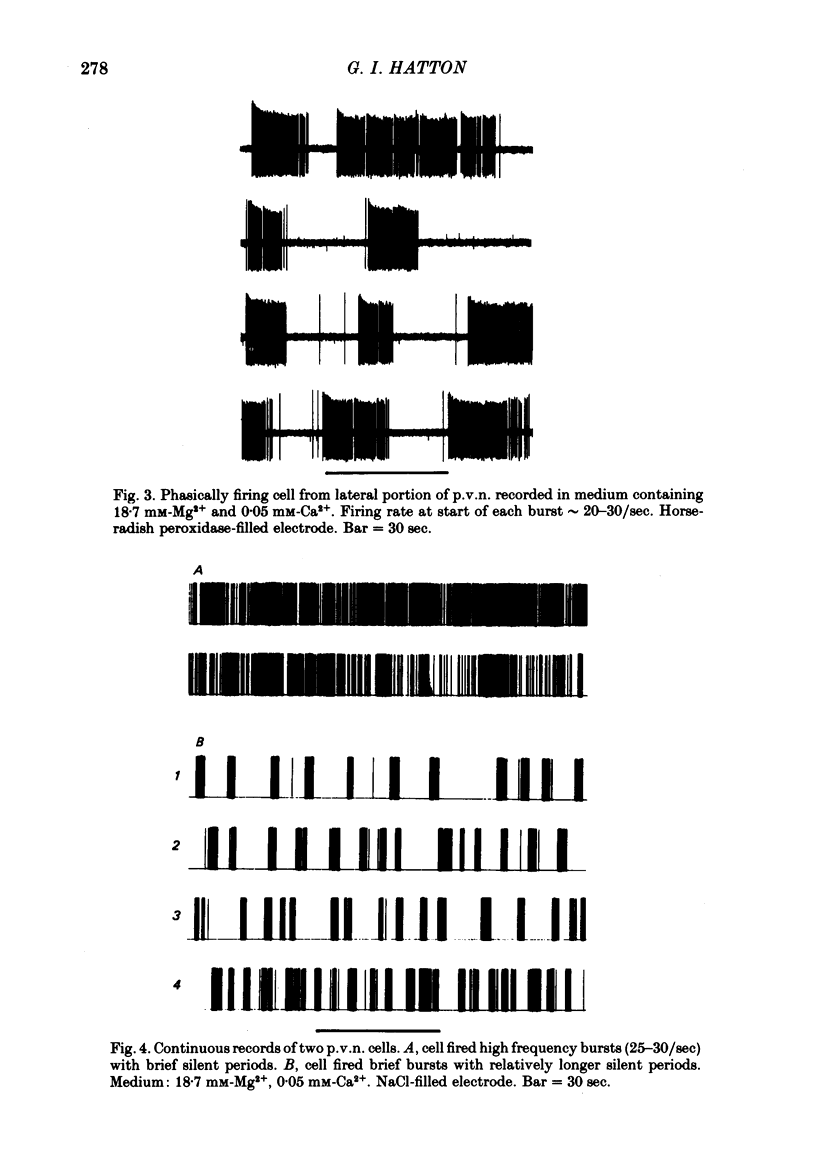
3. The electrical activity of twenty-two phasic bursting neurones in the lateral portion of the paraventricular nucleus (p.v.n.) was recorded. Nineteen of twenty-two phasic p.v.n. neurones were recorded only after synaptic transmission was blocked. The remaining three cells were firing phasically in standard medium when first encountered and continued to display phasic bursting activity for up to 1·25 hr after synaptic blockade. Active cells in nearby hypothalamic areas did not show phasic bursting patterns either before or after synaptic transmission was blocked.
4. The phasic bursting activity of the p.v.n. neurones in this study and that of previously reported p.v.n. cells in vivo were similar in (a) firing rate within bursts (b) burst length and (c) silent period duration.
5. It is concluded that phasic bursting in p.v.n. magnocellular neuropeptidergic cells is not dependent upon synaptically mediated excitation or recurrent inhibition as has been hypothesized earlier.
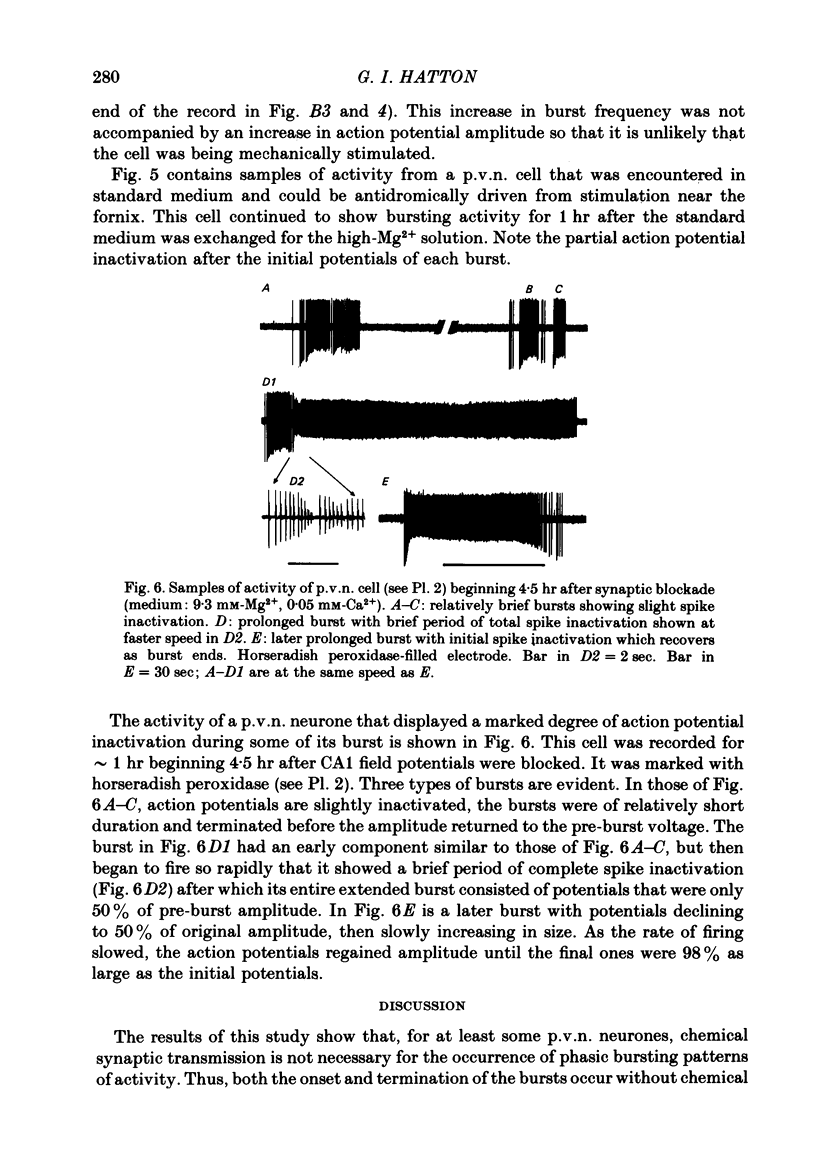
6. Alternative hypotheses, based upon acute changes in [K+]o, endogenous membrane currents and electrotonic coupling are discussed as possible explanations of phasic bursting in these magnocellular neuropeptidergic cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., MacVicar B. A., Dudek F. E., Hatton G. I. Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science. 1981 Mar 13;211(4487):1187–1189. doi: 10.1126/science.7466393. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Warach S., Hatton G. I., McNeill T. H. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Dufy B., Vincent J. D. Hypothalamic supraoptic neurones: rates and patterns of action potential firing during water deprivation in the unanaesthetized monkey. Brain Res. 1975 Dec 19;100(2):315–325. doi: 10.1016/0006-8993(75)90485-0. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Vincent J. D., Dreifuss J. J. Firing patterns of hypothalamic supraoptic neurons during water deprivation in monkeys. Science. 1974 Aug 9;185(4150):535–537. doi: 10.1126/science.185.4150.535. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Antidromic and orthodromic responses of paraventricular and supraoptic neurosecretory cells. Brain Res. 1971 Oct 29;33(2):353–366. doi: 10.1016/0006-8993(71)90108-9. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. The activity of identified supraoptic neurones and their response to acetylcholine applied by iontophoresis. J Physiol. 1972 Jan;220(1):105–118. doi: 10.1113/jphysiol.1972.sp009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek F. E., Hatton G. I., Macvicar B. A. Intracellular recordings from the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1980 Apr;301:101–114. doi: 10.1113/jphysiol.1980.sp013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971 Apr;214(2):245–256. doi: 10.1113/jphysiol.1971.sp009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Single unit activity in the hypothalamo-neurohypophysial system of Brattleboro rats. J Endocrinol. 1974 Jan;60(1):135–143. doi: 10.1677/joe.0.0600135. [DOI] [PubMed] [Google Scholar]
- Finley J. C., Maderdrut J. L., Petrusz P. The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol. 1981 Jun 1;198(4):541–565. doi: 10.1002/cne.901980402. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Intracellular calcium and the control of neuronal pacemaker activity. Fed Proc. 1981 Jun;40(8):2233–2239. [PubMed] [Google Scholar]
- Gregory W. A., Tweedle C. D., Hatton G. I. Ultrastructure of neurons in the paraventricular nucleus of normal, dehydrated and rehydrated rats. Brain Res Bull. 1980 May-Jun;5(3):301–306. doi: 10.1016/0361-9230(80)90173-2. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Dreifuss J. J. Phasically firing neurons in long-term cultures of the rat hypothalamic supraoptic area: pacemaker and follower cells. Brain Res. 1979 Nov 9;177(1):95–103. doi: 10.1016/0006-8993(79)90920-x. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Armstrong W. E., Gregory W. A. Spontaneous and osmotically-stimulated activity in slices of rat hypothalamus. Brain Res Bull. 1978 Sep-Oct;3(5):497–508. doi: 10.1016/0361-9230(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Doran A. D., Salm A. K., Tweedle C. D. Brain slice preparation: hypothalamus. Brain Res Bull. 1980 Jul-Aug;5(4):405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Hutton U. E., Hoblitzell E. R., Armstrong W. E. Morphological evidence for two populations of magnocellular elements in the rat paraventricular nucleus. Brain Res. 1976 May 21;108(1):187–193. doi: 10.1016/0006-8993(76)90176-1. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R. ELECTRICAL PROPERTIES OF HYPOTHALAMIC NEUROENDOCRINE CELLS. J Gen Physiol. 1964 Mar;47:691–717. doi: 10.1085/jgp.47.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Dreifuss J. J. Antidromic inhibition of identified rat supraoptic neurones. Brain Res. 1970 Sep 16;22(3):406–409. doi: 10.1016/0006-8993(70)90483-x. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Yamashita H. Studies of antidromically identified neurosecretory cells of the hypothalamus by intracellular and extracellular recordings. J Physiol. 1972 Mar;221(3):683–705. doi: 10.1113/jphysiol.1972.sp009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E., Reiffenstein R. J. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol. 1980 May;58(5):579–582. doi: 10.1139/y80-097. [DOI] [PubMed] [Google Scholar]
- Martin R., Voigt K. H. Enkephalins co-exist with oxytocin and vasopressin in nerve terminals of rat neurohypophysis. Nature. 1981 Feb 5;289(5797):502–504. doi: 10.1038/289502a0. [DOI] [PubMed] [Google Scholar]
- Negoro H., Holland R. C. Inhibition of unit activity in the hypothalamic paraventricular nucleus following antidromic activation. Brain Res. 1972 Jul 20;42(2):385–402. doi: 10.1016/0006-8993(72)90538-0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. The pharmacology of recurrent inhibition in the supraoptic neurosecretory system. Brain Res. 1971 Dec 24;35(2):501–511. doi: 10.1016/0006-8993(71)90491-4. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Blume H. W., Renaud L. P. Connections of the hypothalamic paraventricular nucleus with the neurohypophysis, median eminence, amygdala, lateral septum and midbrain periaqueductal gray: an electrophysiological study in the rat. Brain Res. 1981 Jun 29;215(1-2):15–28. doi: 10.1016/0006-8993(81)90488-1. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Sar M., Stumpf W. E., Miller R. J., Chang K. J., Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol. 1978 Nov 1;182(1):17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Sladek C. D., Knigge K. M. Osmotic control of vasopressin release by rat hypothalamo-neurohypophyseal explants in organ culture. Endocrinology. 1977 Dec;101(6):1834–1838. doi: 10.1210/endo-101-6-1834. [DOI] [PubMed] [Google Scholar]
- Teyler T. J. Brain slice preparation: hippocampus. Brain Res Bull. 1980 Jul-Aug;5(4):391–403. doi: 10.1016/s0361-9230(80)80009-8. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977 Jun 20;181(1):59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Ultrastructural comparisons of neurons of supraoptic and circularis nuclei in normal and dehydrated rats. Brain Res Bull. 1976 Jan-Feb;1(1):103–121. doi: 10.1016/0361-9230(76)90054-x. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Poulain D. A., Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978 Jun 16;148(2):425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- Walters J. K., Hatton G. I. Supraoptic neuronal activity in rats during five days of water deprivation. Physiol Behav. 1974 Nov;13(5):661–667. doi: 10.1016/0031-9384(74)90237-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Activation of hippocampal neurons by mossy fiber stimulation in thin brain sections in vitro. Exp Brain Res. 1972;14(4):423–435. doi: 10.1007/BF00235037. [DOI] [PubMed] [Google Scholar]